Abstract
1. The intracellular mechanism(s) underlying the decrease of a transient outward K+ current (It) induced by alpha 1-adrenergic agonists was studied in isolated adult rabbit atrial myocytes using whole-cell voltage clamp and cell-attached patch clamp techniques. Experiments were carried out at 22-23 degrees C. 2. Application of the specific alpha 1-adrenergic agonist, methoxamine, produced a decrease in It which was irreversible after the non-hydrolysable GTP analogues, GTP gamma S and Gpp(NH)p, had been introduced into cells via the recording micropipette. 3. Pre-treatment of cells with 0.1-0.15 microgram/ml pertussis toxin (PT) for 8-9 h at 30-34 degrees C did not prevent the alpha 1-induced decrease in It. Yet, this protocol, as measured by the PT-catalysed incorporation of [32P]ADP-ribose in membrane-associated 40 and 41 kDa proteins, effectively caused the ADP-ribosylation of approximately 70% of the PT-sensitive GTP-binding proteins (i.e. Gi) in these treated cells. After taking into account the proportion of non-viable cells (20-30%), the effectiveness of this treatment probably approaches 100% in the viable myocytes from which electrophysiological recordings were made. 4. Cell-attached patch recordings showed that bath application of methoxamine altered the single-channel events underlying It by decreasing their opening probability. Averaged currents from ensemble single-channel openings recorded in the presence of 0.2 mM-methoxamine outside the patch reproduced the features of alpha 1-adrenergic modulation of the macroscopic It observed during whole-cell voltage clamp measurements. This observation provides evidence for the involvement of a diffusible intracellular second messenger in the alpha 1-adrenergic modulation of It. 5. The protein kinase C (PKC) activators, 4 beta-phorbol 12-myristate 13-acetate (PMA) and 1-oleoyl-2-acetylglycerol (OAG) increased It, when included in the bath perfusate, whereas the inactive analogues, 4 alpha-phorbol and 4 alpha-phorbol 12,13-didecanoate, had no effect on It. 6. Exposure of cells to the PKC inhibitors, staurosporine and H-7, either by bath superfusion or intracellularly, via the recording micropipette, did not block the decrease in It produced by methoxamine. 7. Prolonged stimulation of atrial myocytes for 7-9 h at 22 degrees C with 500 nM-PMA produced a 'down-regulation' of endogenous PKC activity, as well as a physical loss of the immunoreactive enzyme, as measured by an in vitro assay, and an anti-PKC monoclonal antibody, respectively.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF








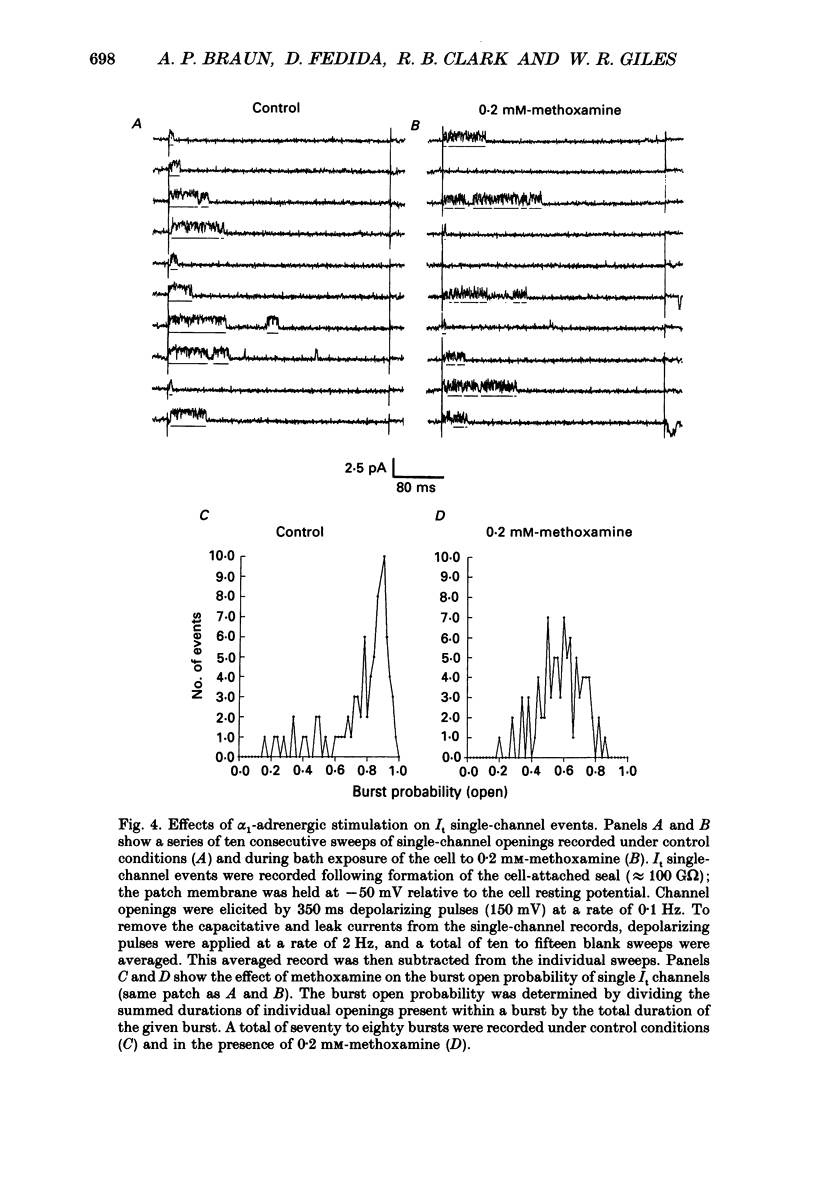






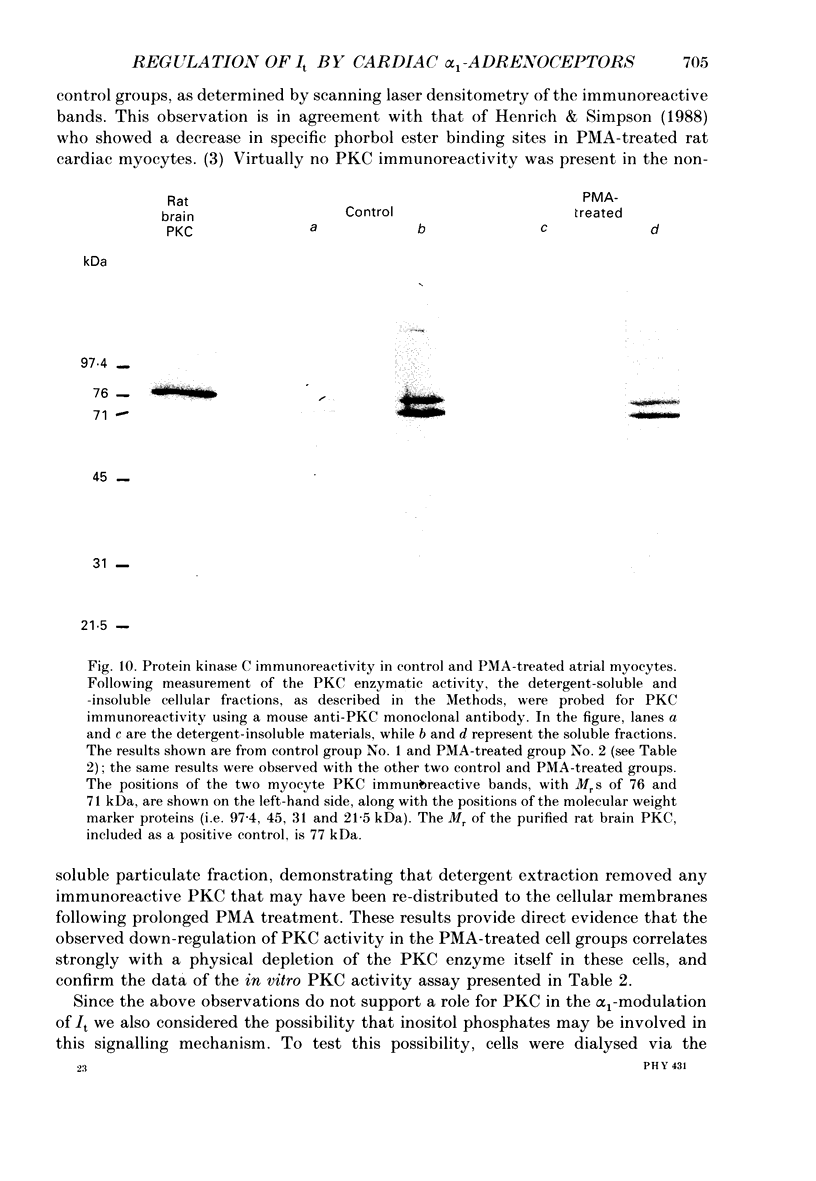







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apkon M., Nerbonne J. M. Alpha 1-adrenergic agonists selectively suppress voltage-dependent K+ current in rat ventricular myocytes. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8756–8760. doi: 10.1073/pnas.85.22.8756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Katada T., Northup J. K., Ui M., Gilman A. G. Purification and properties of the inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. J Biol Chem. 1984 Mar 25;259(6):3560–3567. [PubMed] [Google Scholar]
- Breitwieser G. E., Szabo G. Uncoupling of cardiac muscarinic and beta-adrenergic receptors from ion channels by a guanine nucleotide analogue. Nature. 1985 Oct 10;317(6037):538–540. doi: 10.1038/317538a0. [DOI] [PubMed] [Google Scholar]
- Buxton I. L., Brunton L. L. Action of the cardiac alpha 1-adrenergic receptor. Activation of cyclic AMP degradation. J Biol Chem. 1985 Jun 10;260(11):6733–6737. [PubMed] [Google Scholar]
- Casey P. J., Fong H. K., Simon M. I., Gilman A. G. Gz, a guanine nucleotide-binding protein with unique biochemical properties. J Biol Chem. 1990 Feb 5;265(4):2383–2390. [PubMed] [Google Scholar]
- Clark R. B., Giles W. R., Imaizumi Y. Properties of the transient outward current in rabbit atrial cells. J Physiol. 1988 Nov;405:147–168. doi: 10.1113/jphysiol.1988.sp017326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coraboeuf E., Carmeliet E. Existence of two transient outward currents in sheep cardiac Purkinje fibers. Pflugers Arch. 1982 Feb;392(4):352–359. doi: 10.1007/BF00581631. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Cassel D., Levkovitz H., Lowe M., Selinger Z. Guanosine 5'-O-(2-thiodiphosphate). An inhibitor of adenylate cyclase stimulation by guanine nucleotides and fluoride ions. J Biol Chem. 1979 Oct 10;254(19):9829–9834. [PubMed] [Google Scholar]
- Fedida D., Shimoni Y., Giles W. R. Alpha-adrenergic modulation of the transient outward current in rabbit atrial myocytes. J Physiol. 1990 Apr;423:257–277. doi: 10.1113/jphysiol.1990.sp018021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freissmuth M., Casey P. J., Gilman A. G. G proteins control diverse pathways of transmembrane signaling. FASEB J. 1989 Aug;3(10):2125–2131. [PubMed] [Google Scholar]
- Giles W. R., Imaizumi Y. Comparison of potassium currents in rabbit atrial and ventricular cells. J Physiol. 1988 Nov;405:123–145. doi: 10.1113/jphysiol.1988.sp017325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gross G., Hanft G., Rugevics C. U. Alpha 1-adrenoceptors of rat myocardium: comparison of agonist binding and positive inotropic response. Naunyn Schmiedebergs Arch Pharmacol. 1988 Nov;338(5):582–588. doi: 10.1007/BF00179334. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52(3):165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Henrich C. J., Simpson P. C. Differential acute and chronic response of protein kinase C in cultured neonatal rat heart myocytes to alpha 1-adrenergic and phorbol ester stimulation. J Mol Cell Cardiol. 1988 Dec;20(12):1081–1085. doi: 10.1016/0022-2828(88)90588-3. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hiraoka M., Kawano S. Calcium-sensitive and insensitive transient outward current in rabbit ventricular myocytes. J Physiol. 1989 Mar;410:187–212. doi: 10.1113/jphysiol.1989.sp017528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka M., Kawano S. Mechanism of increased amplitude and duration of the plateau with sudden shortening of diastolic intervals in rabbit ventricular cells. Circ Res. 1987 Jan;60(1):14–26. doi: 10.1161/01.res.60.1.14. [DOI] [PubMed] [Google Scholar]
- Hockberger P., Toselli M., Swandulla D., Lux H. D. A diacylglycerol analogue reduces neuronal calcium currents independently of protein kinase C activation. Nature. 1989 Mar 23;338(6213):340–342. doi: 10.1038/338340a0. [DOI] [PubMed] [Google Scholar]
- Kanaho Y., Katada T., Hoyle K., Crooke S. T., Stadel J. M. Immunochemical comparison of pertussis toxin substrates in brain and peripheral tissues. Cell Signal. 1989;1(6):553–560. doi: 10.1016/0898-6568(89)90063-6. [DOI] [PubMed] [Google Scholar]
- Kushida H., Hiramoto T., Satoh H., Endoh M. Phorbol ester does not mimic, but antagonizes the alpha-adrenoceptor-mediated positive inotropic effect in the rabbit papillary muscle. Naunyn Schmiedebergs Arch Pharmacol. 1988 Feb;337(2):169–176. doi: 10.1007/BF00169245. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leatherman G. F., Kim D., Smith T. W. Effect of phorbol esters on contractile state and calcium flux in cultured chick heart cells. Am J Physiol. 1987 Jul;253(1 Pt 2):H205–H209. doi: 10.1152/ajpheart.1987.253.1.H205. [DOI] [PubMed] [Google Scholar]
- Lemos J. R., Levitan I. B. Intracellular injection of guanyl nucleotides alters the serotonin-induced increase in potassium conductance in Aplysia neuron R15. J Gen Physiol. 1984 Feb;83(2):269–285. doi: 10.1085/jgp.83.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetje C. W., Tietje K. M., Christian J. L., Nathanson N. M. Differential tissue expression and developmental regulation of guanine nucleotide binding regulatory proteins and their messenger RNAs in rat heart. J Biol Chem. 1988 Sep 15;263(26):13357–13365. [PubMed] [Google Scholar]
- Majerus P. W., Connolly T. M., Bansal V. S., Inhorn R. C., Ross T. S., Lips D. L. Inositol phosphates: synthesis and degradation. J Biol Chem. 1988 Mar 5;263(7):3051–3054. [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Irvine R. F., Petersen O. H. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987 Dec 17;330(6149):653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- Nakajima Y., Nakajima S., Inoue M. Pertussis toxin-insensitive G protein mediates substance P-induced inhibition of potassium channels in brain neurons. Proc Natl Acad Sci U S A. 1988 May;85(10):3643–3647. doi: 10.1073/pnas.85.10.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Otani H., Otani H., Das D. K. Alpha 1-adrenoceptor-mediated phosphoinositide breakdown and inotropic response in rat left ventricular papillary muscles. Circ Res. 1988 Jan;62(1):8–17. doi: 10.1161/01.res.62.1.8. [DOI] [PubMed] [Google Scholar]
- Poggioli J., Sulpice J. C., Vassort G. Inositol phosphate production following alpha 1-adrenergic, muscarinic or electrical stimulation in isolated rat heart. FEBS Lett. 1986 Oct 6;206(2):292–298. doi: 10.1016/0014-5793(86)80999-1. [DOI] [PubMed] [Google Scholar]
- Ravens U., Wang X. L., Wettwer E. Alpha adrenoceptor stimulation reduces outward currents in rat ventricular myocytes. J Pharmacol Exp Ther. 1989 Jul;250(1):364–370. [PubMed] [Google Scholar]
- Satoh H., Hashimoto K. Effect of alpha 1-adrenoceptor stimulation with methoxamine and phenylephrine on spontaneously beating rabbit sino-atrial node cells. Naunyn Schmiedebergs Arch Pharmacol. 1988 Apr;337(4):415–422. doi: 10.1007/BF00169533. [DOI] [PubMed] [Google Scholar]
- Schmitz W., Scholz H., Scholz J., Steinfath M., Lohse M., Puurunen J., Schwabe U. Pertussis toxin does not inhibit the alpha 1-adrenoceptor-mediated effect on inositol phosphate production in the heart. Eur J Pharmacol. 1987 Feb 24;134(3):377–378. doi: 10.1016/0014-2999(87)90374-8. [DOI] [PubMed] [Google Scholar]
- Scholz J., Schaefer B., Schmitz W., Scholz H., Steinfath M., Lohse M., Schwabe U., Puurunen J. Alpha-1 adrenoceptor-mediated positive inotropic effect and inositol trisphosphate increase in mammalian heart. J Pharmacol Exp Ther. 1988 Apr;245(1):327–335. [PubMed] [Google Scholar]
- Shah A., Cohen I. S., Rosen M. R. Stimulation of cardiac alpha receptors increases Na/K pump current and decreases gK via a pertussis toxin-sensitive pathway. Biophys J. 1988 Aug;54(2):219–225. doi: 10.1016/S0006-3495(88)82950-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman M. S., Sekiguchi K., Nishizuka Y. Modulation of ion channel activity: a key function of the protein kinase C enzyme family. Pharmacol Rev. 1989 Jun;41(2):211–237. [PubMed] [Google Scholar]
- Sheridan D. J. Alpha adrenoceptors and arrhythmias. J Mol Cell Cardiol. 1986 Nov;18 (Suppl 5):59–68. doi: 10.1016/s0022-2828(86)80461-8. [DOI] [PubMed] [Google Scholar]
- Siegelbaum S. A., Tsien R. W. Calcium-activated transient outward current in calf cardiac Purkinje fibres. J Physiol. 1980 Feb;299:485–506. doi: 10.1113/jphysiol.1980.sp013138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]
- Spicher K., Hinsch K. D., Gausepohl H., Frank R., Rosenthal W., Schultz G. Immunochemical detection of the alpha-subunit of the G-protein, GZ, in membranes and cytosols of mammalian cells. Biochem Biophys Res Commun. 1988 Dec 30;157(3):883–890. doi: 10.1016/s0006-291x(88)80957-4. [DOI] [PubMed] [Google Scholar]
- Steinberg S. F., Kaplan L. M., Inouye T., Zhang J. F., Robinson R. B. Alpha-1 adrenergic stimulation of 1,4,5-inositol trisphosphate formation in ventricular myocytes. J Pharmacol Exp Ther. 1989 Sep;250(3):1141–1148. [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Thandroyen F. T., Flint N. S., Worthington M. G., Opie L. H. Arrhythmogenic action of alpha 1-adrenoceptor stimulation in normoxic rat ventricular myocardium: influence of nisoldipine, reduced extracellular Ca2+ and ryanodine. J Mol Cell Cardiol. 1987 Sep;19(9):841–851. doi: 10.1016/s0022-2828(87)80613-2. [DOI] [PubMed] [Google Scholar]
- Tohse N., Nakaya H., Hattori Y., Endou M., Kanno M. Inhibitory effect mediated by alpha 1-adrenoceptors on transient outward current in isolated rat ventricular cells. Pflugers Arch. 1990 Feb;415(5):575–581. doi: 10.1007/BF02583508. [DOI] [PubMed] [Google Scholar]
- Tseng G. N., Hoffman B. F. Two components of transient outward current in canine ventricular myocytes. Circ Res. 1989 Apr;64(4):633–647. doi: 10.1161/01.res.64.4.633. [DOI] [PubMed] [Google Scholar]
- Walsh K. B., Kass R. S. Regulation of a heart potassium channel by protein kinase A and C. Science. 1988 Oct 7;242(4875):67–69. doi: 10.1126/science.2845575. [DOI] [PubMed] [Google Scholar]
- Walsh M. P., Valentine K. A., Ngai P. K., Carruthers C. A., Hollenberg M. D. Ca2+-dependent hydrophobic-interaction chromatography. Isolation of a novel Ca2+-binding protein and protein kinase C from bovine brain. Biochem J. 1984 Nov 15;224(1):117–127. doi: 10.1042/bj2240117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa K., Kameyama M. Mechanism of receptor-mediated modulation of the delayed outward potassium current in guinea-pig ventricular myocytes. J Physiol. 1990 Feb;421:135–150. doi: 10.1113/jphysiol.1990.sp017937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S. H., Sunahara F. A., Sen A. K. Tumor-promoting phorbol esters inhibit cardiac functions and induce redistribution of protein kinase C in perfused beating rat heart. Circ Res. 1987 Sep;61(3):372–378. doi: 10.1161/01.res.61.3.372. [DOI] [PubMed] [Google Scholar]