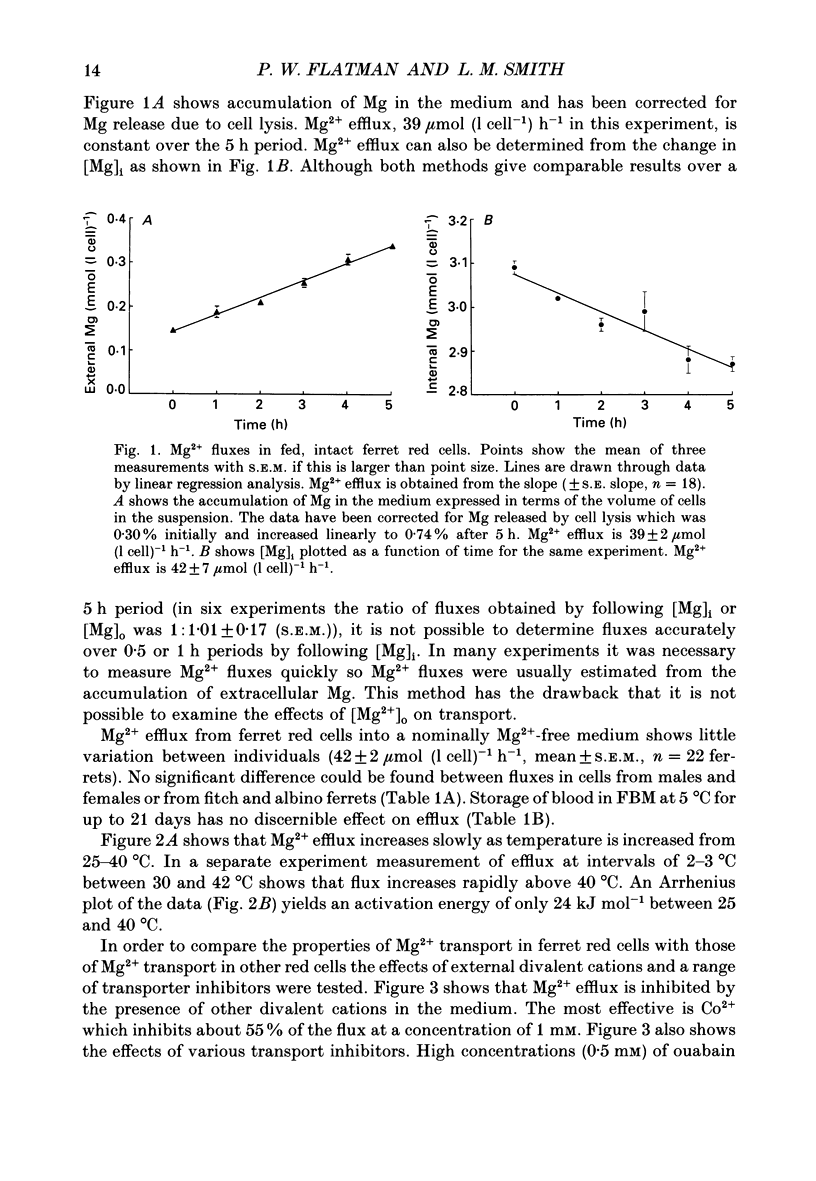
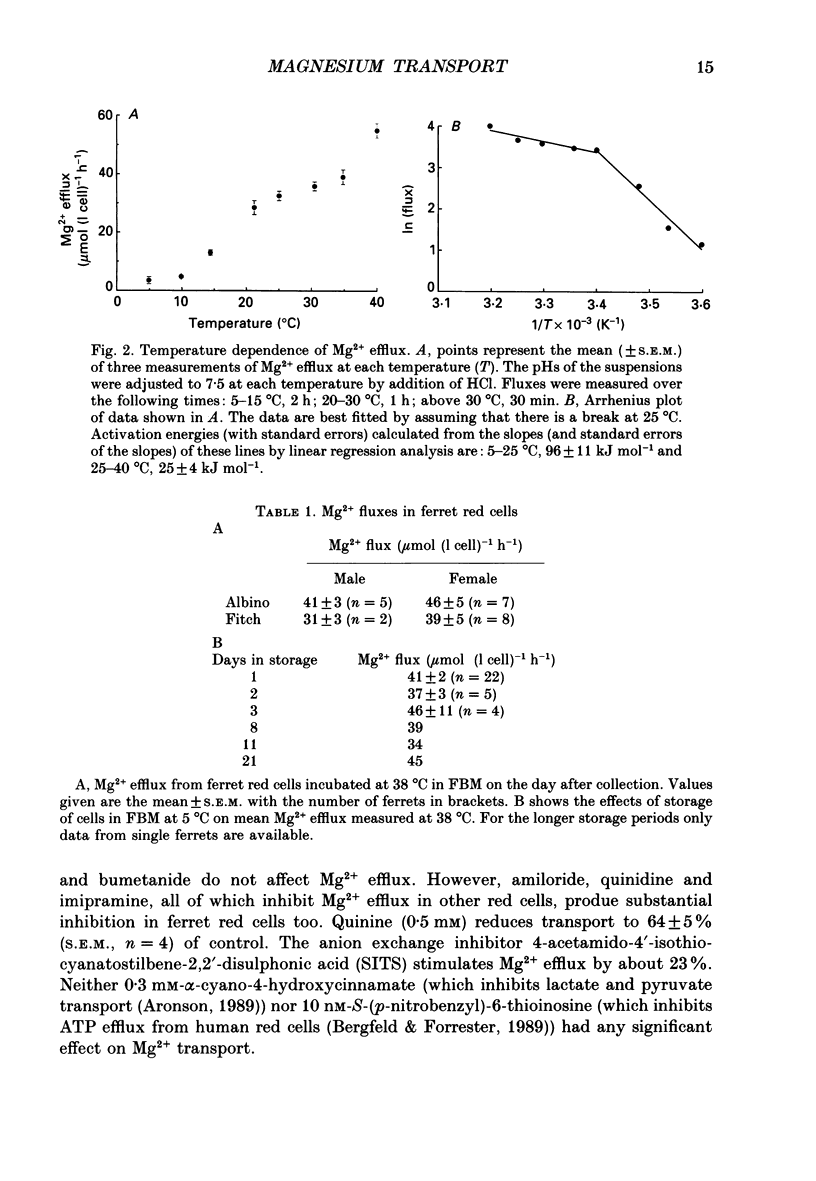
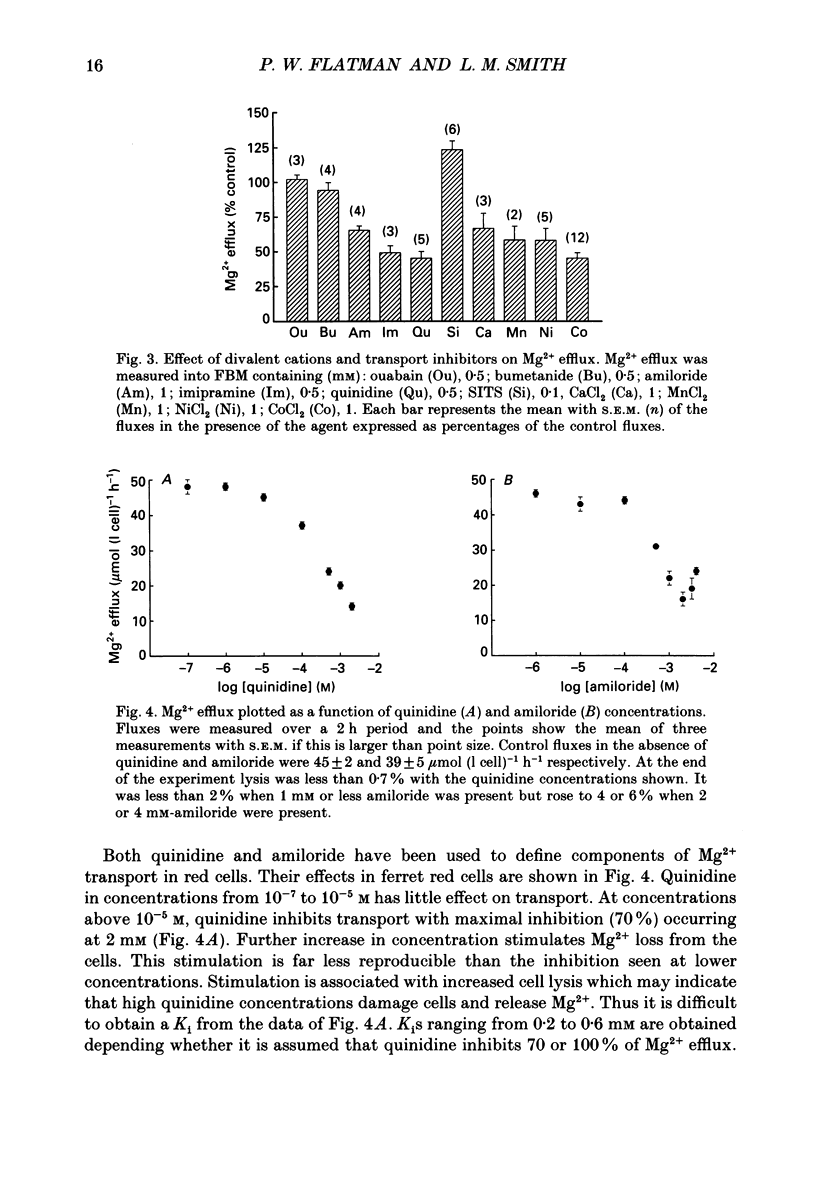
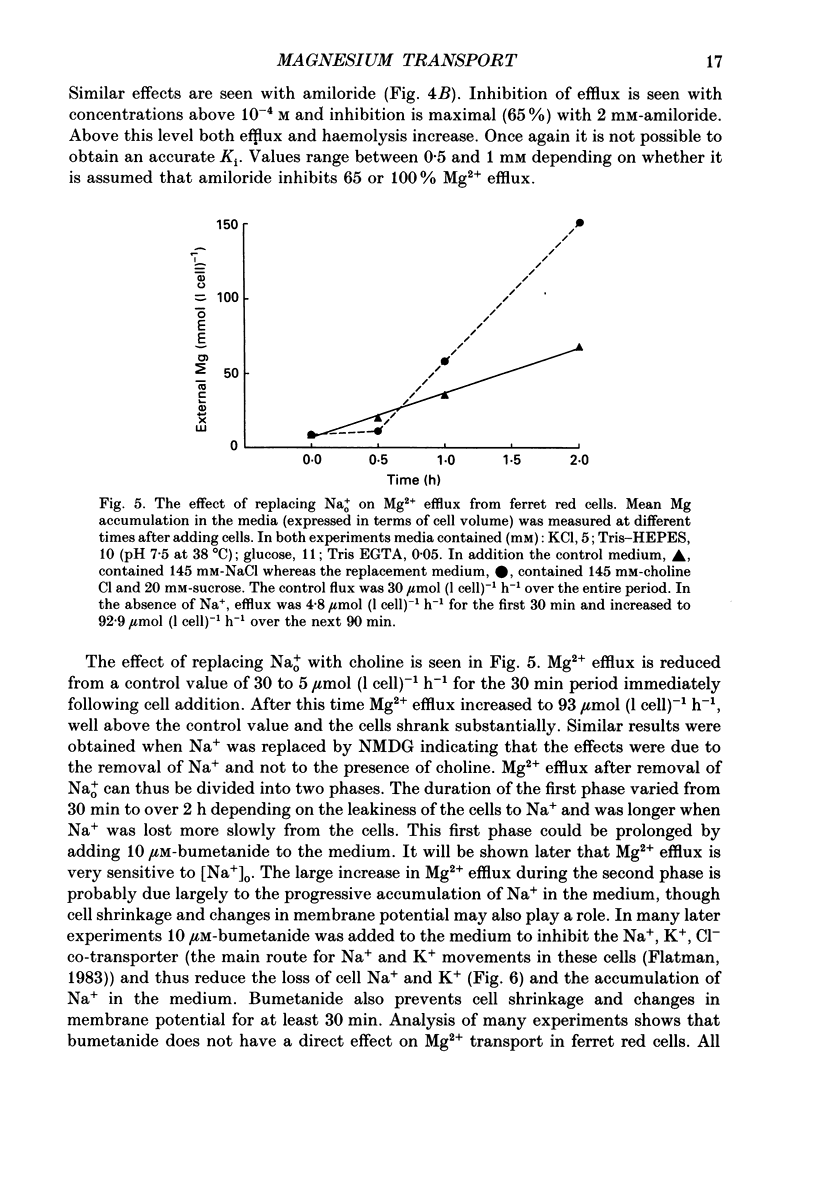
Abstract
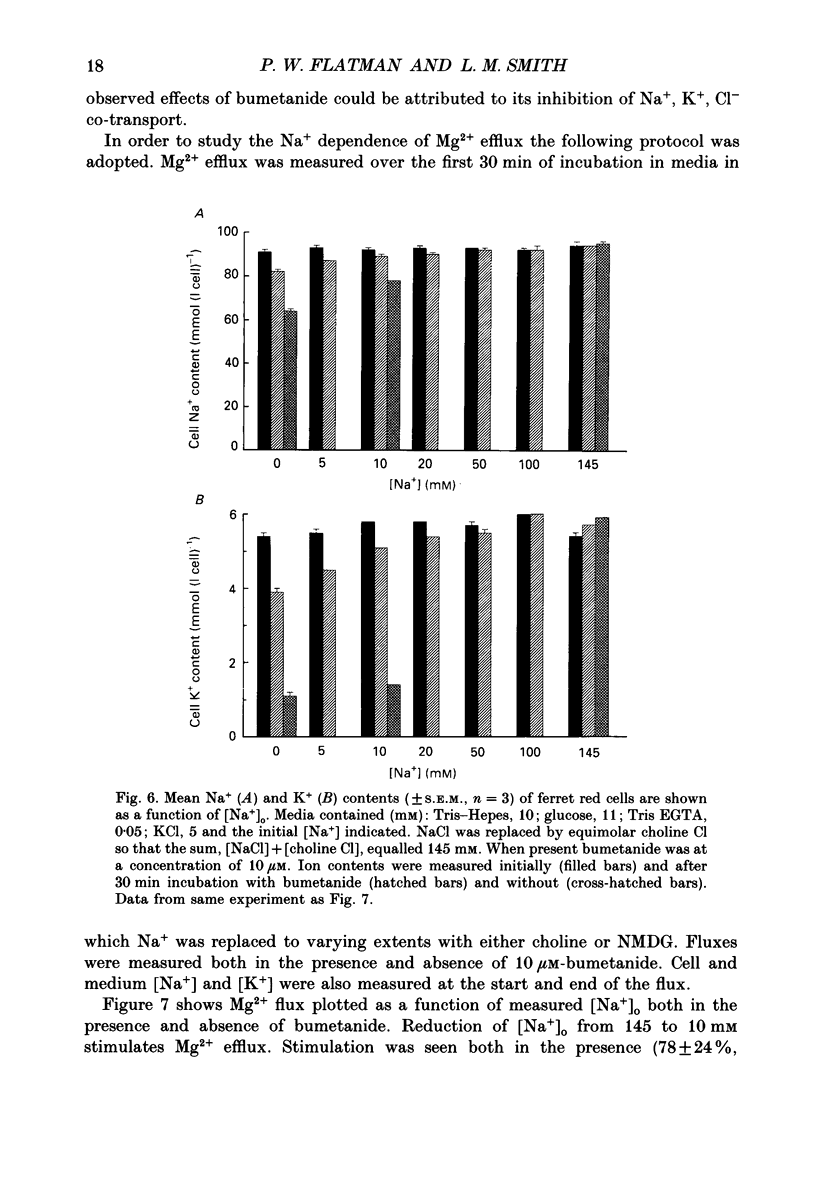
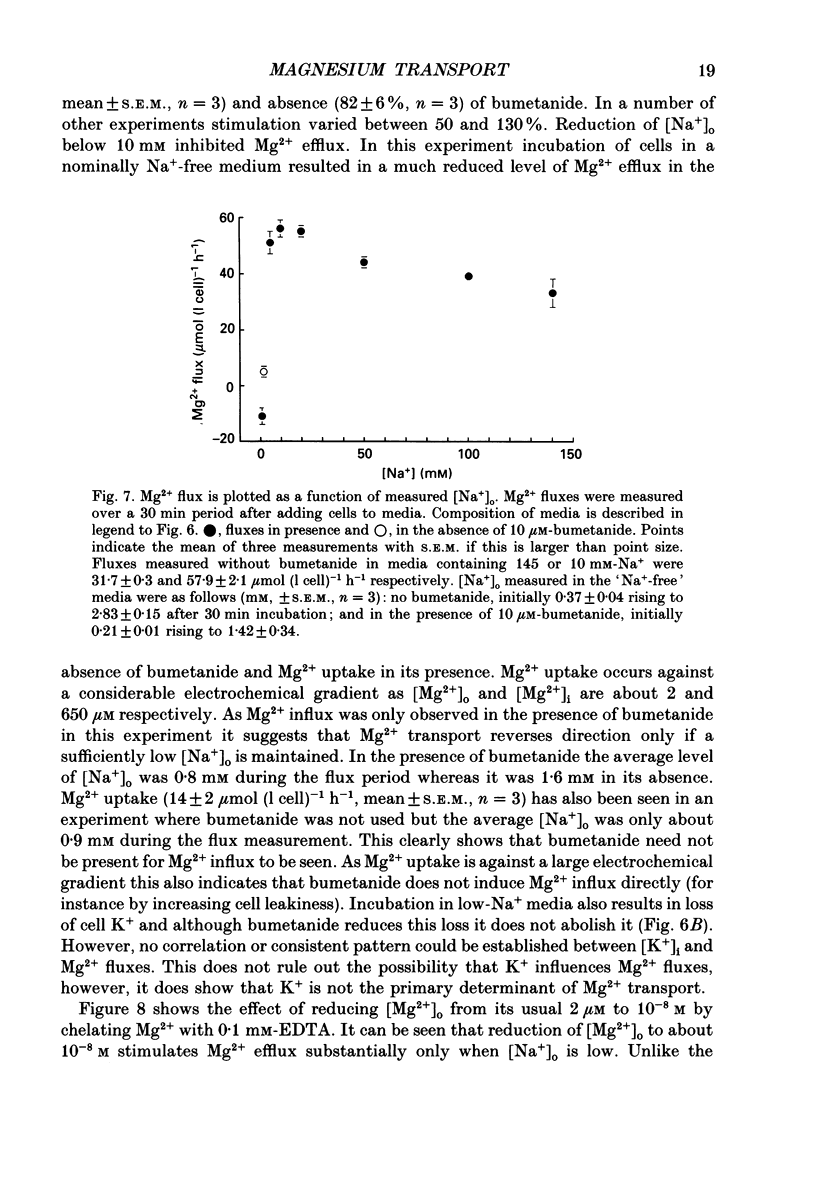
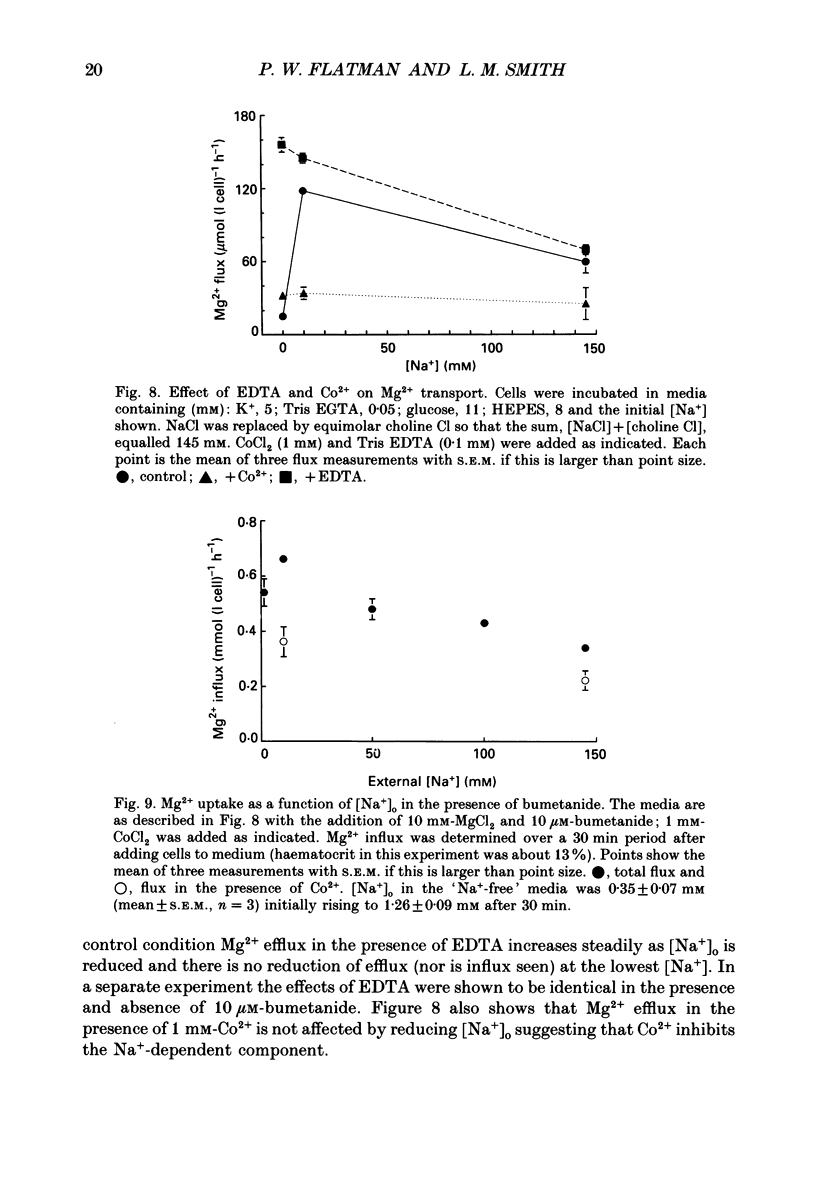
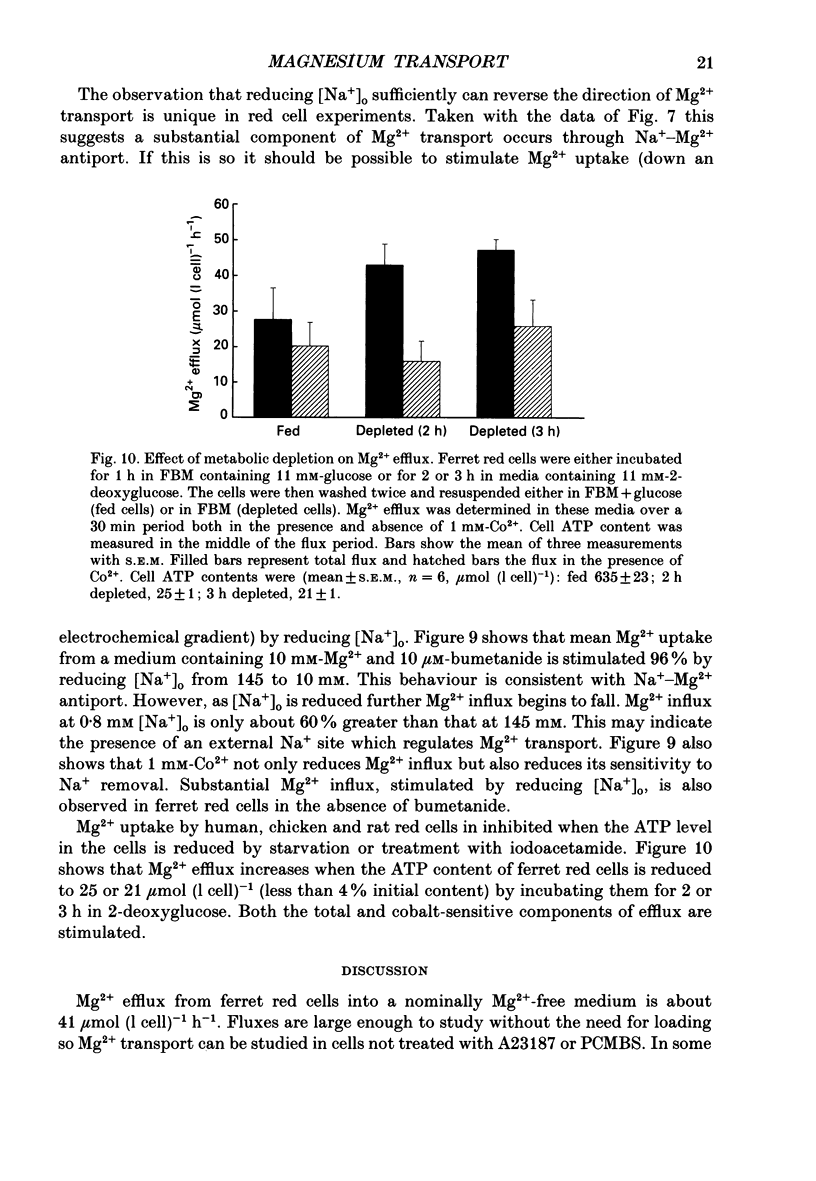
1. Mg2+ efflux from ferret red cells into a nominally Mg2(+)-free medium is 41 +/- 2 mumol (l cell)-1 h-1. The properties of Mg2+ transport can be measured in these cells without the need for Mg2+ loading. 2. Amiloride, quinidine, imipramine and external divalent cations partially inhibit Mg2+ efflux. Maximal inhibition by these agents is about 60-70% suggesting that at least two Mg2+ transport pathways exist. 3. As external Na+ is replaced by choline or N-methyl-D-glucamine Mg2+ efflux is first stimulated, reaching a peak when external [Na+] ([Na+]o) is about 10 mM, and then inhibited. Mg2+ transport reverses direction so net Mg2+ uptake occurs when [Na+]o is reduced below 1 mM. 4. Mg2+ efflux is stimulated when 0.1 mM-EDTA is added to the medium only when [Na+]o is low. 5. Reduction of cell ATP content to about 20 mumol (l cell)-1 by treating cells with 2-deoxyglucose stimulates Mg2+ efflux measured over the 2 h period following depletion. 6. Substantial Mg2+ influx can be observed in ferret red cells when they are incubated in media containing 10 mM-Mg2+. Influx is stimulated by reducing [Na+]o to 10 mM. Further reduction of [Na+]o to below 1 mM reduces Mg2+ uptake. A component of uptake is inhibited by external Co2+. 7. Na(+)-Mg2+ antiport may account for a substantial component of Mg2+ transport in ferret red cells. The direction of transport can be reversed by sufficiently lowering [Na+]o or by increasing external [Mg2+]. Analysis of the conditions at which transport reverses direction suggests transport with a stoichiometry of 1 Na+:1 Mg2+. Antiport with this stoichiometry would also explain maintenance of the physiological level of intracellular ionized Mg2+ in these cells.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson P. S. The renal proximal tubule: a model for diversity of anion exchangers and stilbene-sensitive anion transporters. Annu Rev Physiol. 1989;51:419–441. doi: 10.1146/annurev.ph.51.030189.002223. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Crawford A. C. Mobility and transport of magnesium in squid giant axons. J Physiol. 1972 Dec;227(3):855–874. doi: 10.1113/jphysiol.1972.sp010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. An ATP-dependent Na+/Mg2+ countertransport is the only mechanism for Mg extrusion in squid axons. Biochim Biophys Acta. 1988 Dec 22;946(2):424–428. doi: 10.1016/0005-2736(88)90418-x. [DOI] [PubMed] [Google Scholar]
- Flatman P. W., Andrews P. L. Cation and ATP content of ferret red cells. Comp Biochem Physiol A Comp Physiol. 1983;74(4):939–943. doi: 10.1016/0300-9629(83)90373-0. [DOI] [PubMed] [Google Scholar]
- Flatman P. W., Lew V. L. Magnesium buffering in intact human red blood cells measured using the ionophore A23187. J Physiol. 1980 Aug;305:13–30. doi: 10.1113/jphysiol.1980.sp013346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatman P. W. Sodium and potassium transport in ferret red cells. J Physiol. 1983 Aug;341:545–557. doi: 10.1113/jphysiol.1983.sp014823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatman P. W. The control of red cell magnesium. Magnes Res. 1988 Jul;1(1-2):5–11. [PubMed] [Google Scholar]
- Flatman P. W. The effects of calcium on potassium transport in ferret red cells. J Physiol. 1987 May;386:407–423. doi: 10.1113/jphysiol.1987.sp016541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatman P. W. The effects of magnesium on potassium transport in ferret red cells. J Physiol. 1988 Mar;397:471–487. doi: 10.1113/jphysiol.1988.sp017013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel E. J., Graziani M., Schatzmann H. J. ATP requirement of the sodium-dependent magnesium extrusion from human red blood cells. J Physiol. 1989 Jul;414:385–397. doi: 10.1113/jphysiol.1989.sp017694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Féray J. C., Garay R. A one-to-one Mg2+:Mn2+ exchange in rat erythrocytes. J Biol Chem. 1987 Apr 25;262(12):5763–5768. [PubMed] [Google Scholar]
- Féray J. C., Garay R. An Na+-stimulated Mg2+-transport system in human red blood cells. Biochim Biophys Acta. 1986 Mar 27;856(1):76–84. doi: 10.1016/0005-2736(86)90012-x. [DOI] [PubMed] [Google Scholar]
- Féray J. C., Garay R. Demonstration of a Na+: Mg2+ exchange in human red cells by its sensitivity to tricyclic antidepressant drugs. Naunyn Schmiedebergs Arch Pharmacol. 1988 Sep;338(3):332–337. doi: 10.1007/BF00173409. [DOI] [PubMed] [Google Scholar]
- Günther T., Vormann J. Characterization of Na+/Mg2+ antiport by simultaneous 28Mg2+ influx. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1069–1074. doi: 10.1016/s0006-291x(87)80240-1. [DOI] [PubMed] [Google Scholar]
- Günther T., Vormann J. Mg2+ efflux is accomplished by an amiloride-sensitive Na+/Mg2+ antiport. Biochem Biophys Res Commun. 1985 Jul 31;130(2):540–545. doi: 10.1016/0006-291x(85)90450-4. [DOI] [PubMed] [Google Scholar]
- Güther T., Vormann J., Förster R. Regulation of intracellular magnesium by Mg2+ efflux. Biochem Biophys Res Commun. 1984 Feb 29;119(1):124–131. doi: 10.1016/0006-291x(84)91627-9. [DOI] [PubMed] [Google Scholar]
- Inesi G., Millman M., Eletr S. Temperature-induced transitions of function and structure in sarcoplasmic reticulum membranes. J Mol Biol. 1973 Dec 25;81(4):483–504. doi: 10.1016/0022-2836(73)90518-4. [DOI] [PubMed] [Google Scholar]
- Lüdi H., Schatzmann H. J. Some properties of a system for sodium-dependent outward movement of magnesium from metabolizing human red blood cells. J Physiol. 1987 Sep;390:367–382. doi: 10.1113/jphysiol.1987.sp016706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J., Brinley F. J., Jr, Spangler S. G., Abercrombie R. F. Magnesium efflux in dialyzed squid axons. J Gen Physiol. 1977 Apr;69(4):389–400. doi: 10.1085/jgp.69.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart G. W., Ellory J. C., Klein R. A. Increased human red cell cation passive permeability below 12 degrees C. Nature. 1980 Jul 24;286(5771):403–404. doi: 10.1038/286403a0. [DOI] [PubMed] [Google Scholar]
- Thornton P. C., Wright P. A., Sacra P. J., Goodier T. E. The ferret, Mustela putorius furo, as a new species in toxicology. Lab Anim. 1979 Apr;13(2):119–124. doi: 10.1258/002367779780943422. [DOI] [PubMed] [Google Scholar]


