Abstract
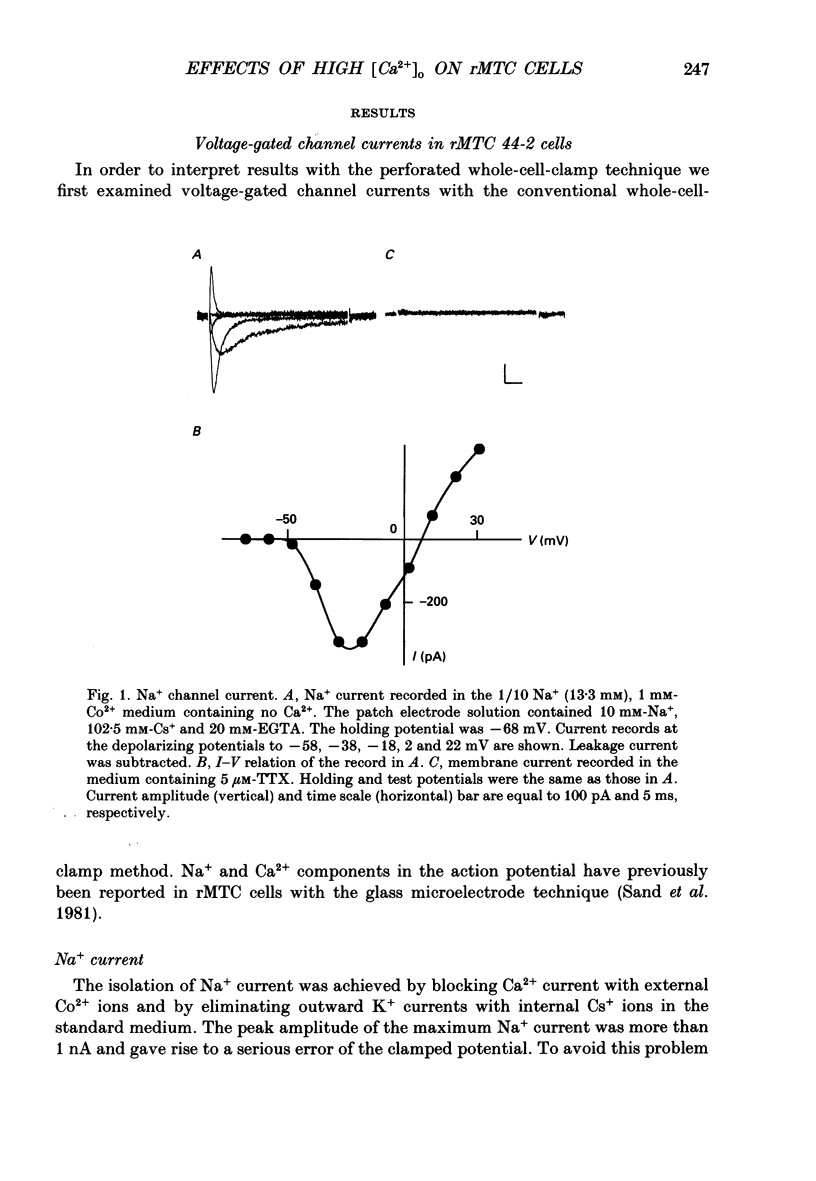
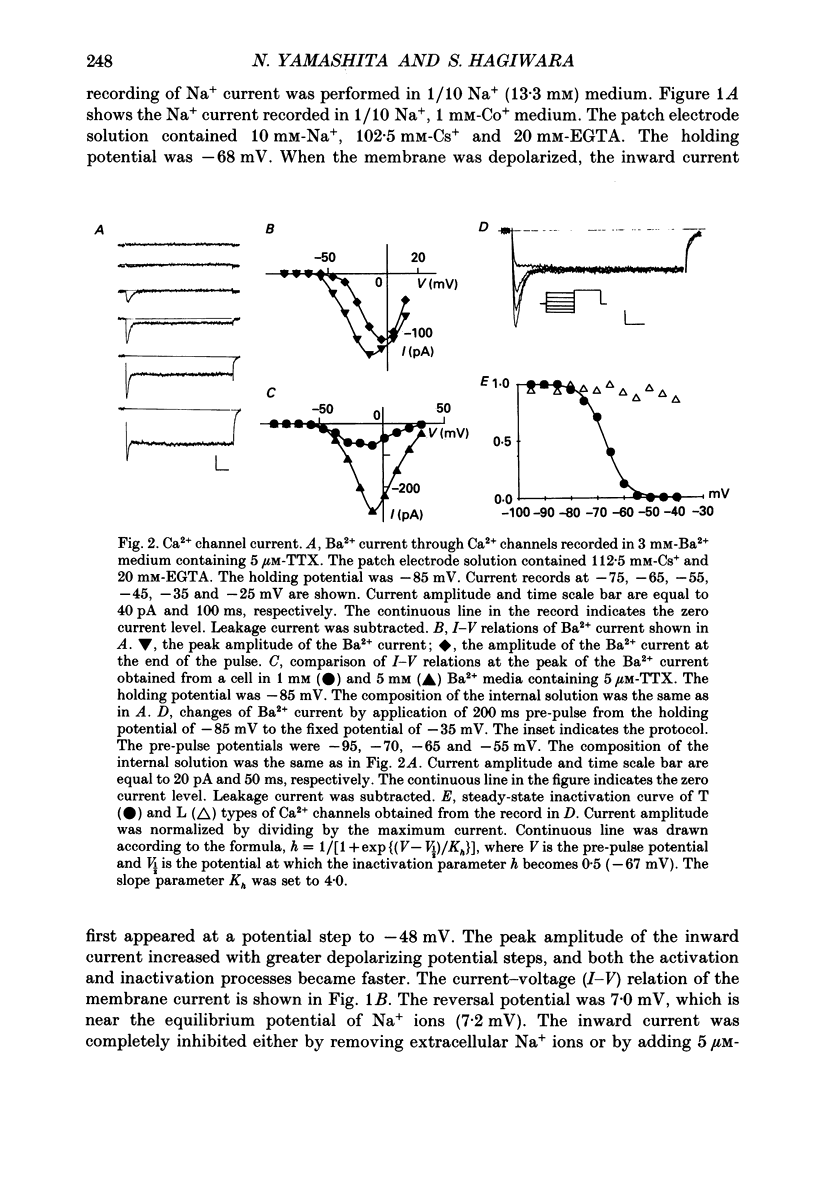
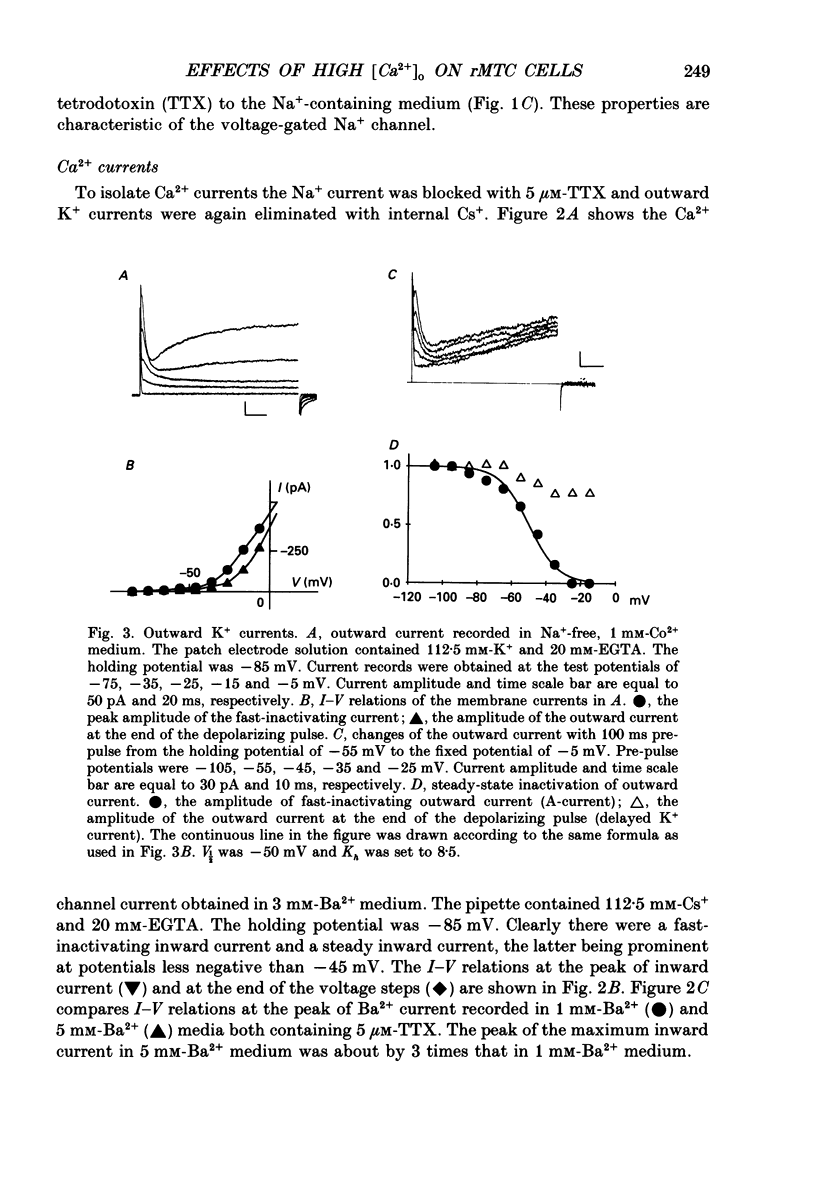
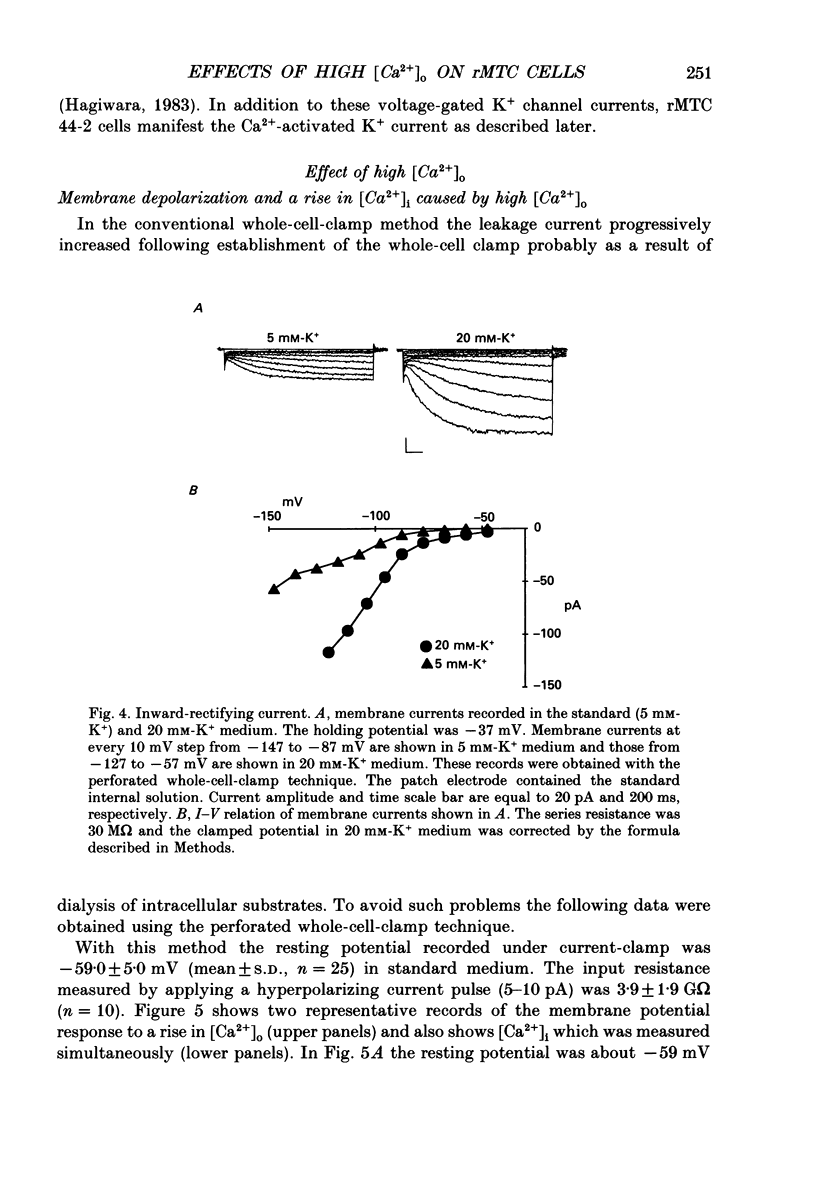
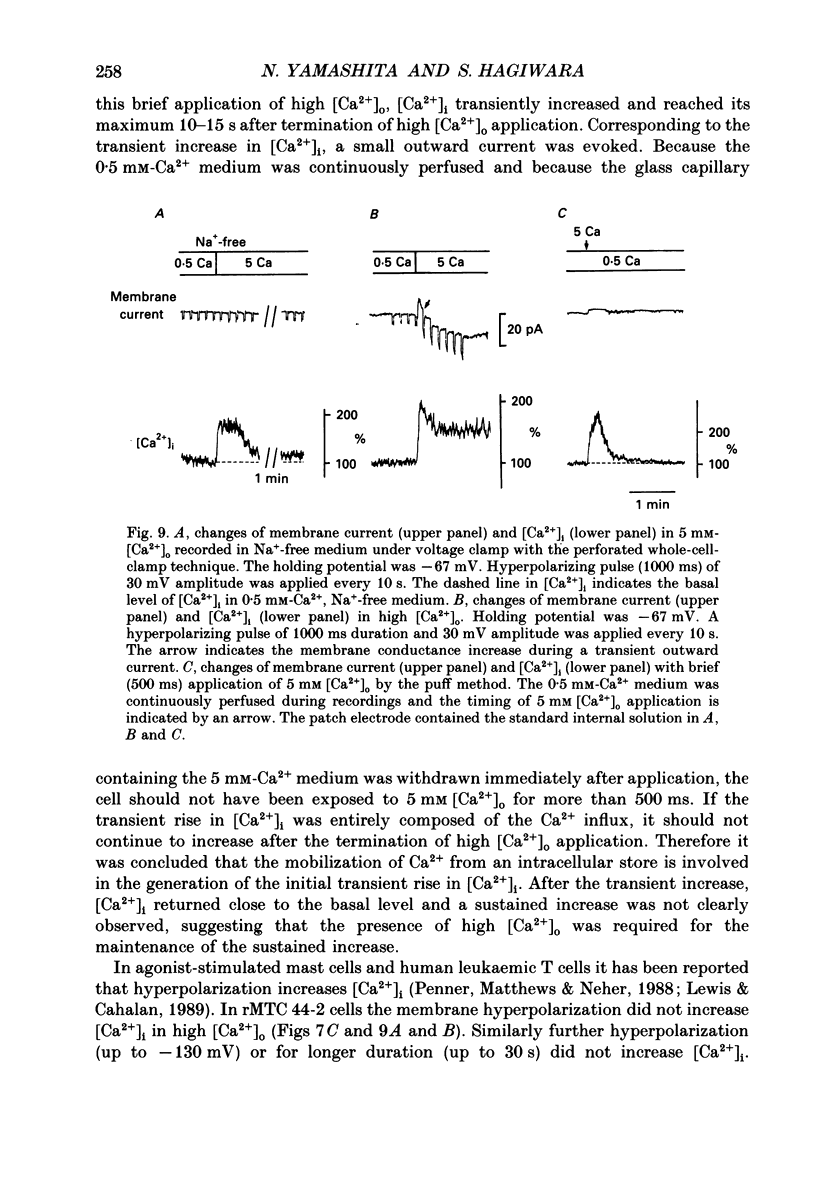
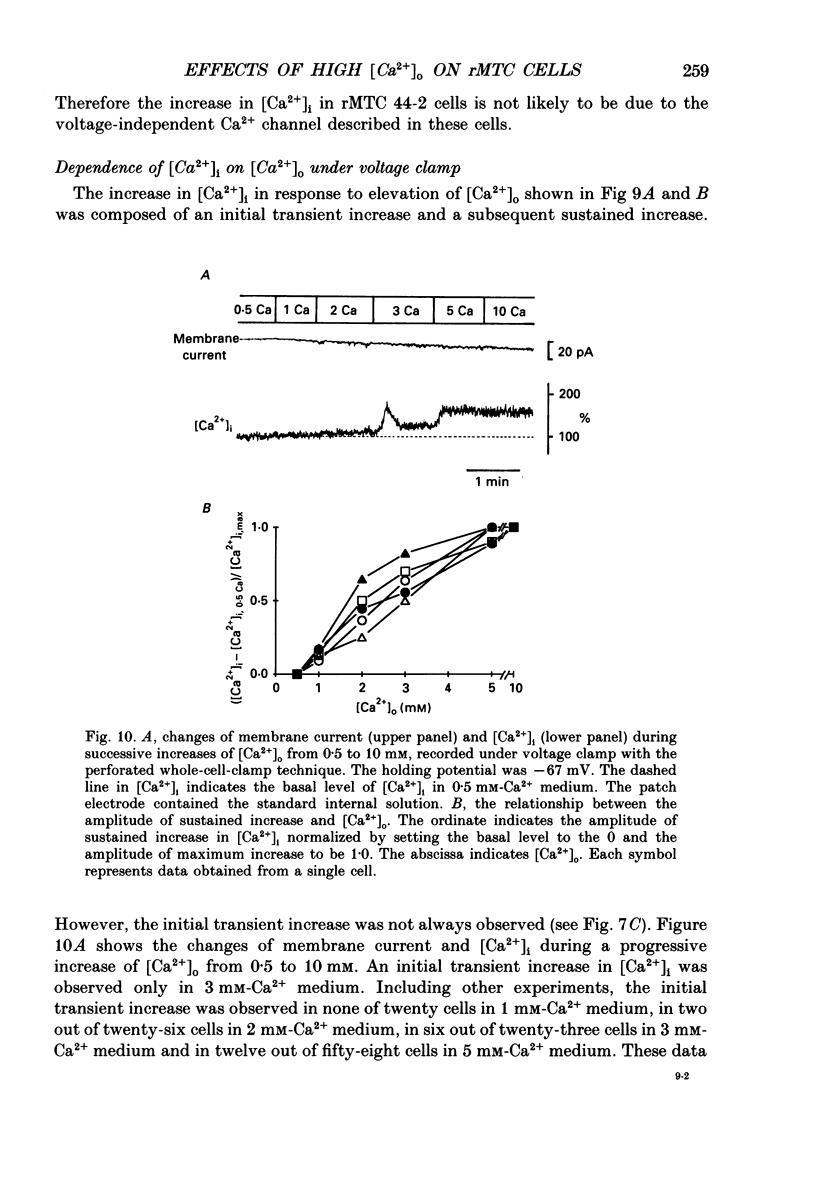
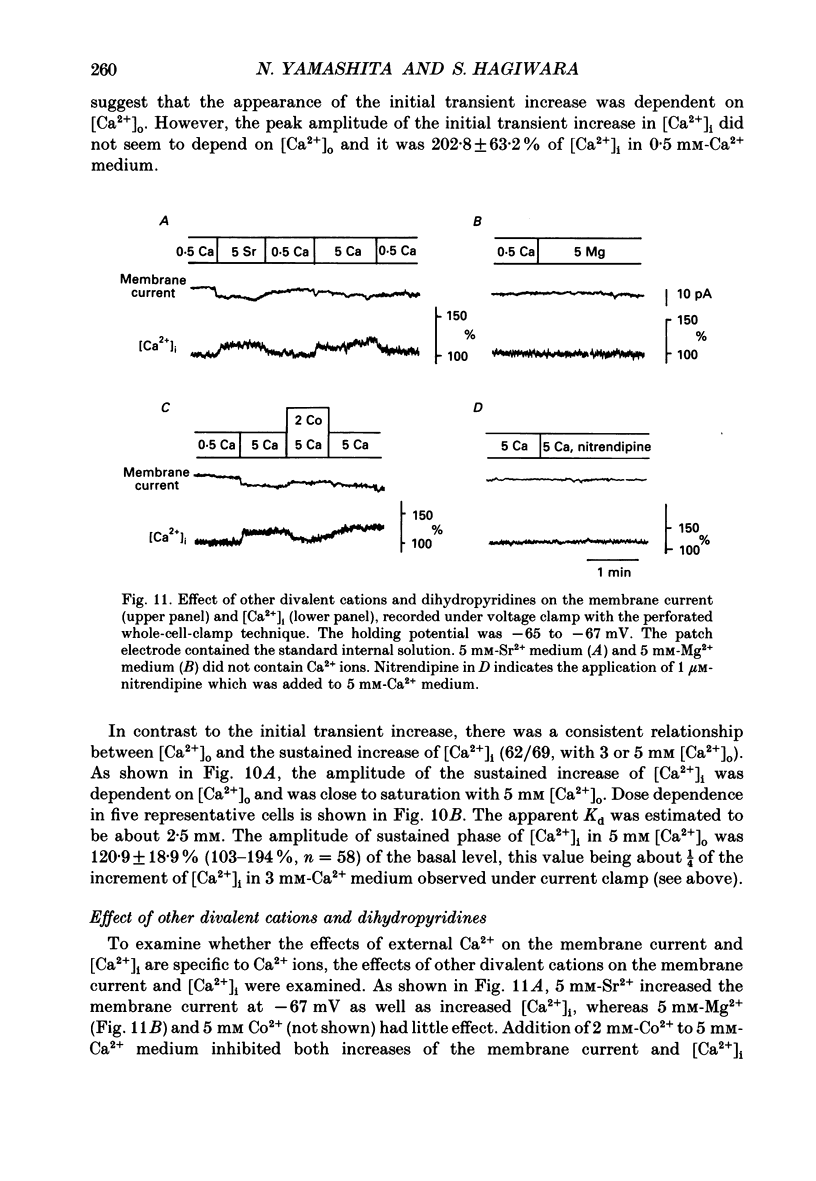
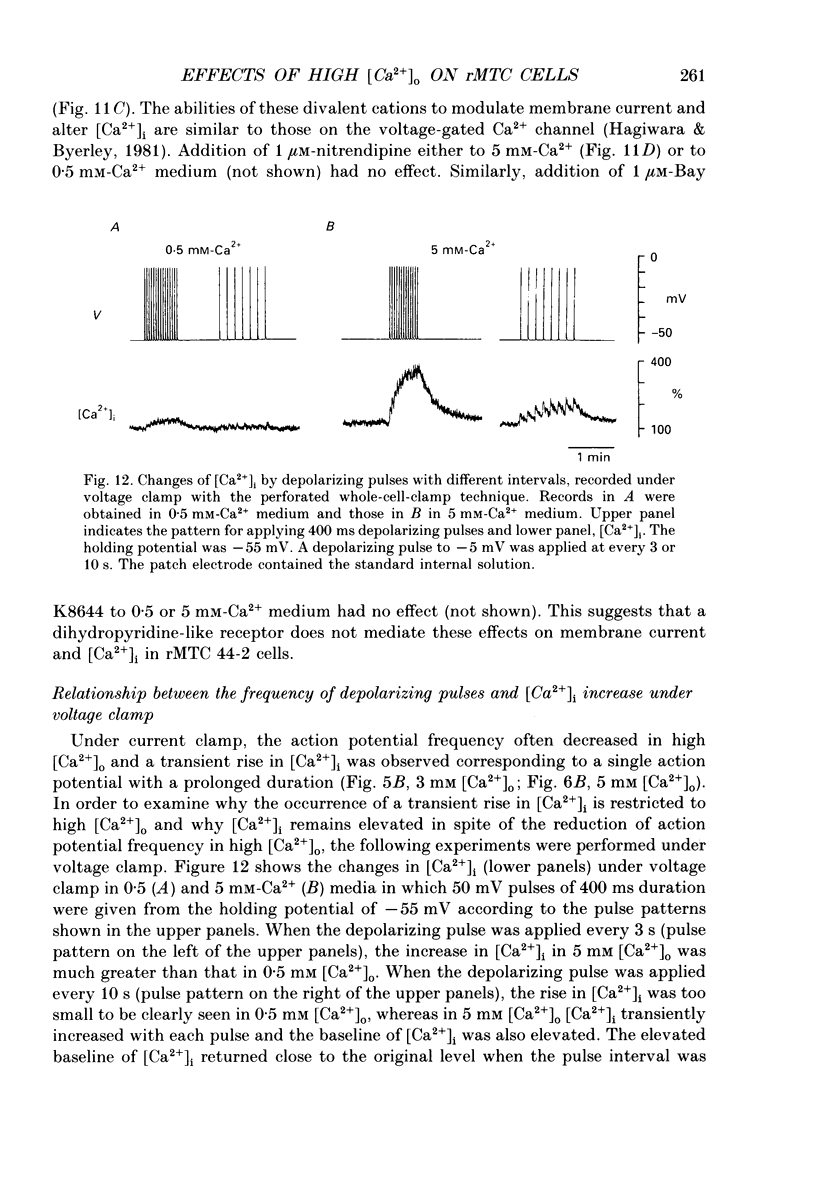
1. Calcitonin secretion is regulated by the external Ca2+ concentration ([Ca2+]o) via a rise in intracellular Ca2+ concentration ([Ca2+]i). The mechanism which couples an increase in [Ca2+]o to an increase in [Ca2+]i was explored in a rat calcitonin-secreting cell line (rMTC 44-2). [Ca2+]i was monitored using Fura-2 AM, and the membrane potential or current was simultaneously measured. 2. Using the conventional whole-cell clamp, tetrodotoxin-sensitive voltage-gated Na+ channels, T- and L-type Ca2+ channels, and three types of K+ channels, the delayed K+ channel, the A-channel and the inward-rectifying channel were observed. 3. Using the nystatin-perforated whole-cell-clamp technique, the resting potential measured under current clamp in standard extracellular medium was -59.0 +/- 5.0 mV (mean +/- S.D., n = 25), and the input resistance was 3.9 +/- 1.9 G omega (n = 10). In 0.5 mM [Ca2+]o most cells (22/25) showed spontaneous action potentials. 4. An increase in [Ca2+]o depolarized the cell membrane and elevated [Ca2+]i even in the presence of 10 microM-tetrodotoxin. The rise in [Ca2+]i was greatly reduced when action potentials were inhibited by applying hyperpolarizing current. The increase in [Ca2+]i saturated with 3-4 mM [Ca2+]o. In 3 mM [Ca2+]o, [Ca2+]i was 188.9 +/- 40.5% (n = 12) of that in 0.5 mM [Ca2+]o. 5. In high [Ca2+]o the duration of action potentials was prolonged, but the action potential frequency did not always increase. In some cases it even decreased in high [Ca2+]o. 6. Two types of action potential were observed in high [Ca2+]o, one with a shorter duration and the other with a longer duration. [Ca2+]i transiently increased in association with the long-duration action potentials. These long-duration action potentials were also accompanied by a larger after-hyperpolarization. 7. Under voltage clamp, high [Ca2+]o caused a membrane conductance increase to Na+ ions. 8. Even when the membrane potential was clamped at a level below the threshold for Ca2+ channel activation, high [Ca2+]o provoked an increase of [Ca2+]i which was composed of an initial transient increase followed by a sustained increase, indicating an involvement of mechanisms other than Ca2+ influx through voltage-gated channels. The sustained increase was more frequently observed than the initial transient increase. The amplitude of the sustained phase was dependent on [Ca2+]o, and in 5 mM [Ca2+]o it was 120.9 +/- 18.9% (103-194%) (n = 58) of that in 0.5 mM [Ca2+]o.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




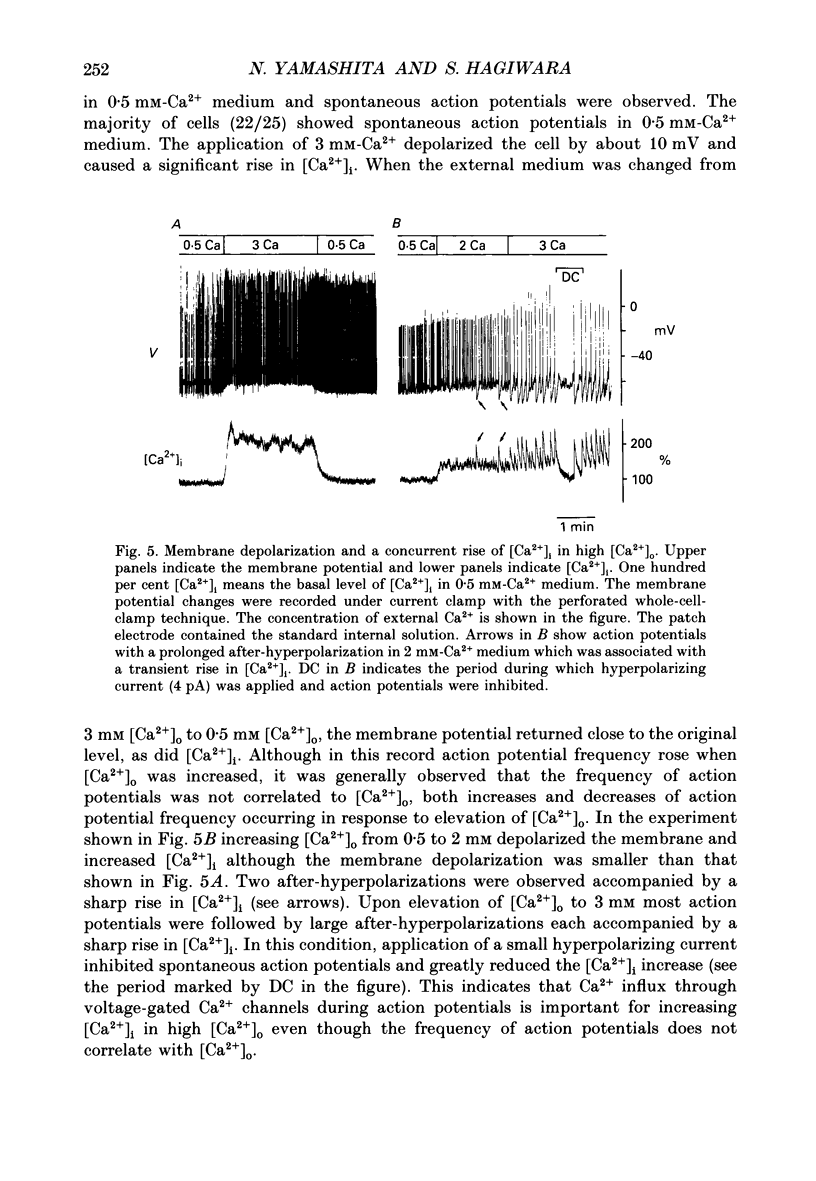







Selected References
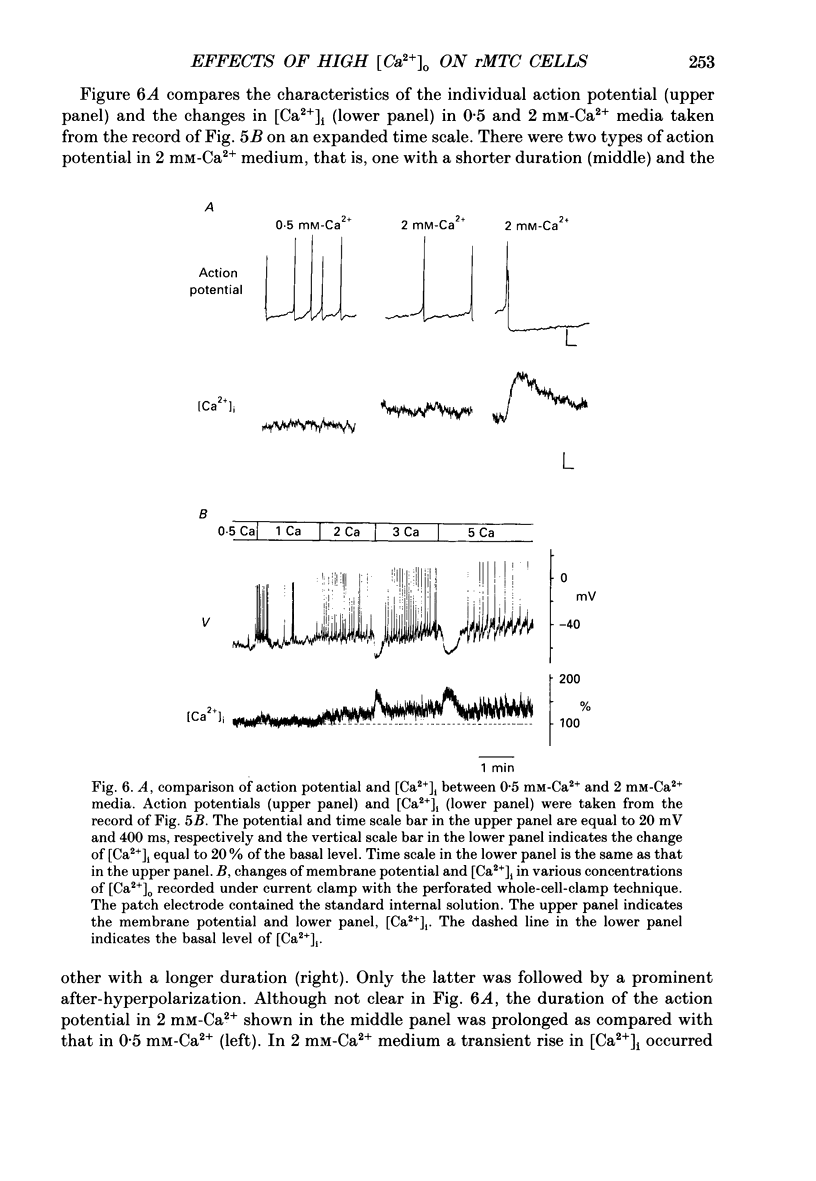
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., McCleskey E. W., Palade P. T. A non-selective cation conductance in frog muscle membrane blocked by micromolar external calcium ions. J Physiol. 1984 Aug;353:565–583. doi: 10.1113/jphysiol.1984.sp015351. [DOI] [PMC free article] [PubMed] [Google Scholar]
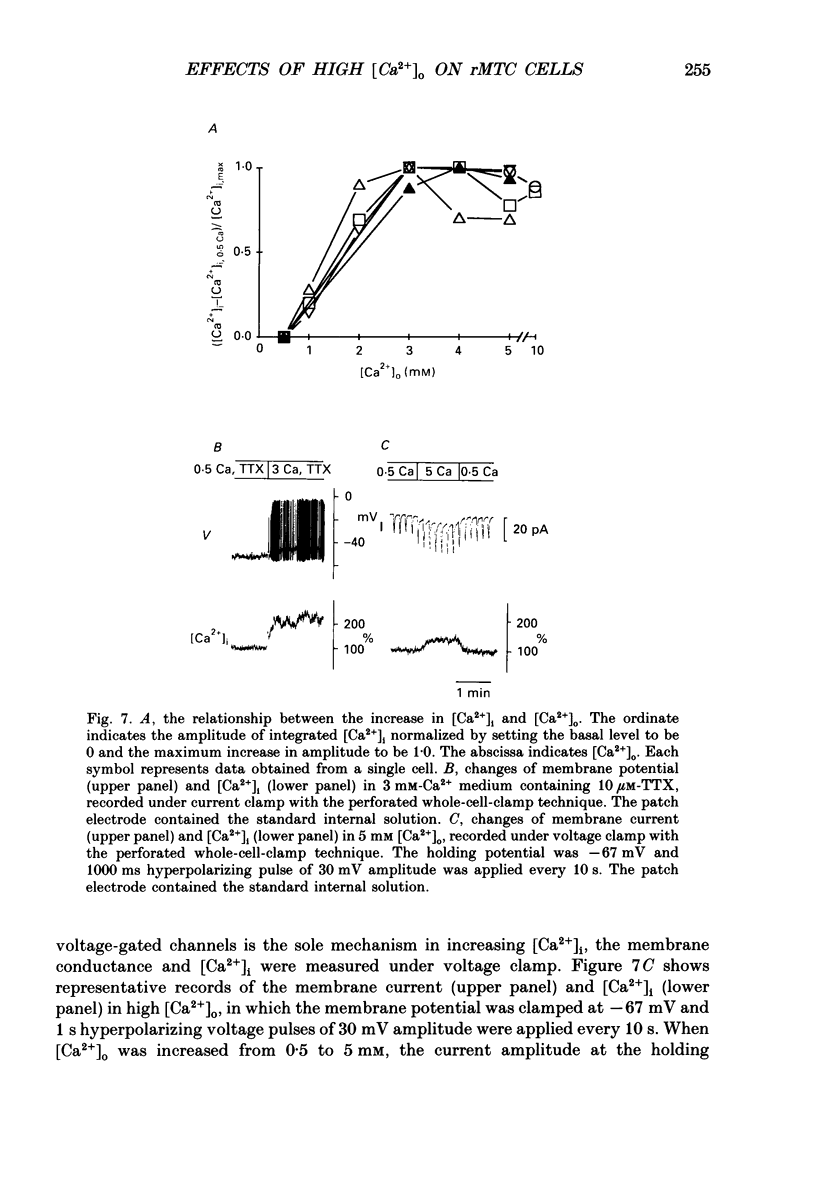
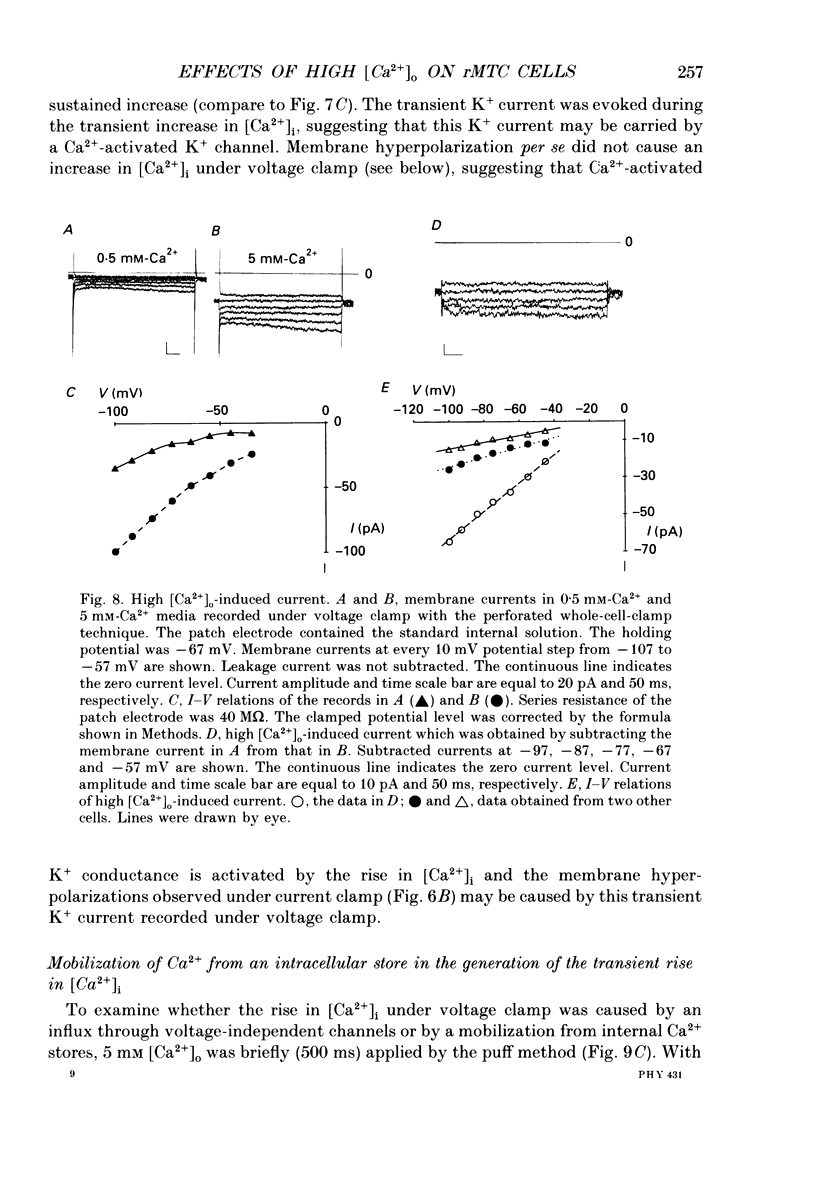
- Benham C. D. Voltage-gated and agonist-mediated rises in intracellular Ca2+ in rat clonal pituitary cells (GH3) held under voltage clamp. J Physiol. 1989 Aug;415:143–158. doi: 10.1113/jphysiol.1989.sp017716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biales B., Dichter M. A., Tischler A. Sodium and calcium action potential in pituitary cells. Nature. 1977 May 12;267(5607):172–174. doi: 10.1038/267172a0. [DOI] [PubMed] [Google Scholar]
- Bruce B. R., Anderson N. C., Jr Hyperpolarization in mouse parathyroid cells by low calcium. Am J Physiol. 1979 Jan;236(1):C15–C21. doi: 10.1152/ajpcell.1979.236.1.C15. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- Cooper C. W., Borosky S. A., Farrell P. E., Steinsland O. S. Effects of the calcium channel activator BAY-K-8644 on in vitro secretion of calcitonin and parathyroid hormone. Endocrinology. 1986 Feb;118(2):545–549. doi: 10.1210/endo-118-2-545. [DOI] [PubMed] [Google Scholar]
- Fried R. M., Tashjian A. H., Jr Unusual sensitivity of cytosolic free Ca2+ to changes in extracellular Ca2+ in rat C-cells. J Biol Chem. 1986 Jun 15;261(17):7669–7674. [PubMed] [Google Scholar]
- Gagel R. F., Zeytinoğlu F. N., Voelkel E. F., Tashjian A. H., Jr Establishment of a calcitonin-producing rat medullary thyroid carcinoma cell line. II. Secretory studies of the tumor and cells in culture. Endocrinology. 1980 Aug;107(2):516–523. doi: 10.1210/endo-107-2-516. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Haller-Brem S., Muff R., Petermann J. B., Born W., Roos B. A., Fischer J. A. Role of cytosolic free calcium concentration in the secretion of calcitonin gene-related peptide and calcitonin from medullary thyroid carcinoma cells. Endocrinology. 1987 Oct;121(4):1272–1277. doi: 10.1210/endo-121-4-1272. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hess P., Tsien R. W. Mechanism of ion permeation through calcium channels. 1984 May 31-Jun 6Nature. 309(5967):453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Hishikawa R., Fukase M., Takenaka M., Fujita T. Effect of calcium channel agonist Bay K 8644 on calcitonin secretion from a rat C-cell line. Biochem Biophys Res Commun. 1985 Jul 16;130(1):454–459. doi: 10.1016/0006-291x(85)90438-3. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R., Murphy E., Lieberman M. Free calcium in isolated chick embryo heart cells measured using quin2 and fura-2. Am J Physiol. 1987 Aug;253(2 Pt 1):C337–C342. doi: 10.1152/ajpcell.1987.253.2.C337. [DOI] [PubMed] [Google Scholar]
- Kawa K. Voltage-gated sodium and potassium currents and their variation in calcitonin-secreting cells of the chick. J Physiol. 1988 May;399:93–113. doi: 10.1113/jphysiol.1988.sp017070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro Y. Spontaneous calcium action potentials in a clonal pituitary cell line and their relationship to prolactin secretion. Nature. 1975 Dec 25;258(5537):741–742. doi: 10.1038/258741a0. [DOI] [PubMed] [Google Scholar]
- Kurtz A., Penner R. Angiotensin II induces oscillations of intracellular calcium and blocks anomalous inward rectifying potassium current in mouse renal juxtaglomerular cells. Proc Natl Acad Sci U S A. 1989 May;86(9):3423–3427. doi: 10.1073/pnas.86.9.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. L., Goodman M. B., St John P. A., Barker J. L. Calcium currents and fura-2 signals in fluorescence-activated cell sorted lactotrophs and somatotrophs of rat anterior pituitary. Endocrinology. 1988 Jul;123(1):611–621. doi: 10.1210/endo-123-1-611. [DOI] [PubMed] [Google Scholar]
- Lewis R. S., Cahalan M. D. Mitogen-induced oscillations of cytosolic Ca2+ and transmembrane Ca2+ current in human leukemic T cells. Cell Regul. 1989 Nov;1(1):99–112. doi: 10.1091/mbc.1.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Barneo J., Armstrong C. M. Depolarizing response of rat parathyroid cells to divalent cations. J Gen Physiol. 1983 Aug;82(2):269–294. doi: 10.1085/jgp.82.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. J., Klahr S. Dissociation of membrane potential and hormone secretion in bovine parathyroid cells. Am J Physiol. 1983 Jul;245(1):E102–E105. doi: 10.1152/ajpendo.1983.245.1.E102. [DOI] [PubMed] [Google Scholar]
- Muff R., Nemeth E. F., Haller-Brem S., Fischer J. A. Regulation of hormone secretion and cytosolic Ca2+ by extracellular Ca2+ in parathyroid cells and C-cells: role of voltage-sensitive Ca2+ channels. Arch Biochem Biophys. 1988 Aug 15;265(1):128–135. doi: 10.1016/0003-9861(88)90378-5. [DOI] [PubMed] [Google Scholar]
- Nemeth E. F., Scarpa A. Rapid mobilization of cellular Ca2+ in bovine parathyroid cells evoked by extracellular divalent cations. Evidence for a cell surface calcium receptor. J Biol Chem. 1987 Apr 15;262(11):5188–5196. [PubMed] [Google Scholar]
- Ozawa S. Biphasic effect of thyrotropin-releasing hormone on membrane K+ permeability in rat clonal pituitary cells. Brain Res. 1981 Mar 23;209(1):240–244. doi: 10.1016/0006-8993(81)91188-4. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Sand O. Electrophysiology of excitable endocrine cells. Physiol Rev. 1986 Oct;66(4):887–952. doi: 10.1152/physrev.1986.66.4.887. [DOI] [PubMed] [Google Scholar]
- Penner R., Matthews G., Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988 Aug 11;334(6182):499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- Sand O., Jonsson L., Nielsen M., Holm R., Gautvik K. M. Electrophysiological properties of calcitonin-secreting cells derived from human medullary thyroid carcinoma. Acta Physiol Scand. 1986 Feb;126(2):173–179. doi: 10.1111/j.1748-1716.1986.tb07803.x. [DOI] [PubMed] [Google Scholar]
- Sand O., Ozawa S., Gautvik K. M. Sodium and calcium action potentials in cells derived from a rat medullary thyroid carcinoma. Acta Physiol Scand. 1981 Jul;112(3):287–291. doi: 10.1111/j.1748-1716.1981.tb06818.x. [DOI] [PubMed] [Google Scholar]
- Sand O., Ozawa S., Hove K. Electrophysiology of cultured parathyroid cells from the goat. Acta Physiol Scand. 1981 Sep;113(1):45–50. doi: 10.1111/j.1748-1716.1981.tb06859.x. [DOI] [PubMed] [Google Scholar]
- Schlegel W., Winiger B. P., Mollard P., Vacher P., Wuarin F., Zahnd G. R., Wollheim C. B., Dufy B. Oscillations of cytosolic Ca2+ in pituitary cells due to action potentials. Nature. 1987 Oct 22;329(6141):719–721. doi: 10.1038/329719a0. [DOI] [PubMed] [Google Scholar]
- Shoback D. M., Membreno L. A., McGhee J. G. High calcium and other divalent cations increase inositol trisphosphate in bovine parathyroid cells. Endocrinology. 1988 Jul;123(1):382–389. doi: 10.1210/endo-123-1-382. [DOI] [PubMed] [Google Scholar]
- Yellen G. Single Ca2+-activated nonselective cation channels in neuroblastoma. Nature. 1982 Mar 25;296(5855):357–359. doi: 10.1038/296357a0. [DOI] [PubMed] [Google Scholar]
- Zeytinoğlu F. N., Gagel R. F., Tashjian A. H., Jr, Hammer R. A., Leeman S. E. Regulation of neurotensin release by a continuous line of mammalian C-cells: the role of biogenic amines. Endocrinology. 1983 Apr;112(4):1240–1246. doi: 10.1210/endo-112-4-1240. [DOI] [PubMed] [Google Scholar]


