Abstract
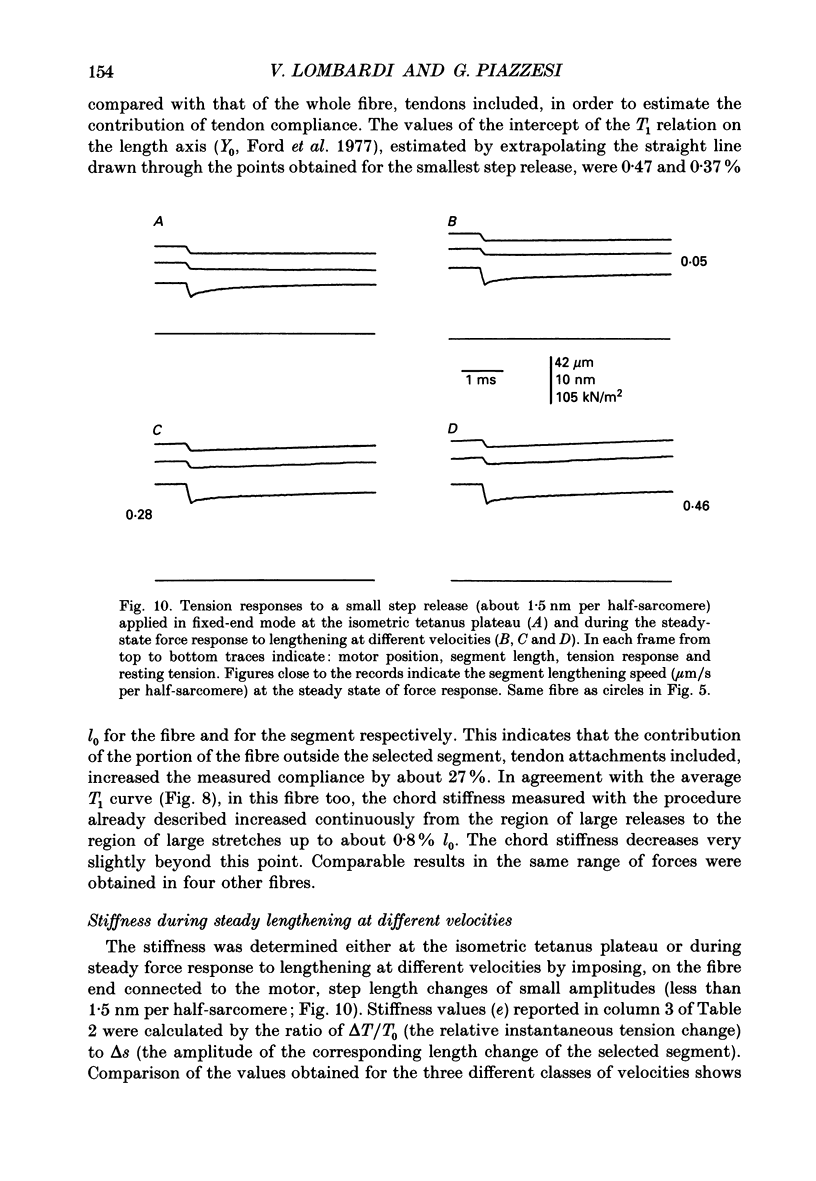
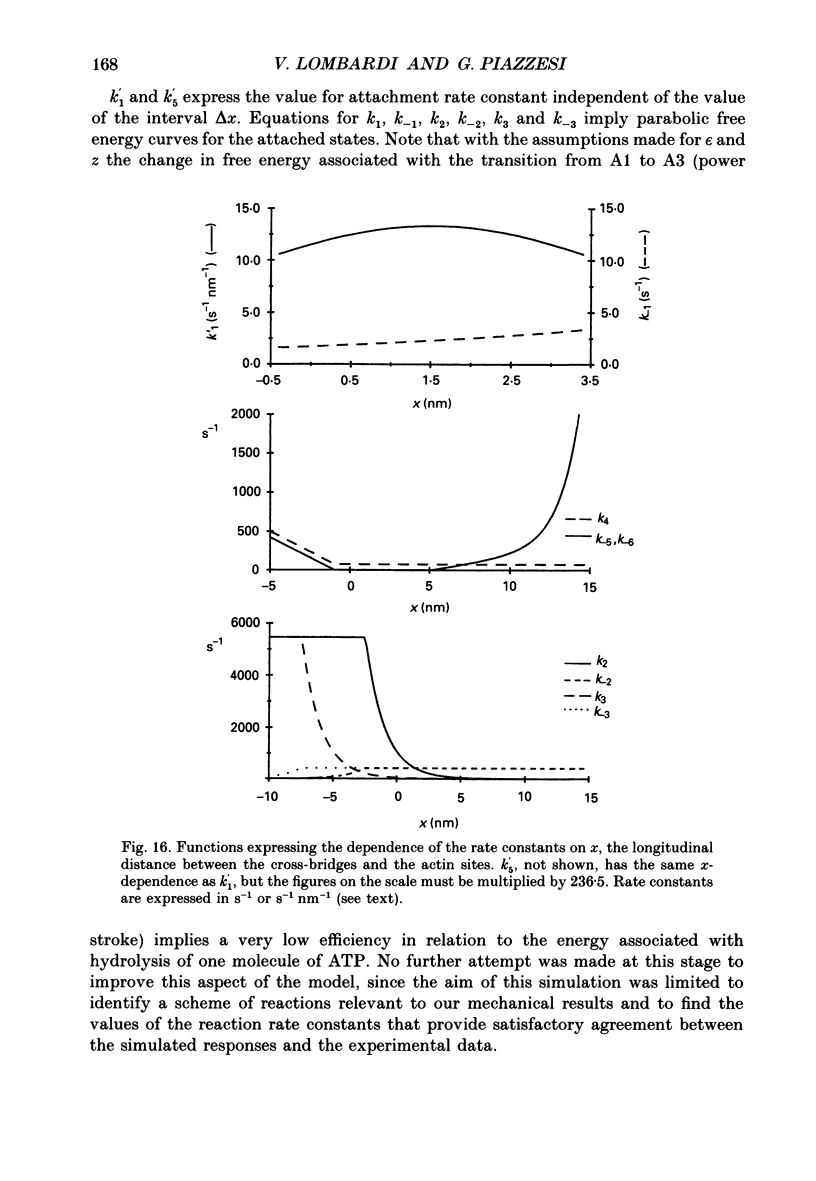
1. Steady lengthenings at different velocities (0.025-1.2 microns/s per half-sarcomere; temperature 2-5.5 degrees C) were imposed on isolated frog muscle fibres at the isometric tetanus plateau by means of a loudspeaker motor. The lengthening at the sarcomere level was measured by means of a striation follower either in fixed-end or in length-clamp mode. The force response was measured by a capacitance gauge transducer (resonance frequency 50 kHz). Preparations showing gross non-homogeneity during lengthening were excluded. 2. A steady tension was in all cases reached after about 20 nm per half-sarcomere of lengthening. Tension during this steady phase rose with speed of elongation up to 0.25-0.4 micron/s per half-sarcomere, when tension was 1.9-2 times isometric tetanic force (T0). Further increase in speed produced only very little increase in the steady tension. 3. During the transitory phase, before steady tension was reached, the tension rose monotonically if speed of lengthening was less than 0.25-0.3 micron/s per half-sarcomere; at higher speed the tension rose above the steady level, reaching a peak when extension was 10-14 nm per half-sarcomere, and then fell to the steady level. Tension at the peak continued to rise with speed of lengthening above 0.3 micron/s per half-sarcomere. 4. During the tension rise within the transitory phase of force response the segment elongated at a speed 15-20% lower than that imposed on the whole fibre, as a consequence of tendon compliance. 5. During the steady phase, non-homogeneity of lengthening speed began above a speed of lengthening which varied from fibre to fibre. At speeds below this value, segments elongated at the same speed as that imposed on the fibre. 6. Tension responses to large step stretches (up to 12 nm per half-sarcomere), applied at the plateau of isometric tetanus, showed that the instantaneous elasticity of contractile machinery is not responsible for the limit in force attained with high-speed lengthening. 7. Instantaneous stiffness was determined during the steady state of force response by superposing small steps (less than 1.5 nm per half-sarcomere) on steady lengthening at different velocities. Stiffness was 10-20% larger during lengthening than at the plateau of isometric tetanus and remained practically constant, independent of lengthening velocity, in the range of velocities used. 8. The results indicate that steady lengthening of a tetanized fibre induces a cross-bridge cycle characterized by fast detachment of the cross-bridge extended beyond a critical level.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABBOTT B. C., AUBERT X. M., HILL A. V. The absorption of work by a muscle stretched during a single twitch or a short tetanus. Proc R Soc Lond B Biol Sci. 1951 Dec 31;139(894):86–104. doi: 10.1098/rspb.1951.0048. [DOI] [PubMed] [Google Scholar]
- AUBERT X. Réversibilité partielle de la contraction musculaire au cours de l'absorption du travail en cycle. Arch Int Physiol. 1948 Jun;55(4):348–361. doi: 10.3109/13813454809144858. [DOI] [PubMed] [Google Scholar]
- Ambrogi-Lorenzini C., Colomo F., Lombardi V. Development of force-velocity relation, stiffness and isometric tension in frog single muscle fibres. J Muscle Res Cell Motil. 1983 Apr;4(2):177–189. doi: 10.1007/BF00712029. [DOI] [PubMed] [Google Scholar]
- Bagni M. A., Cecchi G., Colomo F., Tesi C. Plateau and descending limb of the sarcomere length-tension relation in short length-clamped segments of frog muscle fibres. J Physiol. 1988 Jul;401:581–595. doi: 10.1113/jphysiol.1988.sp017181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler B. H., Dusik L. A., Menard M. R. Tension responses of frog skeletal muscle fibres to rapid shortening and lengthening steps. J Physiol. 1988 Mar;397:631–641. doi: 10.1113/jphysiol.1988.sp017022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavagna G. A., Citterio G. Effect of stretching on the elastic characteristics and the contractile component of frog striated muscle. J Physiol. 1974 May;239(1):1–14. doi: 10.1113/jphysiol.1974.sp010552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi G., Colomo F., Lombardi V., Piazzesi G. Stiffness of frog muscle fibres during rise of tension and relaxation in fixed-end or length-clamped tetani. Pflugers Arch. 1987 Jun;409(1-2):39–46. doi: 10.1007/BF00584747. [DOI] [PubMed] [Google Scholar]
- Colomo F., Lombardi V., Piazzesi G. The mechanisms of force enhancement during constant velocity lengthening in tetanized single fibres of frog muscle. Adv Exp Med Biol. 1988;226:489–502. [PubMed] [Google Scholar]
- Curtin N. A., Gilbert C., Kretzschmar K. M., Wilkie D. R. The effect of the performance of work on total energy output and metabolism during muscular contraction. J Physiol. 1974 May;238(3):455–472. doi: 10.1113/jphysiol.1974.sp010537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Elzinga G., Noble M. I. Critical sarcomere extension required to recruit a decaying component of extra force during stretch in tetanic contractions of frog skeletal muscle fibers. J Gen Physiol. 1981 Oct;78(4):365–382. doi: 10.1085/jgp.78.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Elzinga G., Noble M. I. Enhancement of mechanical performance by stretch during tetanic contractions of vertebrate skeletal muscle fibres. J Physiol. 1978 Aug;281:139–155. doi: 10.1113/jphysiol.1978.sp012413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Elzinga G., Noble M. I. Stretch of contracting muscle fibres: evidence for regularly spaced active sites along the filaments and enhanced mechanical performance. Adv Exp Med Biol. 1984;170:739–751. doi: 10.1007/978-1-4684-4703-3_71. [DOI] [PubMed] [Google Scholar]
- Fenn W. O. The relation between the work performed and the energy liberated in muscular contraction. J Physiol. 1924 May 23;58(6):373–395. doi: 10.1113/jphysiol.1924.sp002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi M. A., Homsher E., Trentham D. R. The kinetics of magnesium adenosine triphosphate cleavage in skinned muscle fibres of the rabbit. J Physiol. 1984 Jul;352:575–599. doi: 10.1113/jphysiol.1984.sp015311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flitney F. W., Hirst D. G. Cross-bridge detachment and sarcomere 'give' during stretch of active frog's muscle. J Physiol. 1978 Mar;276:449–465. doi: 10.1113/jphysiol.1978.sp012246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flitney F. W., Hirst D. G. Filament sliding and energy absorbed by the cross-bridge in active muscle subjected to cycical length changes. J Physiol. 1978 Mar;276:467–479. doi: 10.1113/jphysiol.1978.sp012247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension transients during steady shortening of frog muscle fibres. J Physiol. 1985 Apr;361:131–150. doi: 10.1113/jphysiol.1985.sp015637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. The relation between stiffness and filament overlap in stimulated frog muscle fibres. J Physiol. 1981 Feb;311:219–249. doi: 10.1113/jphysiol.1981.sp013582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths P. J., Güth K., Kuhn H. J., Rüegg J. C. Cross bridge slippage in skinned frog muscle fibres. Biophys Struct Mech. 1980;7(2):107–124. doi: 10.1007/BF00538402. [DOI] [PubMed] [Google Scholar]
- Güth K., Kuhn H. J. Stiffness and tension during and after sudden length changes of glycerinated rabbit psoas muscle fibres. Biophys Struct Mech. 1978 Jul 12;4(3):223–236. doi: 10.1007/BF02426087. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- HUXLEY A. F., PEACHEY L. D. The maximum length for contraction in vertebrate straiated muscle. J Physiol. 1961 Apr;156:150–165. doi: 10.1113/jphysiol.1961.sp006665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill L. A-band length, striation spacing and tension change on stretch of active muscle. J Physiol. 1977 Apr;266(3):677–685. doi: 10.1113/jphysiol.1977.sp011787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- INFANTE A. A., KLAUPIKS D., DAVIES R. E. ADENOSINE TRIPHOSPHATE: CHANGES IN MUSCLES DOING NEGATIVE WORK. Science. 1964 Jun 26;144(3626):1577–1578. doi: 10.1126/science.144.3626.1577. [DOI] [PubMed] [Google Scholar]
- Julian F. J., Morgan D. L. The effect on tension of non-uniform distribution of length changes applied to frog muscle fibres. J Physiol. 1979 Aug;293:379–392. doi: 10.1113/jphysiol.1979.sp012895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Sollins K. R., Sollins M. R. A model for the transient and steady-state mechanical behavior of contracting muscle. Biophys J. 1974 Jul;14(7):546–562. doi: 10.1016/S0006-3495(74)85934-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Sollins M. R. Variation of muscle stiffness with force at increasing speeds of shortening. J Gen Physiol. 1975 Sep;66(3):287–302. doi: 10.1085/jgp.66.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. The relation between force and speed in muscular contraction. J Physiol. 1939 Jun 14;96(1):45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberg M., Brenner B., Chalovich J. M., Greene L. E., Eisenberg E. Cross-bridge attachment in relaxed muscle. Adv Exp Med Biol. 1984;170:269–284. doi: 10.1007/978-1-4684-4703-3_24. [DOI] [PubMed] [Google Scholar]
- Sugi H., Tsuchiya T. Enhancement of mechanical performance in frog muscle fibres after quick increases in load. J Physiol. 1981;319:239–252. doi: 10.1113/jphysiol.1981.sp013904. [DOI] [PMC free article] [PubMed] [Google Scholar]


