Abstract
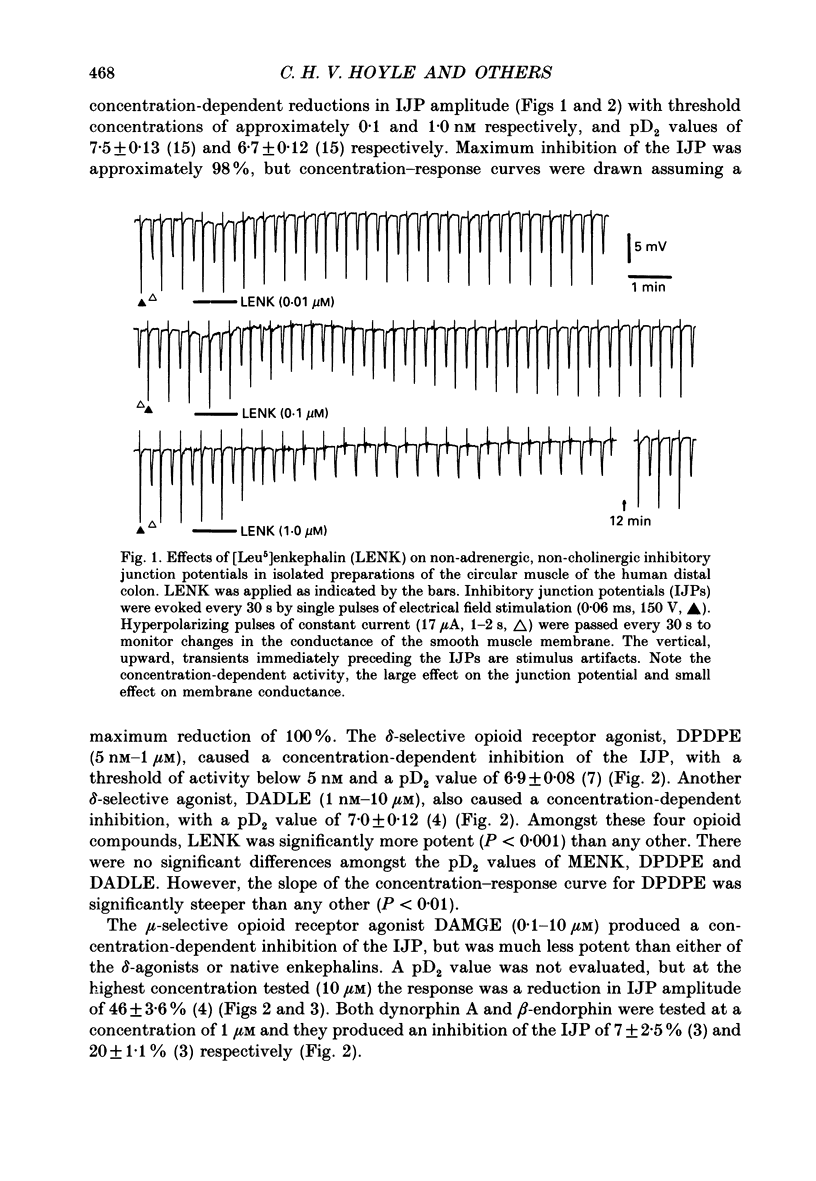
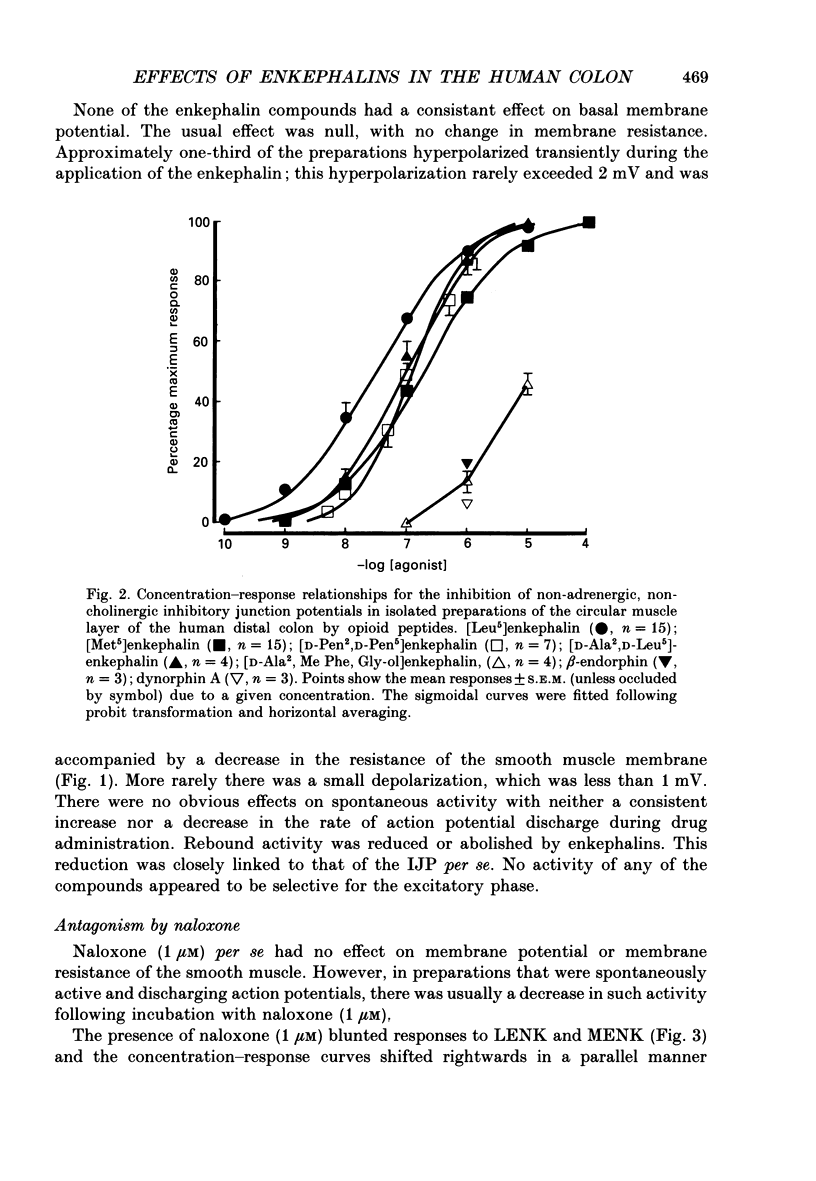
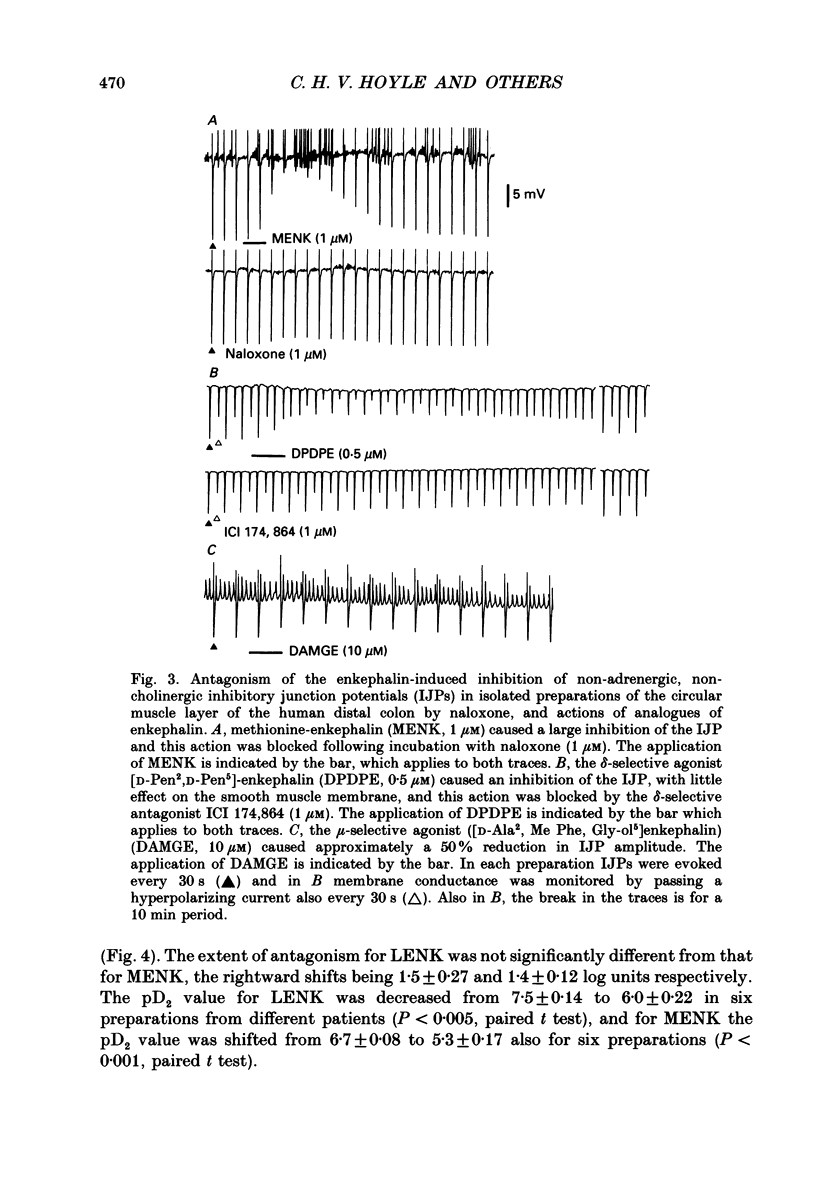
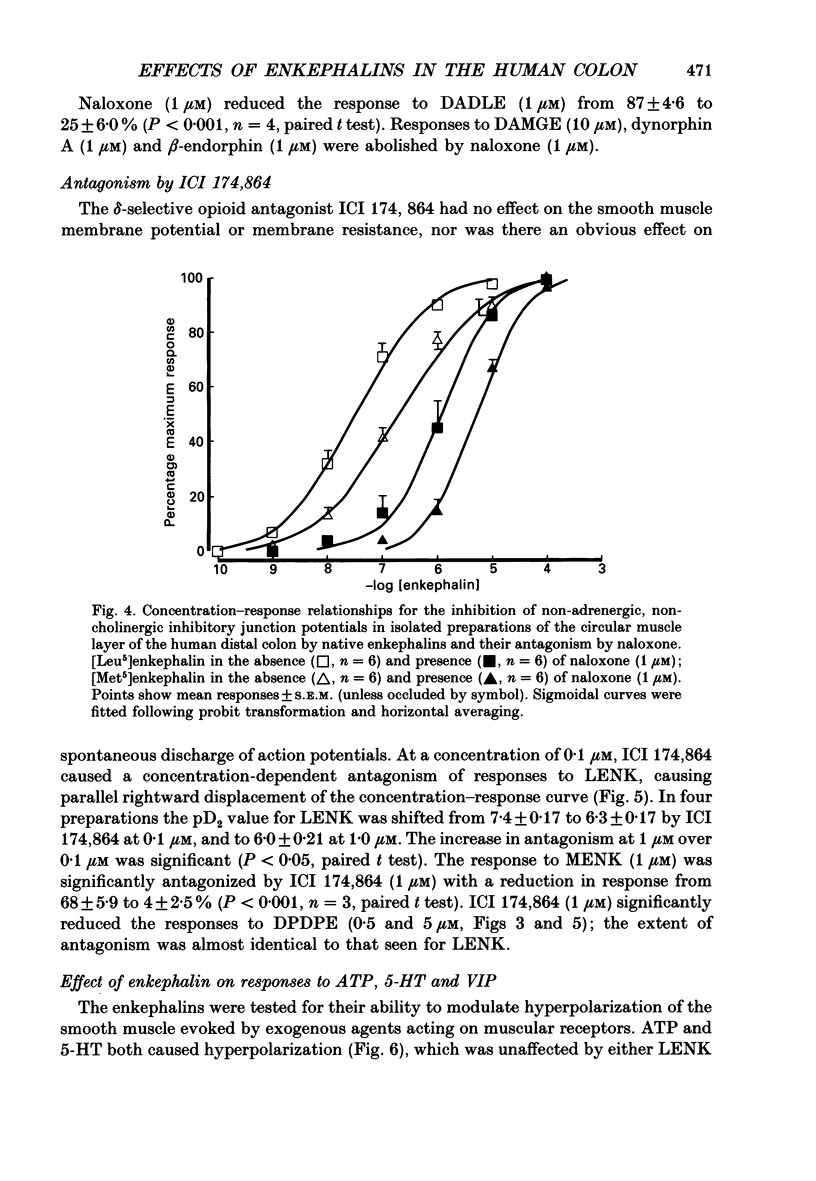
1. A sucrose-gap technique was used to investigate the neuromodulatory actions of enkephalins on non-adrenergic, non-cholinergic inhibitory junction potentials (IJPs) in the circular muscle of the human large intestine. 2. The native enkephalins, [Leu5]enkephalin (LENK) and [Met5]enkephalin (MENK) caused a concentration-dependent reduction in amplitude of IJPs without a significant effect on the smooth muscle membrane. 3. The actions of LENK and MENK were mimicked by the delta-selective opioid receptor agonists [D-Pen2, D-Pen5]enkephalin (DPDPE) and [D-Ala2, D-Leu5]enkephalin (DADLE). 4. The actions of LENK, MENK and DPDPE were antagonized to similar extents by the delta-selective opioid receptor antagonist ICI 174,864. 5. The mu-selective opioid receptor agonist [D-Ala2, Me Phe, Gly-ol5]enkephalin was approximately 100-fold less potent than any of the native or synthetic enkephalins at reducing the amplitude of the IJP. Dynorphin A and beta-endorphin both had very weak activity. 6. Responses to all of the agonists were inhibited by naloxone. The degree of antagonism of DPDPE or DADLE by naloxone (1 microM) was the same as that of LENK or MENK. 7. Neither MENK nor LENK affected hyperpolarization of the smooth muscle membrane induced by ATP or 5-hydroxytryptamine. Vasoactive intestinal polypeptide (1 pM-1 microM) did not produce any observable responses and this lack of reactivity was not affected by the enkephalins. 8. It is concluded that in the circular muscle of the human colon, LENK and MENK can act on prejunctional delta-opioid receptors to produce inhibition of non-adrenergic, non-cholinergic inhibitory neuromuscular transmission. Possible physiological significance of this prejunctional receptor is discussed.
Full text
PDF


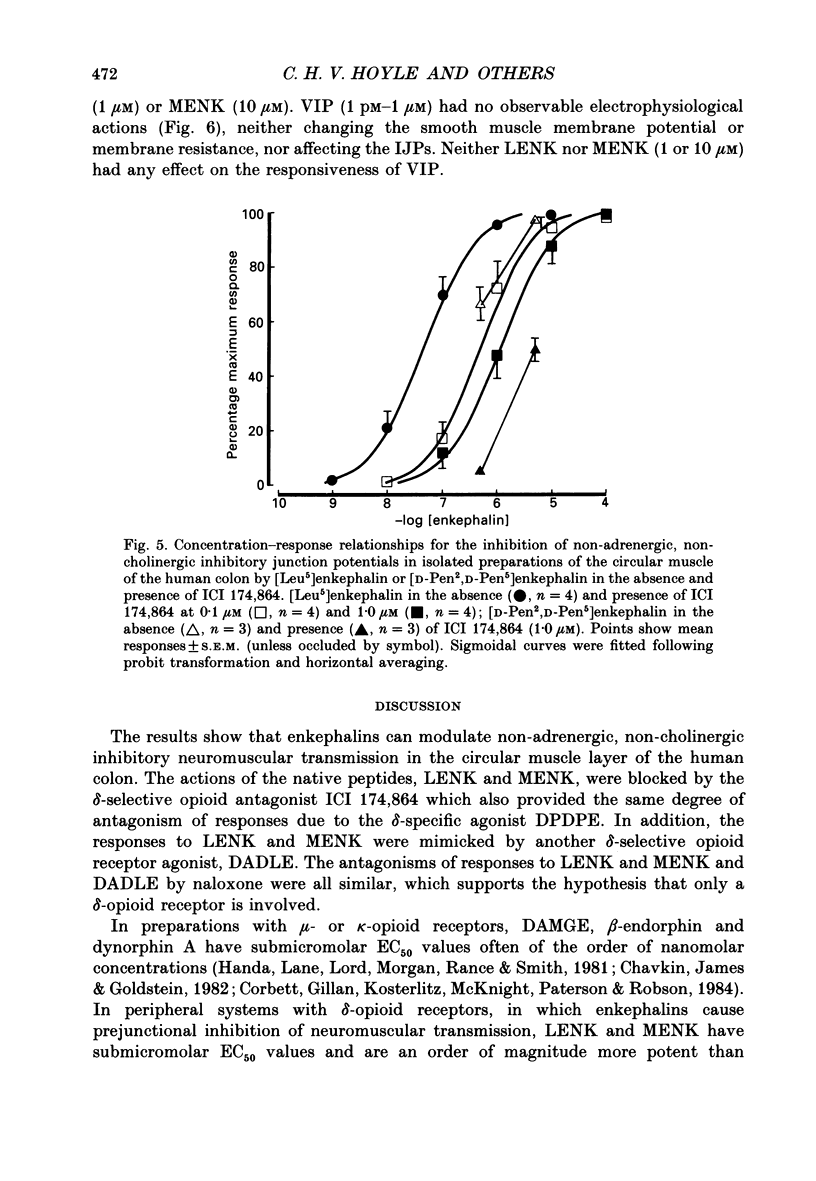
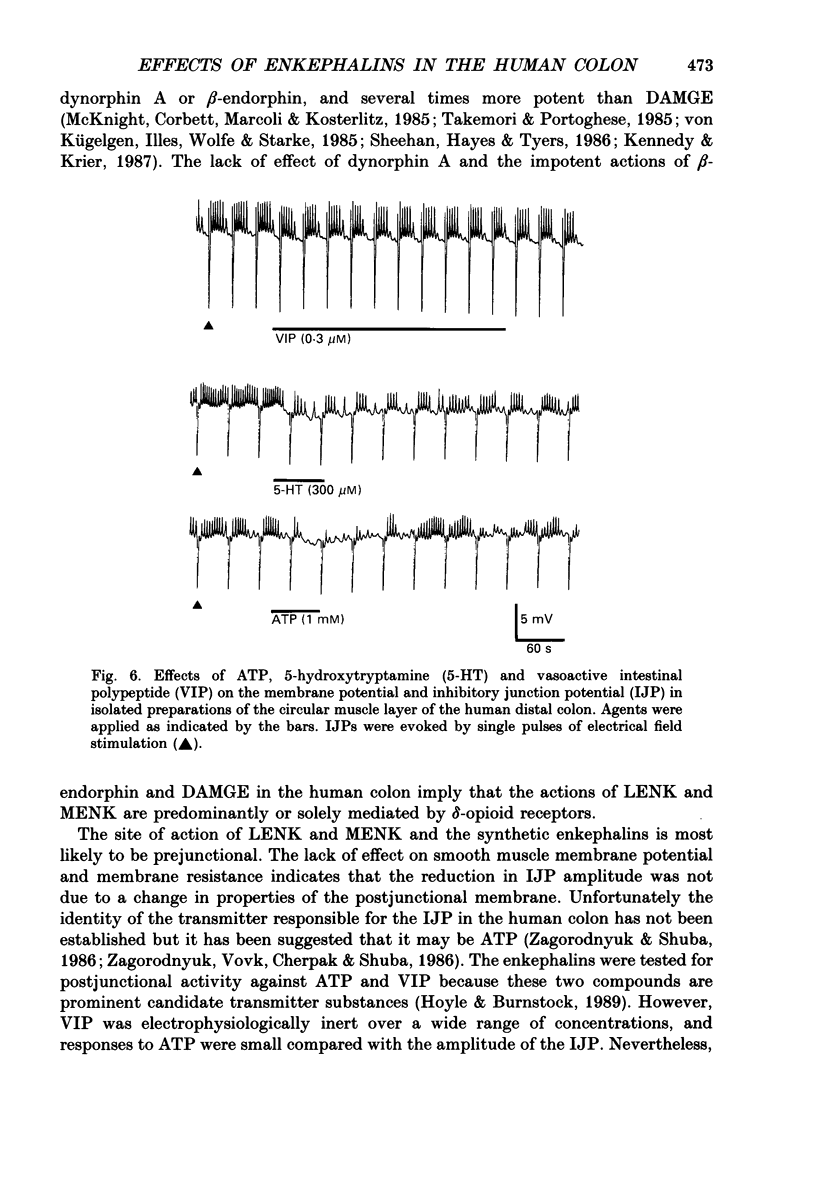





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abadia R., Boschat M., Opolon P. Endorphines et tube digestif. Ann Med Interne (Paris) 1983;134(8):742–753. [PubMed] [Google Scholar]
- Baskin D. S., Hosobuchi Y. Naloxone reversal of ischaemic neurological deficits in man. Lancet. 1981 Aug 8;2(8241):272–275. doi: 10.1016/s0140-6736(81)90524-9. [DOI] [PubMed] [Google Scholar]
- Blanquet F., Bouvier M., Gonella J. Effects of enkephalins and morphine on spontaneous electrical activity and on junction potentials elicited by parasympathetic nerve stimulation in cat and rabbit colon. Br J Pharmacol. 1982 Nov;77(3):419–429. doi: 10.1111/j.1476-5381.1982.tb09314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C., James I. F., Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982 Jan 22;215(4531):413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- Cherubini E., Morita K., North R. A. Opioid inhibition of synaptic transmission in the guinea-pig myenteric plexus. Br J Pharmacol. 1985 Aug;85(4):805–817. doi: 10.1111/j.1476-5381.1985.tb11079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement-Jones V., Lowry P. J., Rees L. H., Besser G. M. Met-enkephalin circulates in human plasma. Nature. 1980 Jan 17;283(5744):295–297. doi: 10.1038/283295a0. [DOI] [PubMed] [Google Scholar]
- Corbett A. D., Gillan M. G., Kosterlitz H. W., McKnight A. T., Paterson S. J., Robson L. E. Selectivities of opioid peptide analogues as agonists and antagonists at the delta-receptor. Br J Pharmacol. 1984 Sep;83(1):271–279. doi: 10.1111/j.1476-5381.1984.tb10143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton R., Giles M. G., Miller L., Shaw J. S., Timms D. ICI 174864: a highly selective antagonist for the opioid delta-receptor. Eur J Pharmacol. 1984 Jan 27;97(3-4):331–332. doi: 10.1016/0014-2999(84)90470-9. [DOI] [PubMed] [Google Scholar]
- Daniel E. E., Gonda T., Domoto T., Oki M. The effects of substance P and met5-enkephalin in dog ileum. Can J Physiol Pharmacol. 1982 Jun;60(6):830–840. doi: 10.1139/y82-116. [DOI] [PubMed] [Google Scholar]
- Edin R. The vagal control of the pyloric motor function: a physiological and immunohistochemical study in cat and man. Acta Physiol Scand Suppl. 1980;485:1–30. [PubMed] [Google Scholar]
- Ekblad E., Winther C., Ekman R., Håkanson R., Sundler F. Projections of peptide-containing neurons in rat small intestine. Neuroscience. 1987 Jan;20(1):169–188. doi: 10.1016/0306-4522(87)90010-8. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M., Miller R. J. Distribution and projections of nerves with enkephalin-like immunoreactivity in the guinea-pig small intestine. Neuroscience. 1983 Apr;8(4):653–664. doi: 10.1016/0306-4522(83)90001-5. [DOI] [PubMed] [Google Scholar]
- Glass J., Chan W. C., Gintzler A. R. Direct analysis of the release of methionine-enkephalin from guinea pig myenteric plexus: modulation by endogenous opioids and exogenous morphine. J Pharmacol Exp Ther. 1986 Dec;239(3):742–747. [PubMed] [Google Scholar]
- Glass J., Clouet D., Gintzler A. R. Short-term nerve stimulation increases enkephalin production and content in the guinea pig myenteric plexus. Brain Res. 1986 Apr 30;372(1):180–184. doi: 10.1016/0006-8993(86)91475-7. [DOI] [PubMed] [Google Scholar]
- Grishina O. Iu, Ovsiannikov V. I. Initsiiruiushchie i moduliruiushchie éffekty met-énkefalina v proiavlenii sokratitel'nykh reaktsii toshchei i podvzdoshnoi kishki. Fiziol Zh SSSR Im I M Sechenova. 1988 May;74(5):728–736. [PubMed] [Google Scholar]
- Handa B. K., Land A. C., Lord J. A., Morgan B. A., Rance M. J., Smith C. F. Analogues of beta-LPH61-64 possessing selective agonist activity at mu-opiate receptors. Eur J Pharmacol. 1981 Apr 9;70(4):531–540. doi: 10.1016/0014-2999(81)90364-2. [DOI] [PubMed] [Google Scholar]
- Hoyle C. H. A modified single sucrose gap. Junction potentials and electrotonic potentials in gastrointestinal smooth muscles. J Pharmacol Methods. 1987 Nov;18(3):219–226. doi: 10.1016/0160-5402(87)90072-6. [DOI] [PubMed] [Google Scholar]
- Hoyle C. H., Burnstock G., Jass J., Lennard-Jones J. E. Enkephalins inhibit non-adrenergic, non-cholinergic neuromuscular transmission in the human colon. Eur J Pharmacol. 1986 Nov 12;131(1):159–160. doi: 10.1016/0014-2999(86)90532-7. [DOI] [PubMed] [Google Scholar]
- James S., Hoyle C. H., Burnstock G., Jass J. R., Jeffrey I. J., Lennard-Jones J. E. Autoradiographic localization of delta-opioid binding sites in human sigmoid colon. Eur J Pharmacol. 1987 Oct 6;142(1):185–186. doi: 10.1016/0014-2999(87)90674-1. [DOI] [PubMed] [Google Scholar]
- Jians R., Janssens J., Vantrappen G., Ceccatelli P. Influence of metenkephalin analogue on motor activity of the gastrointestinal tract. Gastroenterology. 1987 Jul;93(1):114–120. doi: 10.1016/0016-5085(87)90322-2. [DOI] [PubMed] [Google Scholar]
- Kaufman P. N., Krevsky B., Malmud L. S., Maurer A. H., Somers M. B., Siegel J. A., Fisher R. S. Role of opiate receptors in the regulation of colonic transit. Gastroenterology. 1988 Jun;94(6):1351–1356. doi: 10.1016/0016-5085(88)90673-7. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Krier J. [Met5]enkephalin acts via delta-opioid receptors to inhibit pelvic nerve-evoked contractions of cat distal colon. Br J Pharmacol. 1987 Oct;92(2):291–298. doi: 10.1111/j.1476-5381.1987.tb11323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch T. R., Carney J. A., Go V. L. Distribution and quantitation of gut neuropeptides in normal intestine and inflammatory bowel diseases. Dig Dis Sci. 1987 Apr;32(4):369–376. doi: 10.1007/BF01296290. [DOI] [PubMed] [Google Scholar]
- Kosterlitz H. W., Lord J. A., Paterson S. J., Waterfield A. A. Effects of changes in the structure of enkephalins and of narcotic analgesic drugs on their interactions with mu- and delta-receptors. Br J Pharmacol. 1980 Feb;68(2):333–342. doi: 10.1111/j.1476-5381.1980.tb10422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosterlitz H. W. The Wellcome Foundation lecture, 1982. Opioid peptides and their receptors. Proc R Soc Lond B Biol Sci. 1985 Jul 22;225(1238):27–40. doi: 10.1098/rspb.1985.0048. [DOI] [PubMed] [Google Scholar]
- Kromer W. Endogenous and exogenous opioids in the control of gastrointestinal motility and secretion. Pharmacol Rev. 1988 Jun;40(2):121–162. [PubMed] [Google Scholar]
- Leander S., Håkanson R., Sundler F. Nerves containing substance P, vasoactive intestinal polypeptide, enkephalin or somatostatin in the guinea-pig taenia coli. Distribution, ultrastructure and possible functions. Cell Tissue Res. 1981;215(1):21–39. doi: 10.1007/BF00236246. [DOI] [PubMed] [Google Scholar]
- Lolova I., Davidoff M., Itzev D., Apostolov A. Histochemical, immunocytochemical and ultrastructural data on the innervation of the smooth muscle of the large intestine in Hirschsprung's disease. Acta Physiol Pharmacol Bulg. 1986;12(2):55–62. [PubMed] [Google Scholar]
- Lolova I., Itzev D., Davidoff M. Immunocytochemical localization of substance P, methionine-enkephalin and somatostatin in the cat intestinal wall. J Neural Transm. 1984;60(2):71–88. doi: 10.1007/BF01245026. [DOI] [PubMed] [Google Scholar]
- Lord J. A., Waterfield A. A., Hughes J., Kosterlitz H. W. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977 Jun 9;267(5611):495–499. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Hökfelt T., Nilsson G., Terenius L., Rehfeld J., Elde R., Said S. Peptide neurons in the vagus, splanchnic and sciatic nerves. Acta Physiol Scand. 1978 Dec;104(4):499–501. doi: 10.1111/j.1748-1716.1978.tb06307.x. [DOI] [PubMed] [Google Scholar]
- Manara L., Bianchetti A. The central and peripheral influences of opioids on gastrointestinal propulsion. Annu Rev Pharmacol Toxicol. 1985;25:249–273. doi: 10.1146/annurev.pa.25.040185.001341. [DOI] [PubMed] [Google Scholar]
- Martin W. R., Eades C. G., Thompson J. A., Huppler R. E., Gilbert P. E. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976 Jun;197(3):517–532. [PubMed] [Google Scholar]
- McKnight A. T., Corbett A. D., Marcoli M., Kosterlitz H. W. The opioid receptors in the hamster vas deferens are of the delta-type. Neuropharmacology. 1985 Nov;24(11):1011–1017. doi: 10.1016/0028-3908(85)90184-4. [DOI] [PubMed] [Google Scholar]
- Mihara S., North R. A. Opioids increase potassium conductance in submucous neurones of guinea-pig caecum by activating delta-receptors. Br J Pharmacol. 1986 Jun;88(2):315–322. doi: 10.1111/j.1476-5381.1986.tb10207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosberg H. I., Hurst R., Hruby V. J., Gee K., Yamamura H. I., Galligan J. J., Burks T. F. Bis-penicillamine enkephalins possess highly improved specificity toward delta opioid receptors. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5871–5874. doi: 10.1073/pnas.80.19.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura E., Buchan A. M., McIntosh C. H. Autoradiographic localization of mu- and delta-type opioid receptors in the gastrointestinal tract of the rat and guinea pig. Gastroenterology. 1986 Nov;91(5):1084–1094. doi: 10.1016/s0016-5085(86)80002-6. [DOI] [PubMed] [Google Scholar]
- North R. A., Katayama Y., Williams J. T. On the mechanism and site of action of enkephalin on single myenteric neurons. Brain Res. 1979 Apr 6;165(1):67–77. doi: 10.1016/0006-8993(79)90045-3. [DOI] [PubMed] [Google Scholar]
- Ohkawa H. Effects of enkephalin and endorphin on the inhibitory junction potentials in the duodenal smooth muscle cells of the guinea-pig. Jpn J Physiol. 1984;34(1):89–102. doi: 10.2170/jjphysiol.34.89. [DOI] [PubMed] [Google Scholar]
- Paterson S. J., Robson L. E., Kosterlitz H. W. Classification of opioid receptors. Br Med Bull. 1983 Jan;39(1):31–36. doi: 10.1093/oxfordjournals.bmb.a071787. [DOI] [PubMed] [Google Scholar]
- Polak J. M., Bloom S. R., Sullivan S. N., Facer P., Pearse A. G. Enkephalin-like immunoreactivity in the human gastrointestinal tract. Lancet. 1977 May 7;1(8019):972–974. doi: 10.1016/s0140-6736(77)92277-2. [DOI] [PubMed] [Google Scholar]
- Roemer D., Buescher H. H., Hill R. C., Pless J., Bauer W., Cardinaux F., Closse A., Hauser D., Huguenin R. A synthetic enkephalin analogue with prolonged parenteral and oral analgesic activity. Nature. 1977 Aug 11;268(5620):547–549. doi: 10.1038/268547a0. [DOI] [PubMed] [Google Scholar]
- Schang J. C., Hémond M., Hébert M., Pilote M. How does morphine work on colonic motility? An electromyographic study in the human left and sigmoid colon. Life Sci. 1986 Feb 24;38(8):671–676. doi: 10.1016/0024-3205(86)90580-1. [DOI] [PubMed] [Google Scholar]
- Schultzberg M., Hökfelt T., Nilsson G., Terenius L., Rehfeld J. F., Brown M., Elde R., Goldstein M., Said S. Distribution of peptide- and catecholamine-containing neurons in the gastro-intestinal tract of rat and guinea-pig: immunohistochemical studies with antisera to substance P, vasoactive intestinal polypeptide, enkephalins, somatostatin, gastrin/cholecystokinin, neurotensin and dopamine beta-hydroxylase. Neuroscience. 1980;5(4):689–744. doi: 10.1016/0306-4522(80)90166-9. [DOI] [PubMed] [Google Scholar]
- Sheehan M. J., Hayes A. G., Tyers M. B. Pharmacology of delta-opioid receptors in the hamster vas deferens. Eur J Pharmacol. 1986 Oct 14;130(1-2):57–64. doi: 10.1016/0014-2999(86)90183-4. [DOI] [PubMed] [Google Scholar]
- Takemori A. E., Portoghese P. S. Receptors for opioid peptides in the guinea-pig ileum. J Pharmacol Exp Ther. 1985 Nov;235(2):389–392. [PubMed] [Google Scholar]
- Tiengo M. Naloxone in irreversible shock. Lancet. 1980 Sep 27;2(8196):690–690. doi: 10.1016/s0140-6736(80)92723-3. [DOI] [PubMed] [Google Scholar]
- Tonini M., Onori L., Perucca E., Manzo L., De Ponti F., Crema A. Depression by morphine of the excitability of intrinsic inhibitory neurons in the guinea-pig colon. Eur J Pharmacol. 1985 Sep 24;115(2-3):317–320. doi: 10.1016/0014-2999(85)90708-3. [DOI] [PubMed] [Google Scholar]
- Viveros O. H., Wilson S. P. The adrenal chromaffin cell as a model to study the co-secretion of enkephalins and catecholamines. J Auton Nerv Syst. 1983 Jan;7(1):41–58. doi: 10.1016/0165-1838(83)90068-1. [DOI] [PubMed] [Google Scholar]
- Von Kügelgen I., Illes P., Wolf D., Starke K. Presynaptic inhibitory opioid delta- and kappa-receptors in a branch of the rabbit ileocolic artery. Eur J Pharmacol. 1985 Nov 26;118(1-2):97–105. doi: 10.1016/0014-2999(85)90667-3. [DOI] [PubMed] [Google Scholar]
- Wilson S. P., Klein R. L., Chang K. J., Gasparis M. S., Viveros O. H., Yang W. H. Are opioid peptides co-transmitters in noradrenergic vesicles of sympathetic nerves? Nature. 1980 Dec 25;288(5792):707–709. doi: 10.1038/288707a0. [DOI] [PubMed] [Google Scholar]
- Wüster M., Schulz R., Herz A. Specificity of opioids towards the mu-, delta- and epsilon-opiate receptors. Neurosci Lett. 1979 Dec;15(2-3):193–198. doi: 10.1016/0304-3940(79)96112-3. [DOI] [PubMed] [Google Scholar]
- Zagorodniuk V. P., Shuba M. F. Priroda neadrenergicheskogo tormozhaniia v gladkikh myshtsakh kishechnika cheloveka. Neirofiziologiia. 1986;18(3):373–381. [PubMed] [Google Scholar]
- Zagorodniuk V. P., Vovk E. V., Cherpak B. D., Shuba M. F. Issledovanie sinapticheskikh potentsialov v gladkikh myshtsakh tonkogo kishehcnika cheloveka. Fiziol Zh. 1986 Mar-Apr;32(2):172–179. [PubMed] [Google Scholar]


