Abstract
1. The three-microelectrode voltage clamp technique and pharmacological agents were used to examine the properties and functions of potassium currents in squid giant presynaptic terminals. 2. Outward currents consisted of two components: a slow component which activated over hundreds of milliseconds and was blocked by extracellular application of tetraethylammonium (TEA) ions and a more rapidly activating component which was relatively insensitive to extracellular TEA. 3. The more rapid component was studied in isolation by treating presynaptic terminals with extracellular TEA, as well as tetrodotoxin (to block sodium channel currents) and manganese (to block calcium channel currents). The magnitude of this current component was 1-2 mA cm-2 at 0 mV. Rates of activation and deactivation were voltage dependent and little evidence of inactivation was seen for depolarizations less than several seconds in duration. 4. The reversal potential of the current was -70 to -80 mV in normal saline and became more positive with elevated extracellular potassium concentrations, suggesting that potassium is the primary permeant ion. Accumulation of extracellular potassium appeared to be marked during depolarizations that produced significant activation of the current. 5. Extracellular application of 3,4-diaminopyridine (DAP) blocked the current with an apparent dissociation constant of 7 microM at 0 mV. Intracellular applications of DAP and TEA also were effective in reducing this current. These treatments, but not extracellular TEA application, broadened presynaptic action potentials and increased the magnitude and time-to-peak of postsynaptic currents elicited by the broadened presynaptic action potentials. Postsynaptic currents were a sensitive and linear function of action potential duration; a 30% increase in action potential duration increased postsynaptic current amplitude by 190%. 6. Estimation of the magnitude and time course of the presynaptic calcium current, based on previous measurements of calcium channel gating, indicated that action potential broadening produces a large increase in calcium current magnitude. These calculations predict that a 30% increase in presynaptic action potential duration will increase the peak amplitude of the calcium current by approximately 170% and the total amount of calcium entry by approximately 230%. This implies a linear relationship between transmitter release and calcium entry during an action potential and can be explained by assuming that calcium co-operatively triggers release within intracellular domains that do not overlap.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







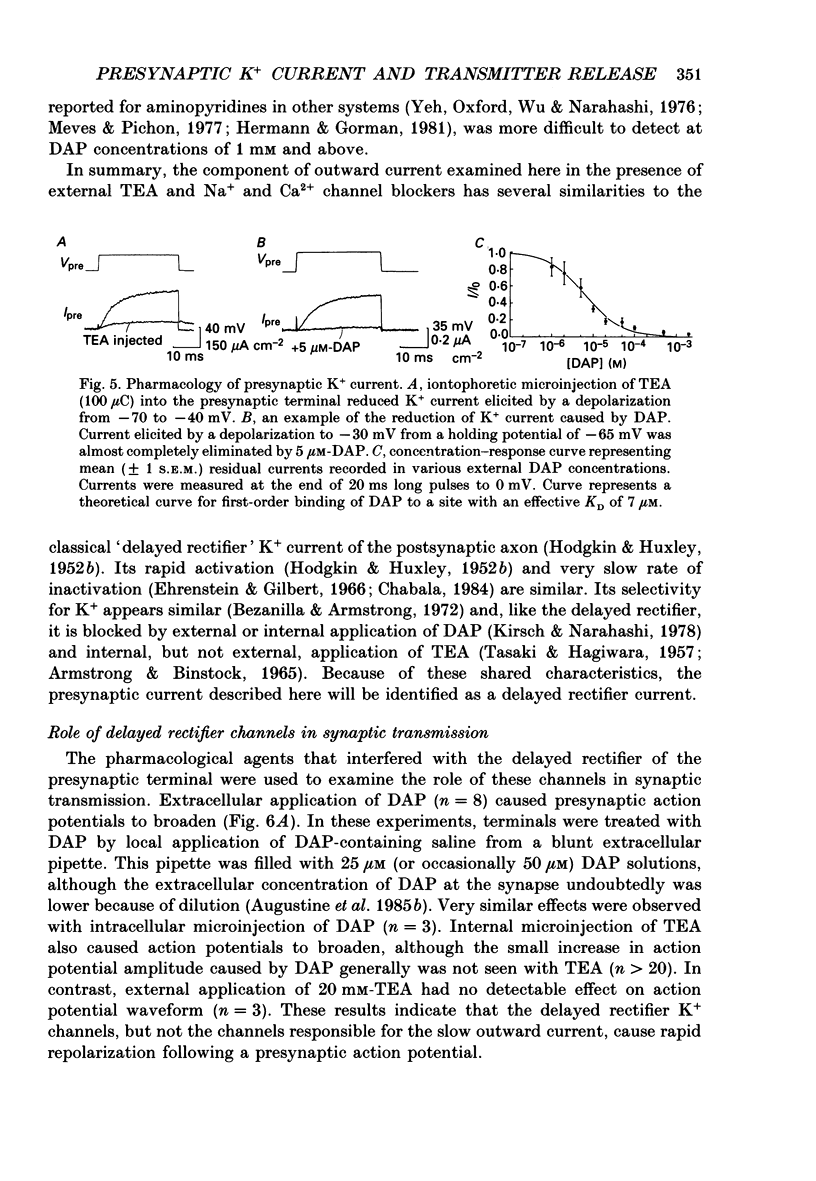
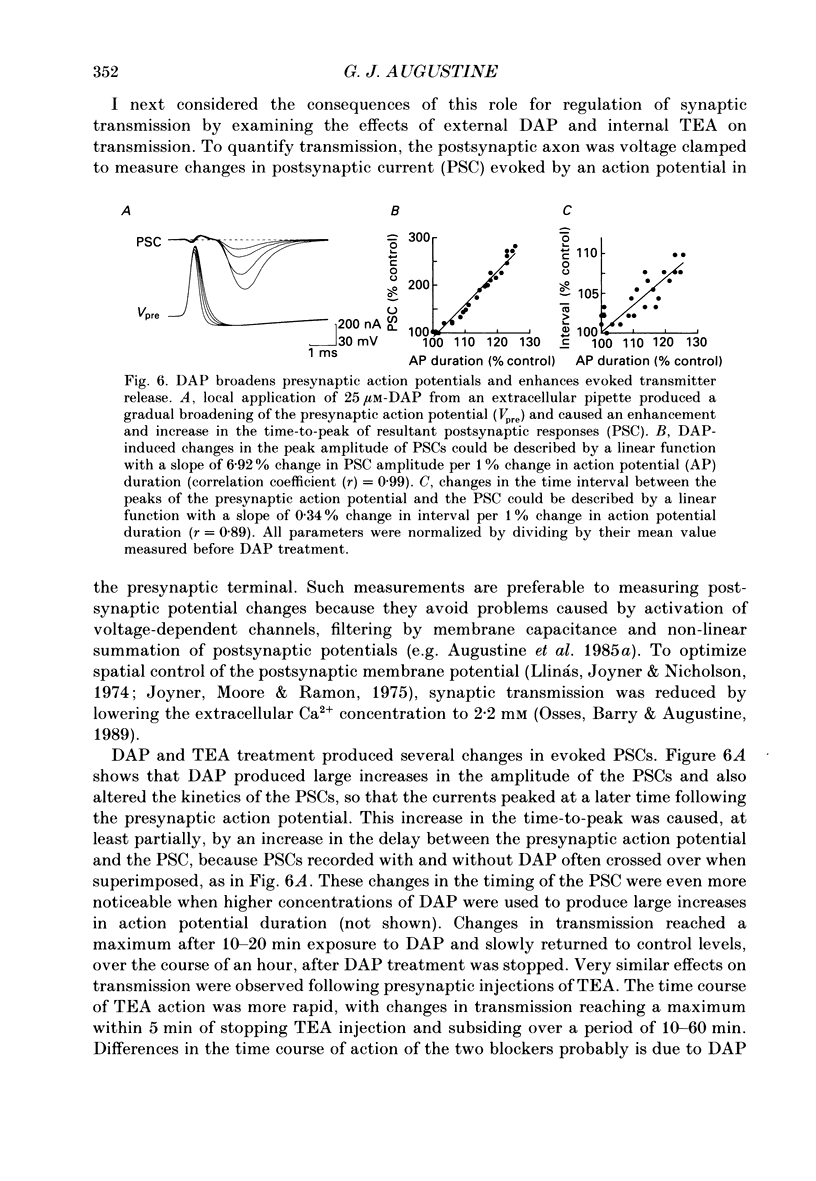












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG C. M., BINSTOCK L. ANOMALOUS RECTIFICATION IN THE SQUID GIANT AXON INJECTED WITH TETRAETHYLAMMONIUM CHLORIDE. J Gen Physiol. 1965 May;48:859–872. doi: 10.1085/jgp.48.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott N. J., Lieberman E. M., Pichon Y., Hassan S., Larmet Y. Periaxonal K+ regulation in the small squid Alloteuthis. Studies on isolated and in situ axons. Biophys J. 1988 Feb;53(2):275–279. doi: 10.1016/S0006-3495(88)83089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelman W. J., Jr, Palti Y., Senft J. P. Potassium ion accumulation in a periaxonal space and its effect on the measurement of membrane potassium ion conductance. J Membr Biol. 1973 Nov 8;13(4):387–410. doi: 10.1007/BF01868237. [DOI] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. Slow changes in potassium permeability in skeletal muscle. J Physiol. 1970 Jul;208(3):645–668. doi: 10.1113/jphysiol.1970.sp009140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkon D. L., Grossman Y. Evidence for nonsynaptic neuronal interaction. J Neurophysiol. 1978 May;41(3):640–653. doi: 10.1152/jn.1978.41.3.640. [DOI] [PubMed] [Google Scholar]
- Almers W. Potassium concentration changes in the transverse tubules of vertebrate skeletal muscle. Fed Proc. 1980 Apr;39(5):1527–1532. [PubMed] [Google Scholar]
- Augustine C. K., Bezanilla F. Phosphorylation modulates potassium conductance and gating current of perfused giant axons of squid. J Gen Physiol. 1990 Feb;95(2):245–271. doi: 10.1085/jgp.95.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P. Calcium dependence of presynaptic calcium current and post-synaptic response at the squid giant synapse. J Physiol. 1986 Dec;381:619–640. doi: 10.1113/jphysiol.1986.sp016347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P., Smith S. J. Calcium entry and transmitter release at voltage-clamped nerve terminals of squid. J Physiol. 1985 Oct;367:163–181. doi: 10.1113/jphysiol.1985.sp015819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P., Smith S. J. Calcium entry into voltage-clamped presynaptic terminals of squid. J Physiol. 1985 Oct;367:143–162. doi: 10.1113/jphysiol.1985.sp015818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Eckert R. Divalent cations differentially support transmitter release at the squid giant synapse. J Physiol. 1984 Jan;346:257–271. doi: 10.1113/jphysiol.1984.sp015020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartschat D. K., Blaustein M. P. Potassium channels in isolated presynaptic nerve terminals from rat brain. J Physiol. 1985 Apr;361:419–440. doi: 10.1113/jphysiol.1985.sp015653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit P. R., Mambrini J. Modification of transmitter release by ions which prolong the presynaptic action potential. J Physiol. 1970 Oct;210(3):681–695. doi: 10.1113/jphysiol.1970.sp009235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Negative conductance caused by entry of sodium and cesium ions into the potassium channels of squid axons. J Gen Physiol. 1972 Nov;60(5):588–608. doi: 10.1085/jgp.60.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque C. W. Intraterminal recordings from the rat neurohypophysis in vitro. J Physiol. 1990 Feb;421:247–262. doi: 10.1113/jphysiol.1990.sp017943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigant J. L., Mallart A. Presynaptic currents in mouse motor endings. J Physiol. 1982 Dec;333:619–636. doi: 10.1113/jphysiol.1982.sp014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burley E. S., Jacobs R. S. Effect of 4-aminopyridine on nerve terminal action potentials. J Pharmacol Exp Ther. 1981 Oct;219(1):268–273. [PubMed] [Google Scholar]
- Chabala L. D. The kinetics of recovery and development of potassium channel inactivation in perfused squid (Loligo pealei) giant axons. J Physiol. 1984 Nov;356:193–220. doi: 10.1113/jphysiol.1984.sp015460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. Calcium domains associated with individual channels can account for anomalous voltage relations of CA-dependent responses. Biophys J. 1984 May;45(5):993–999. doi: 10.1016/S0006-3495(84)84244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton M. P., Augustine G. J. Spatial control of membrane potential: a method for improved voltage clamping of the squid giant synapse. J Neurosci Methods. 1988 Jan;22(3):195–202. doi: 10.1016/0165-0270(88)90040-4. [DOI] [PubMed] [Google Scholar]
- Charlton M. P., Bittner G. D. Facilitation of transmitter release at squid synapses. J Gen Physiol. 1978 Oct;72(4):471–486. doi: 10.1085/jgp.72.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton M. P., Smith S. J., Zucker R. S. Role of presynaptic calcium ions and channels in synaptic facilitation and depression at the squid giant synapse. J Physiol. 1982 Feb;323:173–193. doi: 10.1113/jphysiol.1982.sp014067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J. M. Simultaneous changes in the equilibrium potential and potassium conductance in voltage clamped Ranvier node in the frog. J Physiol. 1981 Sep;318:279–295. doi: 10.1113/jphysiol.1981.sp013864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgington D. R., Stuart A. E. Properties of tetraethylammonium ion-resistant K+ channels in the photoreceptor membrane of the giant barnacle. J Gen Physiol. 1981 Jun;77(6):629–646. doi: 10.1085/jgp.77.6.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenstein G., Gilbert D. L. Slow changes of potassium permeability in the squid giant axon. Biophys J. 1966 Sep;6(5):553–566. doi: 10.1016/S0006-3495(66)86677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erulkar S. D., Weight F. F. Extracellular potassium and trasmitter release at the giant synapse of squid. J Physiol. 1977 Apr;266(2):209–218. doi: 10.1113/jphysiol.1977.sp011764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi J. H., Moore J. W., Stuart A. E. Adaptation in the input-output relation of the synapse made by the barnacle's photoreceptor. J Physiol. 1985 Nov;368:179–195. doi: 10.1113/jphysiol.1985.sp015852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A., Gorman A. L. Effects of 4-aminopyridine on potassium currents in a molluscan neuron. J Gen Physiol. 1981 Jul;78(1):63–86. doi: 10.1085/jgp.78.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochner B., Klein M., Schacher S., Kandel E. R. Action-potential duration and the modulation of transmitter release from the sensory neurons of Aplysia in presynaptic facilitation and behavioral sensitization. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8410–8414. doi: 10.1073/pnas.83.21.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Aldrich R. W. Voltage-dependent K+ currents and underlying single K+ channels in pheochromocytoma cells. J Gen Physiol. 1988 Jan;91(1):73–106. doi: 10.1085/jgp.91.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y., Dennis M. J. Two mutations of synaptic transmission in Drosophila. Proc R Soc Lond B Biol Sci. 1977 Jul 28;198(1130):87–108. doi: 10.1098/rspb.1977.0087. [DOI] [PubMed] [Google Scholar]
- Joyner R. W., Moore J. W., Ramón F. Axon voltage-clamp simulations. III. Postsynaptic region. Biophys J. 1975 Jan;15(1):37–54. doi: 10.1016/S0006-3495(75)85790-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H. Molecular biology of learning: modulation of transmitter release. Science. 1982 Oct 29;218(4571):433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch G. E., Narahashi T. 3,4-diaminopyridine. A potent new potassium channel blocker. Biophys J. 1978 Jun;22(3):507–512. doi: 10.1016/S0006-3495(78)85503-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano K., Livengood D. R., Werman R. Tetraethylammonium ions: effect of presynaptic injection on synaptic transmission. Science. 1967 Mar 10;155(3767):1257–1259. doi: 10.1126/science.155.3767.1257. [DOI] [PubMed] [Google Scholar]
- Latorre R., Miller C. Conduction and selectivity in potassium channels. J Membr Biol. 1983;71(1-2):11–30. doi: 10.1007/BF01870671. [DOI] [PubMed] [Google Scholar]
- Lemos J. R., Nordmann J. J., Cooke I. M., Stuenkel E. L. Single channels and ionic currents in peptidergic nerve terminals. 1986 Jan 30-Feb 5Nature. 319(6052):410–412. doi: 10.1038/319410a0. [DOI] [PubMed] [Google Scholar]
- Lin J. W., Faber D. S. Synaptic transmission mediated by single club endings on the goldfish Mauthner cell. II. Plasticity of excitatory postsynaptic potentials. J Neurosci. 1988 Apr;8(4):1313–1325. doi: 10.1523/JNEUROSCI.08-04-01313.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren C. A., Moore J. W. Identification of ionic currents at presynaptic nerve endings of the lizard. J Physiol. 1989 Jul;414:201–222. doi: 10.1113/jphysiol.1989.sp017684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I., Webb C. K., Bezanilla F. Potassium conductance of the squid giant axon. Single-channel studies. J Gen Physiol. 1988 Aug;92(2):179–196. doi: 10.1085/jgp.92.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Joyner R. W., Nicholson C. Equilibrium potential for the postsynaptic response in the squid giant synapse. J Gen Physiol. 1974 Nov;64(5):519–535. doi: 10.1085/jgp.64.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents in squid giant synapse. Biophys J. 1981 Mar;33(3):289–321. doi: 10.1016/S0006-3495(81)84898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse. Biophys J. 1981 Mar;33(3):323–351. doi: 10.1016/S0006-3495(81)84899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Simon S. M. Transmission by presynaptic spike-like depolarization in the squid giant synapse. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2415–2419. doi: 10.1073/pnas.79.7.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Walton K., Bohr V. Synaptic transmission in squid giant synapse after potassium conductance blockage with external 3- and 4-aminopyridine. Biophys J. 1976 Jan;16(1):83–86. doi: 10.1016/S0006-3495(76)85664-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundh H., Thesleff S. The mode of action of 4-aminopyridine and guanidine on transmitter release from motor nerve terminals. Eur J Pharmacol. 1977 Apr 21;42(4):411–412. doi: 10.1016/0014-2999(77)90176-5. [DOI] [PubMed] [Google Scholar]
- Marty A., Neher E. Potassium channels in cultured bovine adrenal chromaffin cells. J Physiol. 1985 Oct;367:117–141. doi: 10.1113/jphysiol.1985.sp015817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H., Pichon Y. The effect of internal and external 4-aminopyridine on the potassium currents in intracellularly perfused squid giant axons. J Physiol. 1977 Jun;268(2):511–532. doi: 10.1113/jphysiol.1977.sp011869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. The action of calcium on neuronal synapses in the squid. J Physiol. 1966 May;184(2):473–498. doi: 10.1113/jphysiol.1966.sp007927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molgó J., Lemeignan M., Lechat P. Analysis of the action of 4-aminopyridine during repetitive stimulation at the neuromuscular junction. Eur J Pharmacol. 1979 Jan 15;53(3):307–311. doi: 10.1016/0014-2999(79)90138-9. [DOI] [PubMed] [Google Scholar]
- Moore J. W., Ramon F. On numerical integration of the Hodgkin and Huxley equations for a membrane action potential. J Theor Biol. 1974 May;45(1):249–273. doi: 10.1016/0022-5193(74)90054-x. [DOI] [PubMed] [Google Scholar]
- Nicholls J., Wallace B. G. Modulation of transmission at an inhibitory synapse in the central nervous system of the leech. J Physiol. 1978 Aug;281:157–170. doi: 10.1113/jphysiol.1978.sp012414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant H. C., Gallant P. E., Cohen R., Neary J. T., Gainer H. Calcium-dependent 4-aminopyridine stimulation of protein phosphorylation in squid optic lobe synaptosomes. Cell Mol Neurobiol. 1983 Sep;3(3):223–238. doi: 10.1007/BF00710949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perozo E., Bezanilla F., Dipolo R. Modulation of K channels in dialyzed squid axons. ATP-mediated phosphorylation. J Gen Physiol. 1989 Jun;93(6):1195–1218. doi: 10.1085/jgp.93.6.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumplin D. W., Reese T. S., Llinás R. Are the presynaptic membrane particles the calcium channels? Proc Natl Acad Sci U S A. 1981 Nov;78(11):7210–7213. doi: 10.1073/pnas.78.11.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski M. A., Barker J. L. Effects of 4-aminopyridine on calcium action potentials and calcium current under voltage clamp in spinal neurons. Brain Res. 1983 Nov 28;280(1):180–185. doi: 10.1016/0006-8993(83)91190-3. [DOI] [PubMed] [Google Scholar]
- Salzberg B. M., Obaid A. L., Senseman D. M., Gainer H. Optical recording of action potentials from vertebrate nerve terminals using potentiometric probes provides evidence for sodium and calcium components. Nature. 1983 Nov 3;306(5938):36–40. doi: 10.1038/306036a0. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M. The biophysical pharmacology of calcium-dependent acetylcholine secretion. Pharmacol Rev. 1985 Mar;37(1):81–132. [PubMed] [Google Scholar]
- Simon S. M., Llinás R. R. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985 Sep;48(3):485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. O. Restricted diffusion of extracellular potassium at the neuromuscular junction of aged rats. Brain Res. 1982 May 13;239(2):668–673. doi: 10.1016/0006-8993(82)90548-0. [DOI] [PubMed] [Google Scholar]
- Smith S. J., Charlton M. P., Augustine G. J. The voltage-dependence of transmitter release. J Physiol (Paris) 1986;81(4):332–339. [PubMed] [Google Scholar]
- Stühmer W., Ruppersberg J. P., Schröter K. H., Sakmann B., Stocker M., Giese K. P., Perschke A., Baumann A., Pongs O. Molecular basis of functional diversity of voltage-gated potassium channels in mammalian brain. EMBO J. 1989 Nov;8(11):3235–3244. doi: 10.1002/j.1460-2075.1989.tb08483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson R. P., Jr, Armstrong C. M. K+ channels close more slowly in the presence of external K+ and Rb+. Nature. 1981 Jun 4;291(5814):427–429. doi: 10.1038/291427a0. [DOI] [PubMed] [Google Scholar]
- TASAKI I., HAGIWAR A. S. Demonstration of two stable potential states in the squid giant axon under tetraethylammonium chloride. J Gen Physiol. 1957 Jul 20;40(6):859–885. doi: 10.1085/jgp.40.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesleff S. Aminopyridines and synaptic transmission. Neuroscience. 1980;5(8):1413–1419. doi: 10.1016/0306-4522(80)90002-0. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Erulkar S. D. Synaptic transmission and effects of temperature at the squid giant synapse. Nature. 1976 Jun 24;261(5562):720–722. doi: 10.1038/261720a0. [DOI] [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Dynamics of aminopyridine block of potassium channels in squid axon membrane. J Gen Physiol. 1976 Nov;68(5):519–535. doi: 10.1085/jgp.68.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikami D., Bagabaldo Z., Olivera B. M. The inhibitory effects of omega-conotoxins on Ca channels and synapses. Ann N Y Acad Sci. 1989;560:230–248. doi: 10.1111/j.1749-6632.1989.tb24100.x. [DOI] [PubMed] [Google Scholar]
- Zucker R. S., Fogelson A. L. Relationship between transmitter release and presynaptic calcium influx when calcium enters through discrete channels. Proc Natl Acad Sci U S A. 1986 May;83(9):3032–3036. doi: 10.1073/pnas.83.9.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]


