Abstract
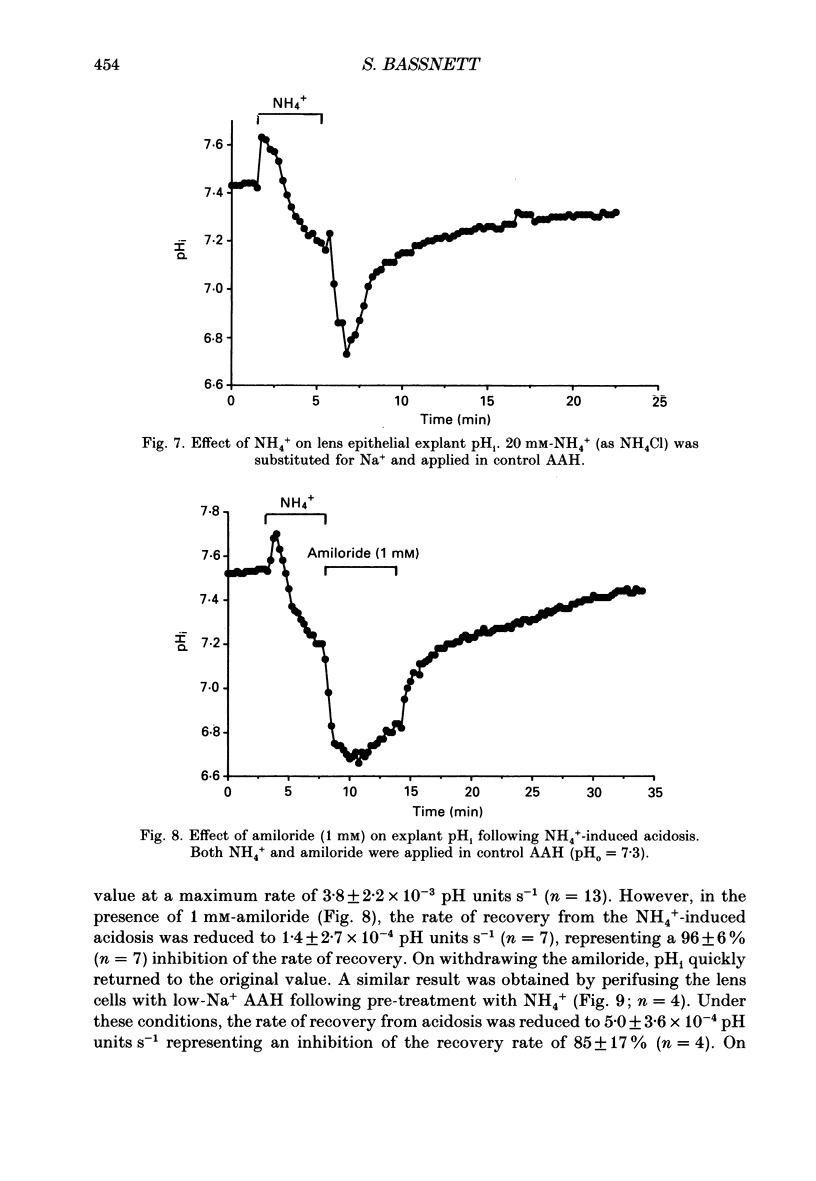
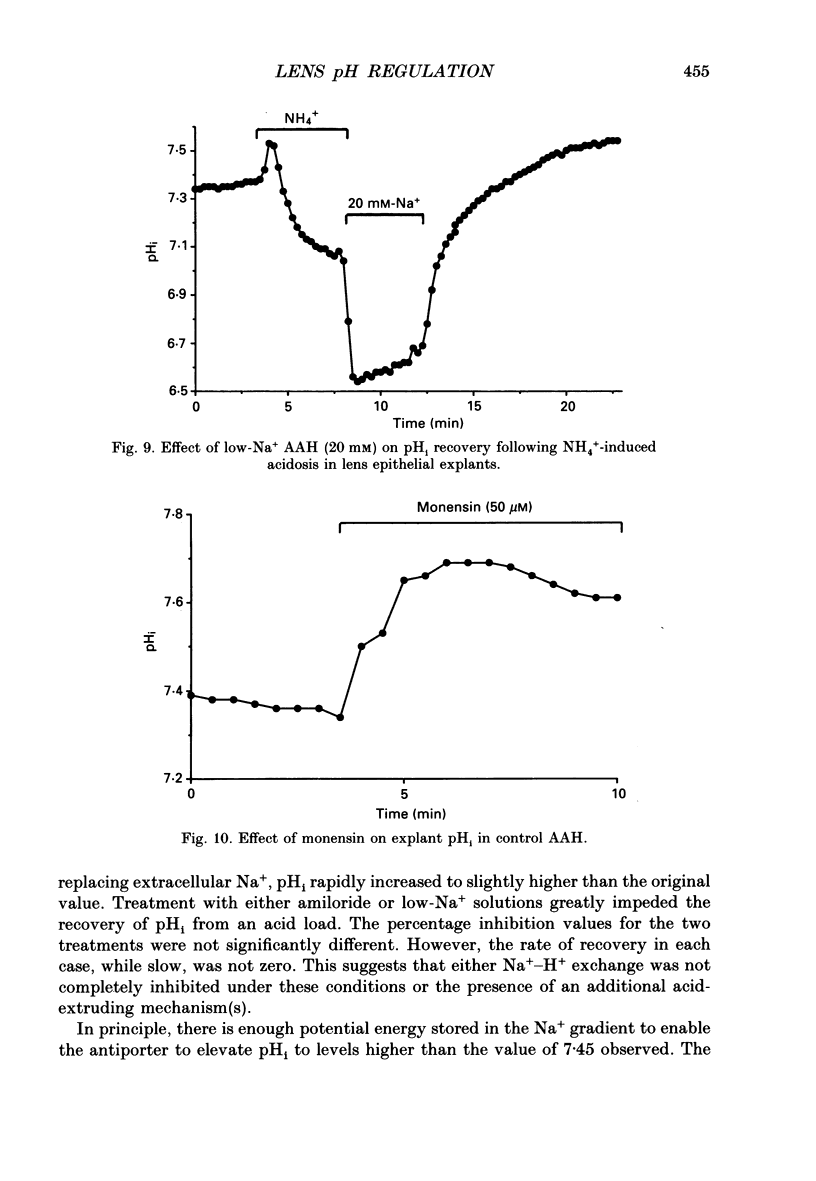
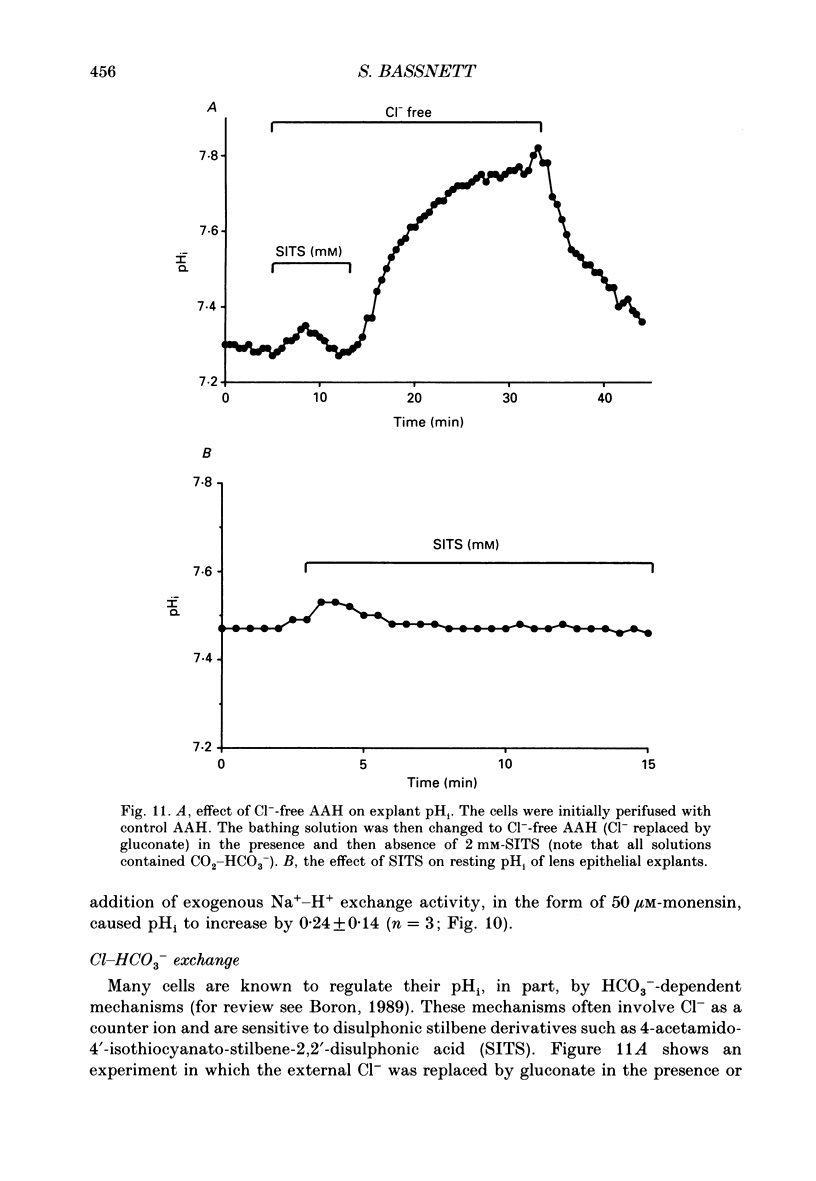
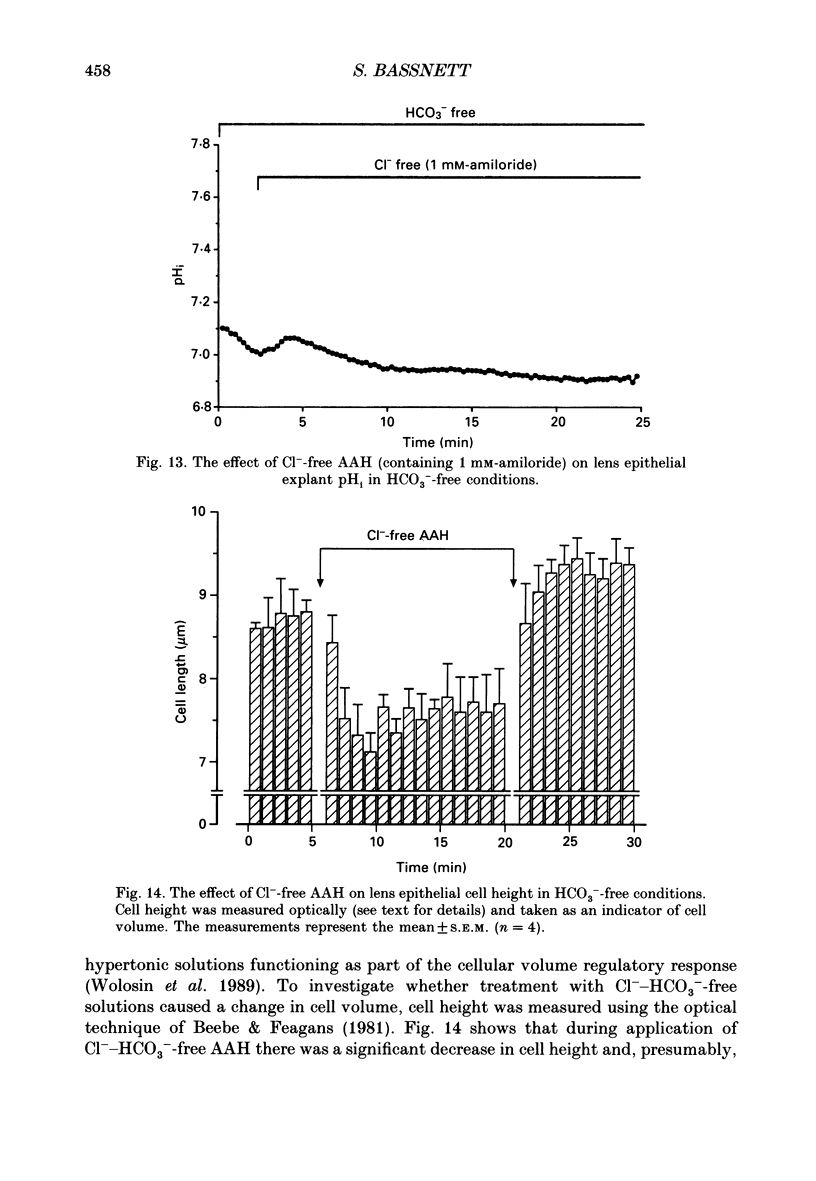
1. The intracellular pH (pHi) of embryonic lens epithelia was measured by the emission ratio technique using the fluorescent pH probe carboxy-seminaphthorhodafluor-1 (Snarf-1). 2. In artificial aqueous humour solutions (AAH) containing HCO3-, pHi was 7.45, a value more alkaline than that of the bathing medium (pH = 7.3). In HCO3- -free AAH, pHi was 7.29. 3. Acetazolamide, an inhibitor of carbonic anhydrase, had no effect on resting pHi. 4. The pHi could be manipulated experimentally by changing the external pH (pHo) of HEPES-buffered AAH, the addition or withdrawal of CO2-HCO3-, or by perfusion with the weak bases NH4Cl and procaine. 5. The pHi change induced by withdrawal of 5 mM-procaine was used to calculate a value for the intrinsic cytoplasmic buffering capacity (beta i) of 16.5 mM. 6. The addition of amiloride (1 mM) or treatment with low-Na+ AAH solutions led to a decrease in pHi of 0.23 over the 10 min exposure. In addition, these treatments inhibited pHi recovery from NH4(+)-induced acidosis. These observations are consistent with the presence of amiloride-sensitive N(+)-H+ antiport. 7. Addition of exogenous antiport activity in the form of 50 microM-monensin caused an increase in pHi of 0.24. 8. In HCO3(-)-containing media, replacing Cl- by gluconate or isothionate led to an immediate, reversible increase in pHi which could be completely inhibited by 2 mM-4-acetamido-4'-isothiocyanato-stillbene-2,2'-disulphonic acid (SITS). This indicates the presence of Cl(-)-HCO3- exchange in this tissue. 9. Under HCO3(-)-free conditions, replacement of Cl- by gluconate or isothionate caused a small transient acidification followed, 5 min later, by a large sustained alkalinization. The delayed increase in pHi could be completely blocked by 1 mM-amiloride and may reflect volume-sensitive stimulation of the Na(+)-H+ antiporter as cell volume (estimated by cell height measurements) was shown to decrease significantly during this period.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. P., Low P. S., Dola A., Maisel H. Band 3 and ankyrin homologues are present in eye lens: evidence for all major erythrocyte membrane components in same non-erythroid cell. Biochem Biophys Res Commun. 1987 Nov 30;149(1):266–275. doi: 10.1016/0006-291x(87)91634-2. [DOI] [PubMed] [Google Scholar]
- Bassnett S., Croghan P. C., Duncan G. Diffusion of lactate and its role in determining intracellular pH in the lens of the eye. Exp Eye Res. 1987 Jan;44(1):143–147. doi: 10.1016/s0014-4835(87)80032-5. [DOI] [PubMed] [Google Scholar]
- Bassnett S., Duncan G. Direct measurement of pH in the rat lens by ion-sensitive microelectrodes. Exp Eye Res. 1985 Apr;40(4):585–590. doi: 10.1016/0014-4835(85)90080-6. [DOI] [PubMed] [Google Scholar]
- Bassnett S., Duncan G. The influence of pH on membrane conductance and intercellular resistance in the rat lens. J Physiol. 1988 Apr;398:507–521. doi: 10.1113/jphysiol.1988.sp017054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassnett S., Reinisch L., Beebe D. C. Intracellular pH measurement using single excitation-dual emission fluorescence ratios. Am J Physiol. 1990 Jan;258(1 Pt 1):C171–C178. doi: 10.1152/ajpcell.1990.258.1.C171. [DOI] [PubMed] [Google Scholar]
- Bassnett S., Stewart S., Duncan G., Croghan P. C. Efflux of chloride from the rat lens: influence of membrane potential and intracellular acidification. Q J Exp Physiol. 1988 Nov;73(6):941–949. doi: 10.1113/expphysiol.1988.sp003228. [DOI] [PubMed] [Google Scholar]
- Beebe D. C., Feagans D. E. A tissue culture system for studying lens cell differentiation. Vision Res. 1981;21(1):113–118. doi: 10.1016/0042-6989(81)90143-7. [DOI] [PubMed] [Google Scholar]
- Beebe D. C., Parmelee J. T., Belcher K. S. Volume regulation in lens epithelial cells and differentiating lens fiber cells. J Cell Physiol. 1990 Jun;143(3):455–459. doi: 10.1002/jcp.1041430308. [DOI] [PubMed] [Google Scholar]
- Boyarsky G., Ganz M. B., Sterzel R. B., Boron W. F. pH regulation in single glomerular mesangial cells. I. Acid extrusion in absence and presence of HCO3-. Am J Physiol. 1988 Dec;255(6 Pt 1):C844–C856. doi: 10.1152/ajpcell.1988.255.6.C844. [DOI] [PubMed] [Google Scholar]
- Boyarsky G., Ganz M. B., Sterzel R. B., Boron W. F. pH regulation in single glomerular mesangial cells. II. Na+-dependent and -independent Cl(-)-HCO3- exchangers. Am J Physiol. 1988 Dec;255(6 Pt 1):C857–C869. doi: 10.1152/ajpcell.1988.255.6.C857. [DOI] [PubMed] [Google Scholar]
- Clayton R. M., Cuthbert J., Seth J., Phillips C. I., Bartholomew R. S., Reid J. M. Epidemiological and other studies in the assessment of factors contributing to cataractogenesis. Ciba Found Symp. 1984;106:25–47. doi: 10.1002/9780470720875.ch3. [DOI] [PubMed] [Google Scholar]
- Duncan G. The site of the ion restricting membranes in the toad lens. Exp Eye Res. 1969 Oct;8(4):406–412. doi: 10.1016/s0014-4835(69)80006-0. [DOI] [PubMed] [Google Scholar]
- Friedland B. R., Maren T. H. The relation between carbonic anhydrase activity and ion transport in elasmobranch and rabbit lens. Exp Eye Res. 1981 Nov;33(5):545–561. doi: 10.1016/s0014-4835(81)80129-7. [DOI] [PubMed] [Google Scholar]
- Fröhlich O. Antiporters. Curr Opin Cell Biol. 1989 Aug;1(4):729–734. doi: 10.1016/0955-0674(89)90041-0. [DOI] [PubMed] [Google Scholar]
- Goodenough D. A., Dick J. S., 2nd, Lyons J. E. Lens metabolic cooperation: a study of mouse lens transport and permeability visualized with freeze-substitution autoradiography and electron microscopy. J Cell Biol. 1980 Aug;86(2):576–589. doi: 10.1083/jcb.86.2.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner J. V., Kopp S. J., Sanders D. R., Glonek T. Organophosphates of the crystalline lens: a nuclear magnetic resonance spectroscopic study. Invest Ophthalmol Vis Sci. 1981 Nov;21(5):700–713. [PubMed] [Google Scholar]
- Hoffmann E. K., Simonsen L. O. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiol Rev. 1989 Apr;69(2):315–382. doi: 10.1152/physrev.1989.69.2.315. [DOI] [PubMed] [Google Scholar]
- Hudgins P. M., Putney J. W., Jr Distribution of local anesthetics and the intracellular pH in vascular smooth muscle. J Pharmacol Exp Ther. 1972 Jun;181(3):538–546. [PubMed] [Google Scholar]
- KINOSHITA J. H. PATHWAYS OF GLUCOSE METABOLISM IN THE LENS. Invest Ophthalmol. 1965 Aug;4:619–628. [PubMed] [Google Scholar]
- Kuszak J., Maisel H., Harding C. V. Gap junctions of chick lens fiber cells. Exp Eye Res. 1978 Oct;27(4):495–498. doi: 10.1016/0014-4835(78)90026-x. [DOI] [PubMed] [Google Scholar]
- Peracchia C., Peracchia L. L. Gap junction dynamics: reversible effects of hydrogen ions. J Cell Biol. 1980 Dec;87(3 Pt 1):719–727. doi: 10.1083/jcb.87.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae J. L., Kuszak J. R. The electrical coupling of epithelium and fibers in the frog lens. Exp Eye Res. 1983 Mar;36(3):317–326. doi: 10.1016/0014-4835(83)90114-8. [DOI] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Shinohara T., Piatigorsky J. Regulation of protein synthesis, intracellular electrolytes and cataract formation in vitro. Nature. 1977 Dec 1;270(5636):406–411. doi: 10.1038/270406a0. [DOI] [PubMed] [Google Scholar]
- Szatkowski M. S., Thomas R. C. The intrinsic intracellular H+ buffering power of snail neurones. J Physiol. 1989 Feb;409:89–101. doi: 10.1113/jphysiol.1989.sp017486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. The effect of carbon dioxide on the intracellular pH and buffering power of snail neurones. J Physiol. 1976 Mar;255(3):715–735. doi: 10.1113/jphysiol.1976.sp011305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosin J. M., Alvarez L. J., Candia O. A. Cellular pH and Na+-H+ exchange activity in lens epithelium of Bufo marinus toad. Am J Physiol. 1988 Nov;255(5 Pt 1):C595–C602. doi: 10.1152/ajpcell.1988.255.5.C595. [DOI] [PubMed] [Google Scholar]
- Wolosin J. M., Alvarez L. J., Candia O. A. Stimulation of toad lens epithelial Na+/H+ exchange activity by hypertonicity. Exp Eye Res. 1989 Jun;48(6):855–862. doi: 10.1016/0014-4835(89)90068-7. [DOI] [PubMed] [Google Scholar]



