Abstract
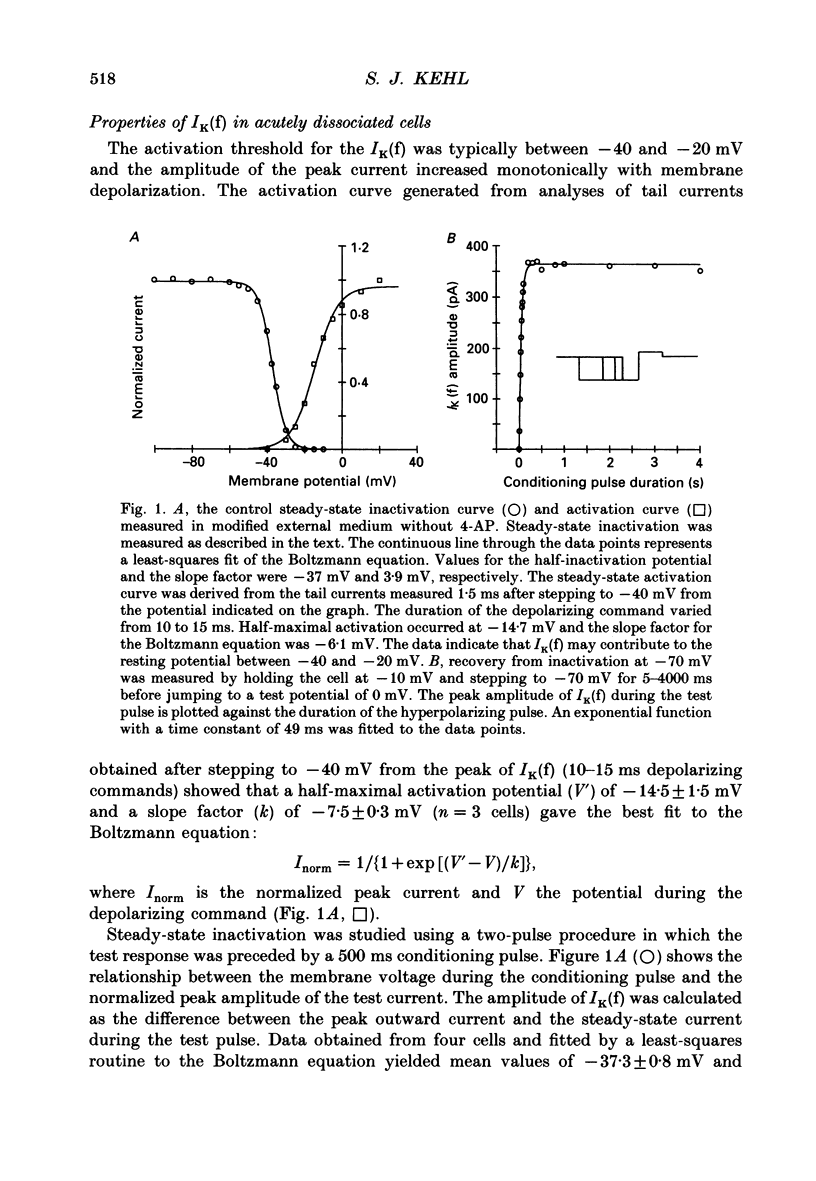
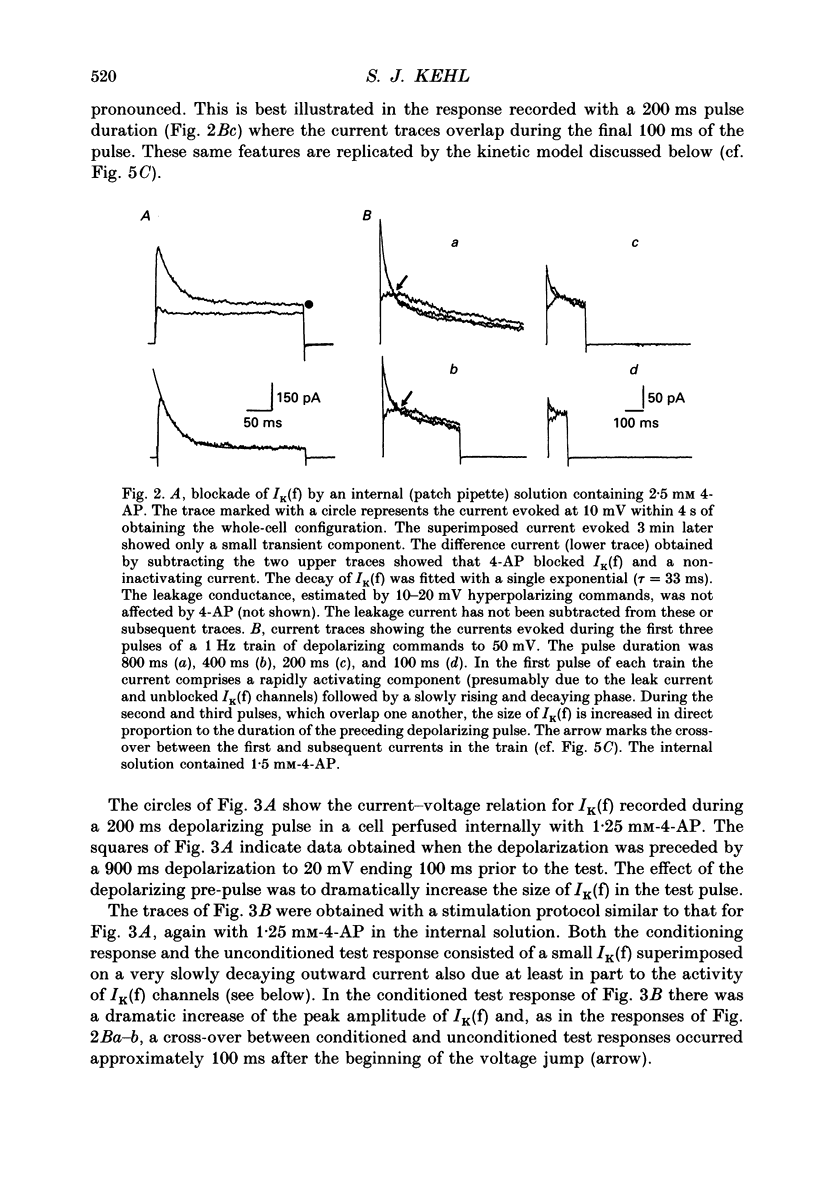
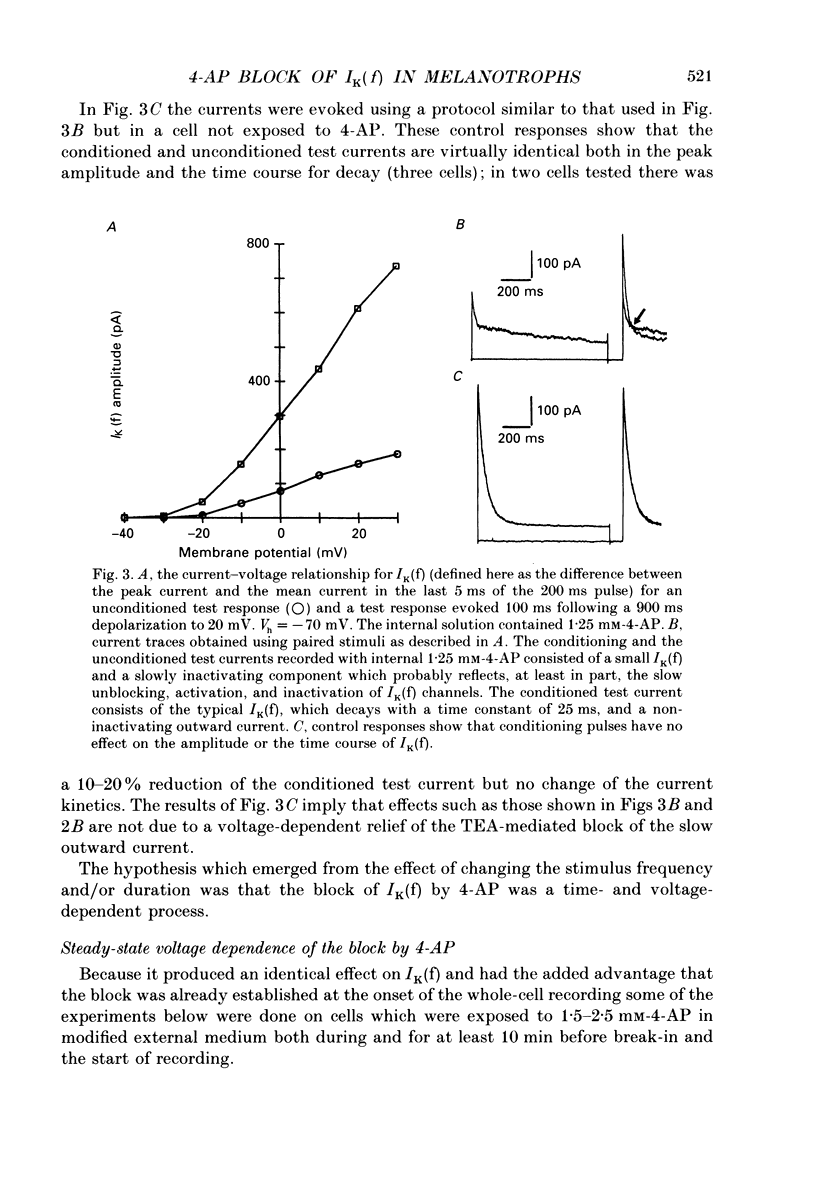
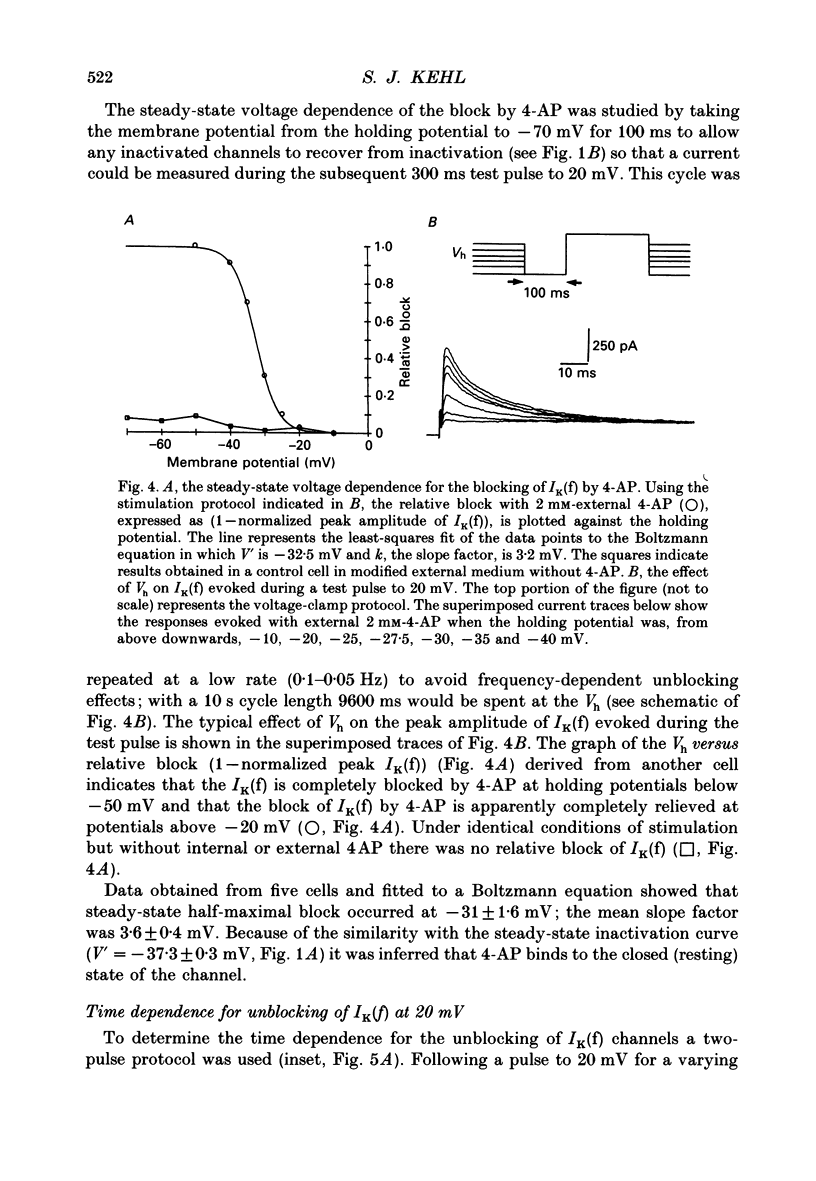
1. Whole-cell voltage-clamp recordings were made from acutely dissociated melanotrophs obtained from adult rats. 2. In the presence of external Na+ and Ca2+ channel blockers and 20 mM-tetraethylammonium (TEA) depolarizations to -40 mV or more evoked a fast-activating fast-inactivating outward K+ current (IK(f)). Double-pulse experiments showed that steady-state half-inactivation occurred near -37 mV; half-maximal activation of IK(f) occurred at -15 mV. Recovery from inactivation in most cells fitted a single exponential with a time constant of 40-50 ms. 3. When applied either internally or externally, 1-2.5 mM-4-aminopyridine (4-AP) substantially reduced IK(f) but the degree of block was affected by the intensity, duration and frequency of depolarizing commands. 4. Analysis of the steady-state voltage dependence of the block by 4-AP showed that half-maximal blocking occurred at approximately -31 mV. This implied that 4-AP binds to the resting state of the IK(f) channel. 5. Studies of the time dependence for the blocking or unblocking of IK(f) showed that both processes were exponential with mean time constants of 1942 ms (at -70 mV) and 726 ms (at 20 mV), respectively. Recovery from inactivation was apparently unaffected by 4-AP. 6. A four-state sequential model in which 4-AP reversibly binds to the resting state of the channel replicates the frequency dependence of the 4-AP blockade.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bäck N., Rechardt L. The effect of reserpine on the pars intermedia of the rat pituitary. An electron-microscopic, fluorescence-histochemical and immunohistochemical study. Cell Tissue Res. 1985;241(1):1–8. doi: 10.1007/BF00214619. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French R. J., Horn R. Sodium channel gating: models, mimics, and modifiers. Annu Rev Biophys Bioeng. 1983;12:319–356. doi: 10.1146/annurev.bb.12.060183.001535. [DOI] [PubMed] [Google Scholar]
- Hermann A., Gorman A. L. Effects of 4-aminopyridine on potassium currents in a molluscan neuron. J Gen Physiol. 1981 Jul;78(1):63–86. doi: 10.1085/jgp.78.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondeghem L. M., Katzung B. G. Antiarrhythmic agents: the modulated receptor mechanism of action of sodium and calcium channel-blocking drugs. Annu Rev Pharmacol Toxicol. 1984;24:387–423. doi: 10.1146/annurev.pa.24.040184.002131. [DOI] [PubMed] [Google Scholar]
- Kasai H., Kameyama M., Yamaguchi K., Fukuda J. Single transient K channels in mammalian sensory neurons. Biophys J. 1986 Jun;49(6):1243–1247. doi: 10.1016/S0006-3495(86)83754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl S. J. Cultured melanotrophs of the adult rat pituitary possess a voltage-activated fast transient outward current. J Physiol. 1989 Apr;411:457–468. doi: 10.1113/jphysiol.1989.sp017583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl S. J., Hughes D., McBurney R. N. A patch clamp study of gamma-aminobutyric acid (GABA)-induced macroscopic currents in rat melanotrophs in cell culture. Br J Pharmacol. 1987 Nov;92(3):573–585. doi: 10.1111/j.1476-5381.1987.tb11359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch G. E., Narahashi T. Site of action and active form of aminopyridines in squid axon membranes. J Pharmacol Exp Ther. 1983 Jul;226(1):174–179. [PubMed] [Google Scholar]
- Mayer M. L., Sugiyama K. A modulatory action of divalent cations on transient outward current in cultured rat sensory neurones. J Physiol. 1988 Feb;396:417–433. doi: 10.1113/jphysiol.1988.sp016970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurney R. N., Kehl S. J. Electrophysiology of neurosecretory cells from the pituitary intermediate lobe. J Exp Biol. 1988 Sep;139:317–328. doi: 10.1242/jeb.139.1.317. [DOI] [PubMed] [Google Scholar]
- Numann R. E., Wadman W. J., Wong R. K. Outward currents of single hippocampal cells obtained from the adult guinea-pig. J Physiol. 1987 Dec;393:331–353. doi: 10.1113/jphysiol.1987.sp016826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford G. S., Wagoner P. K. The inactivating K+ current in GH3 pituitary cells and its modification by chemical reagents. J Physiol. 1989 Mar;410:587–612. doi: 10.1113/jphysiol.1989.sp017550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Thompson S. Aminopyridine block of transient potassium current. J Gen Physiol. 1982 Jul;80(1):1–18. doi: 10.1085/jgp.80.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbricht W., Wagner H. H. Block of potassium channels of the nodal membrane by 4-aminopyridine and its partial removal on depolarization. Pflugers Arch. 1976 Nov 30;367(1):77–87. doi: 10.1007/BF00583659. [DOI] [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Dynamics of aminopyridine block of potassium channels in squid axon membrane. J Gen Physiol. 1976 Nov;68(5):519–535. doi: 10.1085/jgp.68.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]


