Abstract
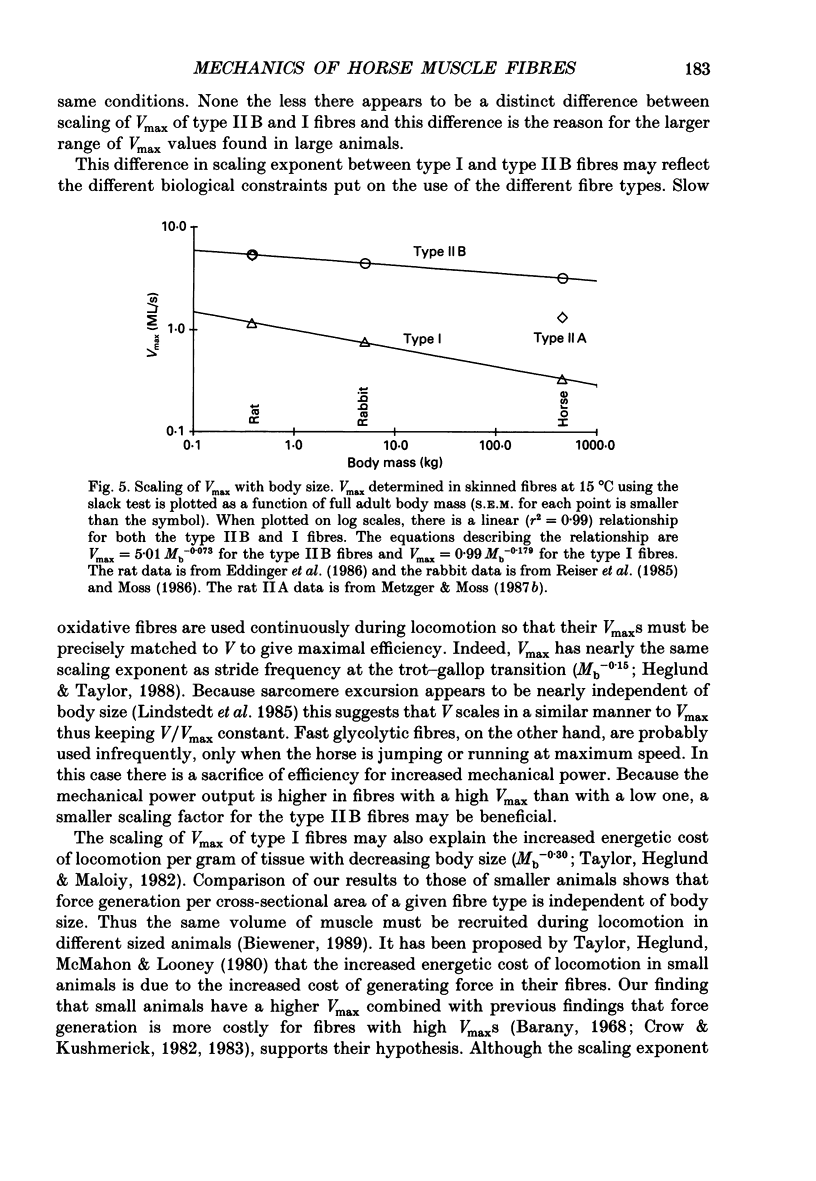
1. To explore how maximum velocity of shortening (Vmax) of fibres varies within one muscle and how Vmax varies with body size, we measured Vmax of muscle fibres from soleus muscle of a large animal, the horse. 2. Vmax was determined by the slack test on skinned single muscle fibres at 15 degrees C during maximal activation (pCa = 5.2). The fibre type was subsequently determined by a combination of single-cell histochemistry and gel electrophoresis of the myosin light chains. 3. Vmax values for the type I, IIA and IIB muscle fibres were 0.33 +/- 0.04 muscle lengths/s (ML/s) (+/- S.E.M., n = 6), 1.33 +/- 0.08 ML/s (n = 7) and 3.20 +/- 0.26 ML/s (n = 6), respectively. It is likely that the large range in Vmax is due to differences observed in the myosin heavy chains and light chains associated with the three fibre types. 4. Comparison of Vmax over a 1200-fold range (450 kg horse vs. 0.38 kg rat) of body mass (Mb) suggests that slow fibres scale more dramatically (Mb-0.18) than do fast glycolytic fibres (Mb-0.07). This difference may enable the slow fibres to work at high efficiencies in the large animal while the fast fibres can still generate a large mechanical power when necessary.
Full text
PDF


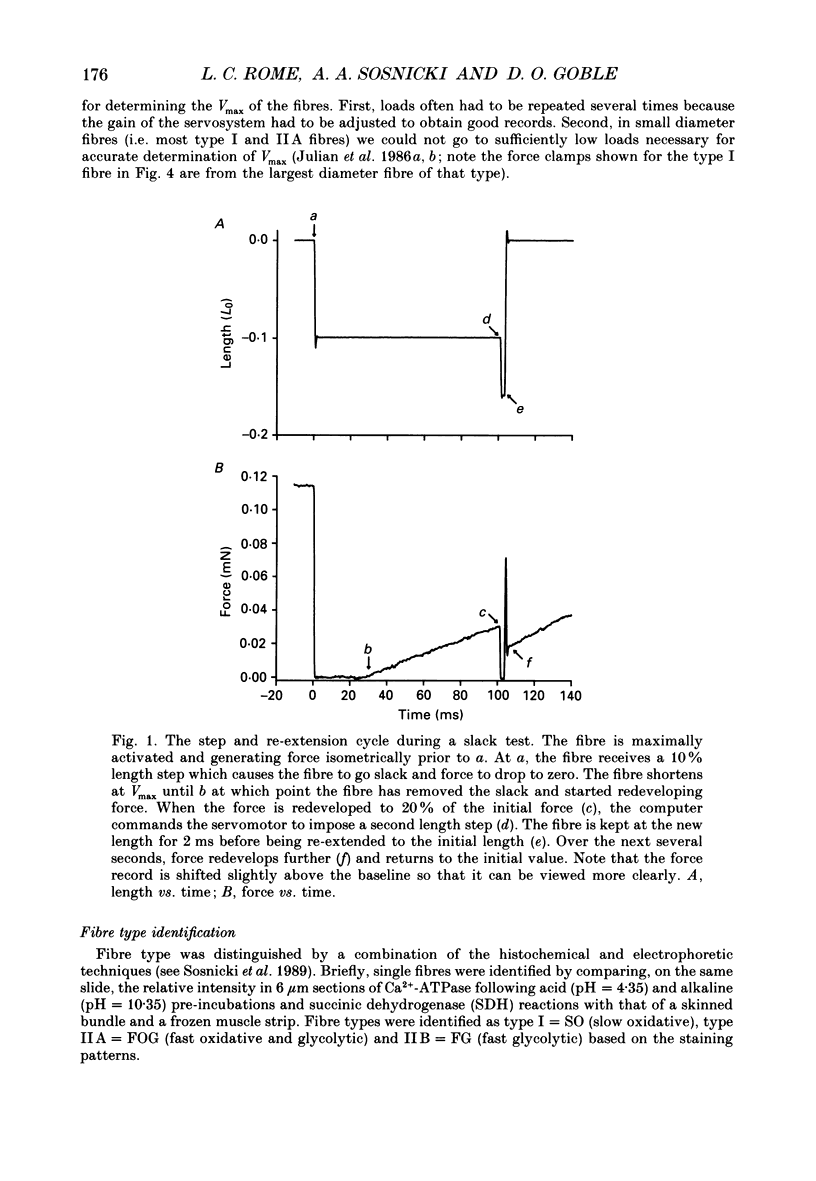

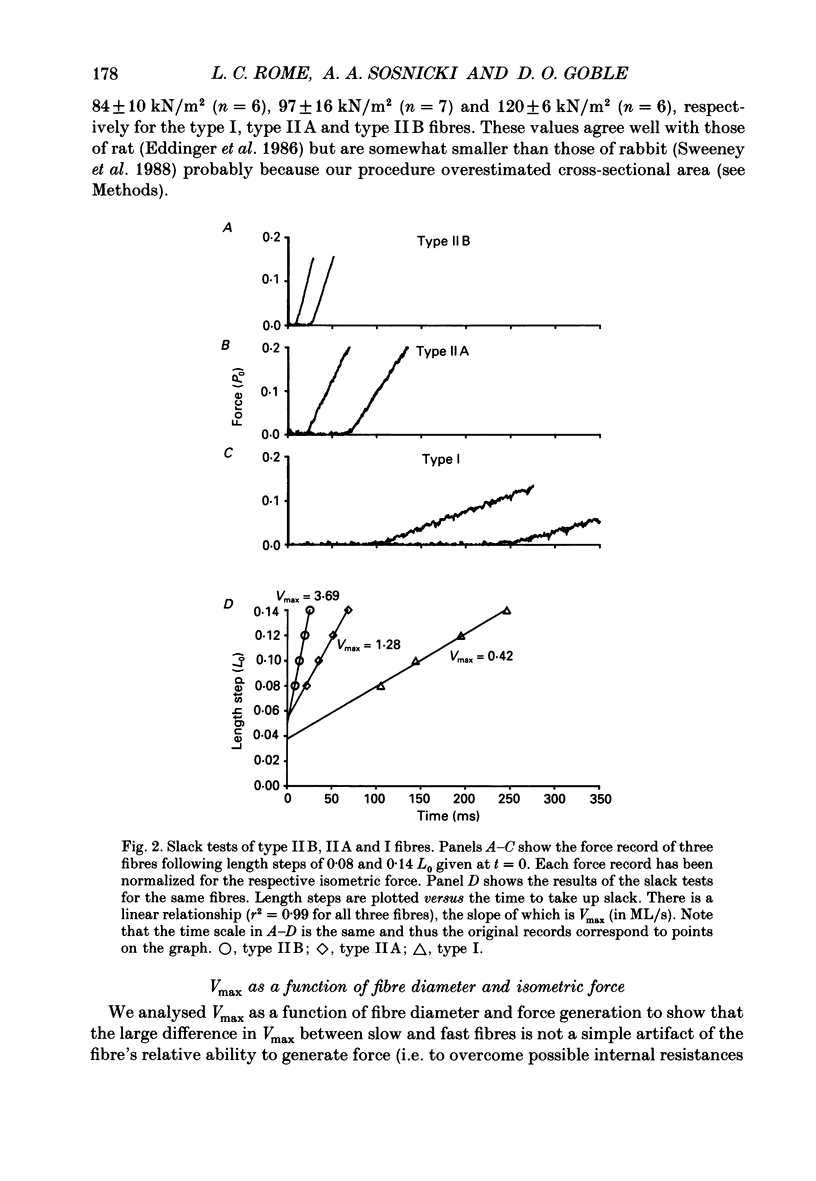
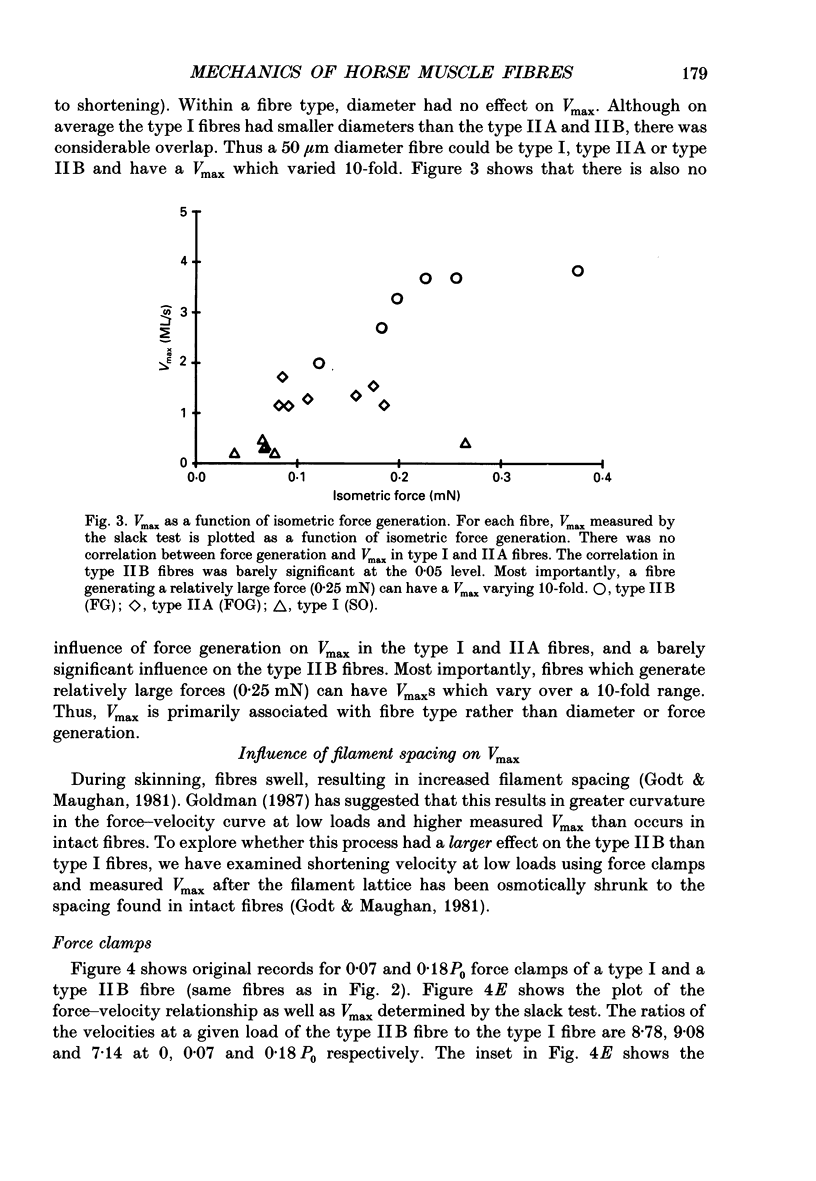
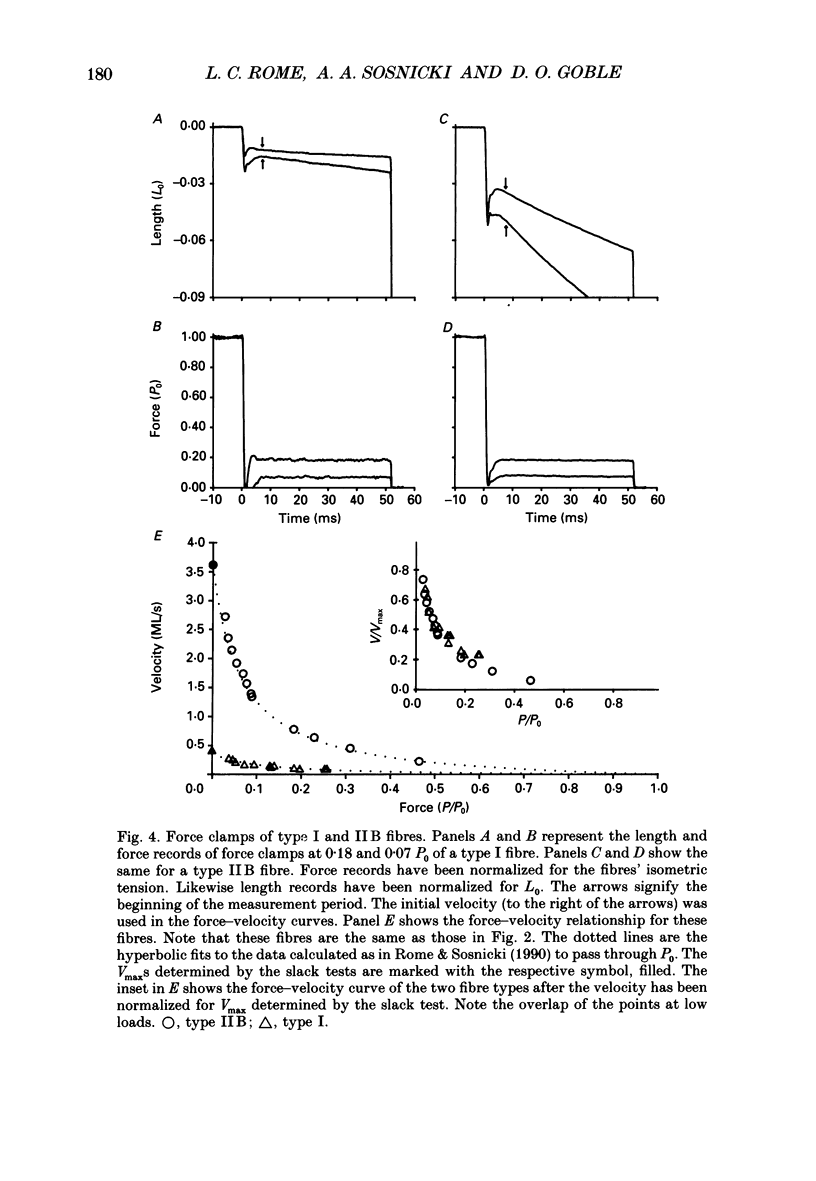




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biewener A. A. Scaling body support in mammals: limb posture and muscle mechanics. Science. 1989 Jul 7;245(4913):45–48. doi: 10.1126/science.2740914. [DOI] [PubMed] [Google Scholar]
- Brenner B. Technique for stabilizing the striation pattern in maximally calcium-activated skinned rabbit psoas fibers. Biophys J. 1983 Jan;41(1):99–102. doi: 10.1016/S0006-3495(83)84411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967 Jul;50(6 Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Crow M. T., Kushmerick M. J. Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol. 1982 Jan;79(1):147–166. doi: 10.1085/jgp.79.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow M. T., Kushmerick M. J. Correlated reduction of velocity of shortening and the rate of energy utilization in mouse fast-twitch muscle during a continuous tetanus. J Gen Physiol. 1983 Nov;82(5):703–720. doi: 10.1085/jgp.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddinger T. J., Cassens R. G., Moss R. L. Mechanical and histochemical characterization of skeletal muscles from senescent rats. Am J Physiol. 1986 Sep;251(3 Pt 1):C421–C430. doi: 10.1152/ajpcell.1986.251.3.C421. [DOI] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitts R. H., Costill D. L., Gardetto P. R. Effect of swim exercise training on human muscle fiber function. J Appl Physiol (1985) 1989 Jan;66(1):465–475. doi: 10.1152/jappl.1989.66.1.465. [DOI] [PubMed] [Google Scholar]
- Gardetto P. R., Schluter J. M., Fitts R. H. Contractile function of single muscle fibers after hindlimb suspension. J Appl Physiol (1985) 1989 Jun;66(6):2739–2749. doi: 10.1152/jappl.1989.66.6.2739. [DOI] [PubMed] [Google Scholar]
- Godt R. E., Maughan D. W. Influence of osmotic compression on calcium activation and tension in skinned muscle fibers of the rabbit. Pflugers Arch. 1981 Oct;391(4):334–337. doi: 10.1007/BF00581519. [DOI] [PubMed] [Google Scholar]
- Goldman Y. E., Hibberd M. G., Trentham D. R. Relaxation of rabbit psoas muscle fibres from rigor by photochemical generation of adenosine-5'-triphosphate. J Physiol. 1984 Sep;354:577–604. doi: 10.1113/jphysiol.1984.sp015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E. Measurement of sarcomere shortening in skinned fibers from frog muscle by white light diffraction. Biophys J. 1987 Jul;52(1):57–68. doi: 10.1016/S0006-3495(87)83188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaser M. L., Moss R. L., Reiser P. J. Variations in contractile properties of rabbit single muscle fibres in relation to troponin T isoforms and myosin light chains. J Physiol. 1988 Dec;406:85–98. doi: 10.1113/jphysiol.1988.sp017370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heglund N. C., Taylor C. R. Speed, stride frequency and energy cost per stride: how do they change with body size and gait? J Exp Biol. 1988 Sep;138:301–318. doi: 10.1242/jeb.138.1.301. [DOI] [PubMed] [Google Scholar]
- Julian F. J., Rome L. C., Stephenson D. G., Striz S. The influence of free calcium on the maximum speed of shortening in skinned frog muscle fibres. J Physiol. 1986 Nov;380:257–273. doi: 10.1113/jphysiol.1986.sp016284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Rome L. C., Stephenson D. G., Striz S. The maximum speed of shortening in living and skinned frog muscle fibres. J Physiol. 1986 Jan;370:181–199. doi: 10.1113/jphysiol.1986.sp015929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstedt S. L., Hoppeler H., Bard K. M., Thronson H. A., Jr Estimate of muscle-shortening rate during locomotion. Am J Physiol. 1985 Dec;249(6 Pt 2):R699–R703. doi: 10.1152/ajpregu.1985.249.6.R699. [DOI] [PubMed] [Google Scholar]
- Maughan D. W., Godt R. E. Inhibition of force production in compressed skinned muscle fibers of the frog. Pflugers Arch. 1981 May;390(2):161–163. doi: 10.1007/BF00590200. [DOI] [PubMed] [Google Scholar]
- McMahon T. A. Using body size to understand the structural design of animals: quadrupedal locomotion. J Appl Physiol. 1975 Oct;39(4):619–627. doi: 10.1152/jappl.1975.39.4.619. [DOI] [PubMed] [Google Scholar]
- Metzger J. M., Moss R. L. Greater hydrogen ion-induced depression of tension and velocity in skinned single fibres of rat fast than slow muscles. J Physiol. 1987 Dec;393:727–742. doi: 10.1113/jphysiol.1987.sp016850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. M., Moss R. L. Shortening velocity in skinned single muscle fibers. Influence of filament lattice spacing. Biophys J. 1987 Jul;52(1):127–131. doi: 10.1016/S0006-3495(87)83197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. M., Moss R. L. Thin filament regulation of shortening velocity in rat skinned skeletal muscle: effects of osmotic compression. J Physiol. 1988 Apr;398:165–175. doi: 10.1113/jphysiol.1988.sp017036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L. Effects on shortening velocity of rabbit skeletal muscle due to variations in the level of thin-filament activation. J Physiol. 1986 Aug;377:487–505. doi: 10.1113/jphysiol.1986.sp016199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser P. J., Moss R. L., Giulian G. G., Greaser M. L. Shortening velocity in single fibers from adult rabbit soleus muscles is correlated with myosin heavy chain composition. J Biol Chem. 1985 Aug 5;260(16):9077–9080. [PubMed] [Google Scholar]
- Rome L. C., Funke R. P., Alexander R. M., Lutz G., Aldridge H., Scott F., Freadman M. Why animals have different muscle fibre types. Nature. 1988 Oct 27;335(6193):824–827. doi: 10.1038/335824a0. [DOI] [PubMed] [Google Scholar]
- Rome L. C., Sosnicki A. A. The influence of temperature on mechanics of red muscle in carp. J Physiol. 1990 Aug;427:151–169. doi: 10.1113/jphysiol.1990.sp018165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosnicki A. A., Lutz G. J., Rome L. C., Goble D. O. Histochemical and molecular determination of fiber types in chemically skinned single equine skeletal muscle fibers. J Histochem Cytochem. 1989 Nov;37(11):1731–1738. doi: 10.1177/37.11.2530270. [DOI] [PubMed] [Google Scholar]
- Sweeney H. L., Corteselli S. A., Kushmerick M. J. Measurements on permeabilized skeletal muscle fibers during continuous activation. Am J Physiol. 1987 May;252(5 Pt 1):C575–C580. doi: 10.1152/ajpcell.1987.252.5.C575. [DOI] [PubMed] [Google Scholar]
- Sweeney H. L., Kushmerick M. J., Mabuchi K., Sréter F. A., Gergely J. Myosin alkali light chain and heavy chain variations correlate with altered shortening velocity of isolated skeletal muscle fibers. J Biol Chem. 1988 Jun 25;263(18):9034–9039. [PubMed] [Google Scholar]
- Taylor C. R., Heglund N. C., Maloiy G. M. Energetics and mechanics of terrestrial locomotion. I. Metabolic energy consumption as a function of speed and body size in birds and mammals. J Exp Biol. 1982 Apr;97:1–21. doi: 10.1242/jeb.97.1.1. [DOI] [PubMed] [Google Scholar]


