Abstract
Background
Notch signaling pathways are conserved across species and traditionally have been implicated in cell fate determination during embryonic development. Notch signaling components are also expressed postdevelopmentally in the brains of adult mice and Drosophila. Recent studies suggest that Notch signaling may play a role in the physiological, rather than developmental, regulation of neurons. Here, we investigate a new non-developmental role for Caenorhabditis elegans lin-12 Notch signaling in neurons regulating the spontaneous reversal rate during locomotion.
Results
The spontaneous reversal rate of C. elegans during normal locomotion is constant. Both lin-12 gain and loss of function mutant animals had significantly increased reversal rates compared to wild type controls. These defects were caused by lin-12 activity, because the loss of function defect could be rescued by a wild type lin-12 transgene. Furthermore, overexpression of lin-12 recapitulated the gain-of-function defect. Increasing or decreasing lin-12 activity in the postdevelopmental adult animal was sufficient to rapidly and reversibly increase reversals, thereby excluding a developmental role for lin-12. Although lin-12 is expressed in the vulval and somatic gonad lineages, we find that these tissues play no role in regulating reversal rates. In contrast, altering lin-12 activity specifically in the nervous system was sufficient to increase reversals. These behavioral changes require components of the canonical lin-12 signaling cascade, including the ligand lag-2 and the transcriptional effector lag-1. Finally, the C. elegans AMPA/kainate glutamate receptor homolog glr-1 shows strong genetic interactions with lin-12, suggesting that glr-1 and/or other glutamate gated channels may be targets of lin-12 regulation.
Conclusion
Our results demonstrate a neuronal role for lin-12 Notch in C. elegans and suggest that lin-12 acutely regulates neuronal physiology to modulate animal behavior, without altering neuronal cell fate specification or neurite outgrowth. This is consistent with a role for Notch signaling in neurological disease with late onset symptoms.
Background
The conserved Notch signaling pathway has well established roles in cell fate determination during development. Transmembrane Notch receptors are activated by transmembrane DSL (Delta/Serrate/LAG-2) family ligands [1-6]. The intracellular (IC) domain of Notch is proteolytically released by presenilins and translocates to the nucleus [7-11], where it acts as a transcriptional activator abetted by CSL (CBF1/Su(H)/LAG-1) proteins [12-15]. In C. elegans, the LIN-12 Notch receptor is activated by LAG-2 and related DSL ligands [16-19], and proteolytically processed by the presenilins SEL-12 and HOP-1 [20-22]. The CSL protein LAG-1 interacts with LIN-12IC to activate transcription of target genes [23].
Notch receptors and ligands are expressed in adult vertebrate neurons [24,25]; recent studies in Drosophila and mice suggest that altering Notch signaling results in defective neuronal function [Costa, 2003 #50; Ge, 2004 #46; Presente, 2004 #31; Saura, 2004 #32; Wang, 2004 #51;Yoon, 2005 #69]. The importance of these findings is underscored by the fact that several genetic diseases associated with neuronal defects and/or late onset symptoms map to mutations in Notch pathway genes [32-36]. However, it remains unclear from these studies whether Notch signaling is acutely affecting neuronal physiology or if it is causing permanent changes in cell fate and/or structure due to developmental defects or aberrant growth.
Here, we report a new role for lin-12 signaling in the adult C. elegans nervous system, using behavior as an indicator of neuronal activity. C. elegans predominantly move forward, but they spontaneously initiate backward locomotion. Genetically modulating lin-12 activity alters the rate of initiation of spontaneous reversals. Using inducible RNAi and a conditional, gain-of-function allele of lin-12, we show that this behavioral change can occur within a few hours of altering lin-12 activity in post-developmental adults. We also show that these inducible behavioral changes are rapidly reversible, strongly suggesting that lin-12 mediated behavioral changes are unlikely due to changes in cell fate. Altering lin-12 activity in a subset of interneurons is sufficient to alter behavior. glr-1, an AMPA/kainate receptor homolog gene expressed in these interneurons, genetically interacts with lin-12. Our results demonstrate a novel, post-developmental role for lin-12 signaling that is clearly distinct from its role in cell fate determination.
Results
Altering lin-12 activity increases spontaneous reversals during locomotion
To assess a role for lin-12 Notch signaling in behavior, we first examined spontaneous reversal rates during locomotion in lin-12 mutant animals (Fig. 1). Normal animals moving forward consistently initiate backward locomotion approximately 10 times per 3 minutes. Reversal rates were significantly increased in lin-12(n941) loss of function (lf) animals, which completely lack lin-12 gene function. The behavioral defect of lin-12(lf) animals was rescued by a previously described transgene containing a lin-12 cDNA driven by the lin-12 promoter [37]. Furthermore, lin-12(lf) behavioral defects could be recapitulated by RNAi (see below). These results indicated that loss of function in lin-12 caused increased reversal rates.
Figure 1.
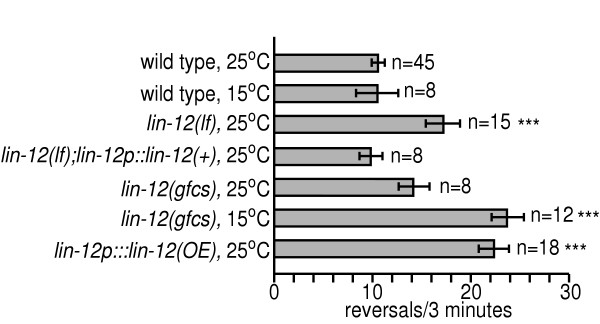
Altering lin-12 activity increases spontaneous reversal rates. Animals of the genotypes indicated were tested for mean number of reversals per 3 minutes on NGM agar plates (see Methods for details). lin-12(lf) is lin-12(n941), a complete loss of function allele. lin-12(gfcs) is lin-12(n137n460), a cold sensitive, gain of function allele. lin-12p::lin-12(+) and lin-12p::lin-12(OE) are transgenic animals that have been injected (at different concentrations, see Methods) with plin-12::gfp, a plasmid that expresses a functional lin-12 cDNA fused to gfp under the control of the lin-12 promoter 37. *** p<0.0001 vs. wild type.
The effect of increased lin-12 activity on reversal rates was then assessed using lin-12(n137n460) (Fig. 1), a gain of function, cold sensitive (gfcs) allele [38,39]. Reversals were not significantly increased in lin-12(gfcs) animals raised at the permissive temperature (25°C), but were dramatically increased in animals raised at the restrictive temperature (15°C). Cultivation temperature had no effect on reversal rate in wild type animals. The increased reversal rate of lin-12(gfcs) animals was due to increased lin-12 activity, as transgenic animals that overexpress LIN-12 (lin-12p::lin-12(OE)) also had increased reversals. Thus, both gain and loss of function in lin-12 causes increased reversal rates.
An allelic series of lin-12 mutants reveals complex regulation of behavior
To further characterize the relationship between lin-12 activity and reversal rates, we assessed behavior across the lin-12 allelic series ordered based on the severity of previously determined vulval defects (Table 1). lin-12 alleles can be grouped into 4 classes: strong loss of function, weak gain of function, moderate gain of function, and strong gain of function. lin-12(n941) null animals are sterile and display protruding vulva that usually burst in adult animals; these animals had increased reversals. The vulval and reversal phenotypes of lin-12(n941)/+ animals were normal, indicating that the lin-12(n941) mutation is recessive. lin-12(n302), lin-12(n379), and lin-12(n676) are weak gain of function alleles [39]; these animals were fertile, vulvaless, and had slightly decreased reversal rates. lin-12(n137n460) acts as a moderate gain of function allele; these animals are cold-sensitive, display multiple pseudovulvae at the restrictive temperature, and had increased reversals. Finally, lin-12(n137) and lin-12(n427) are strong gain of function alleles that display multiple pseudovulvae and had strongly decreased reversals. We note that lin-12(n137)/lin-12(n941) hemizygote animals have multiple pseudovulvae and have high reversal rates consistent with lin-12(n137n460) phenotypes. Since lin-12p::lin-12(OE) (Fig. 1) and other transgenic animals overexpressing lin-12 (see Fig. 5B) recapitulate the moderate lin-12 gain of function allele, we expected that injection of the lin-12p::lin-12 construct at a higher concentration would lead to transgenic animals that recapitulate the strong lin-12 gain of function alleles. However, we were unable to generate viable transgenic lines using higher concentrations of lin-12p::lin-12 (data not shown; see Methods for details). We conclude that altering lin-12 activity results in complex changes in the pattern of reversal behavior; the implications of this allelic series are discussed below.
Table 1.
Allelic series of lin-12 mutants. Vulval phenotype abbreviations are as follows: Pvl, protruding vulva; WT, wild type; Vul, vulvaless; and Muv, multiple pseudovulvae. All animals were tested at 25°C except animals carrying the cold-sensitive lin-12(n137n460) allele were raised at the non-permissive temperature 15°C. Control animals raised at 15 or 25°C had wild type reversal rates (see Figs. 1 and 2).
| strain | vulval phenotype | reversals/3 min. ± S.E.M. | n |
| lin-12(n941) (null) | Pvl | 17.3 ± 1.7 | 15 |
| lin-12(n941)/+ | WT | 10.8 ± 1.5 | 15 |
| lin-12(+) | WT | 10.5 ± 0.7 | 45 |
| lin-12(n302)/lin-12(n941) | Vul | 9.3 ± 1.3 | 15 |
| lin-12(n302) | Vul | 8.8 ± 1.1 | 13 |
| lin-12(n379) | Vul | 7.7 ± 0.5 | 15 |
| lin-12(n676) | Vul | 6.0 ± 0.3 | 5 |
| lin-12(n137n460)/lin-12(n941) | WT | 22.9 ± 1.5 | 12 |
| lin-12(n137n460)/+ | WT | 20.1 ± 1.8 | 9 |
| lin-12(n137n460) | Muv | 23.7 ± 1.8 | 12 |
| lin-12(n137)/lin-12(n941) | Muv | 17.6 ± 1.2 | 5 |
| lin-12(n137)/+ | Muv | 6.0 ± 1.6 | 7 |
| lin-12(n427) | Muv | 3.9 ± 0.2 | 15 |
| lin-12(n137) | Muv | 3.6 ± 1.1 | 10 |
Figure 5.
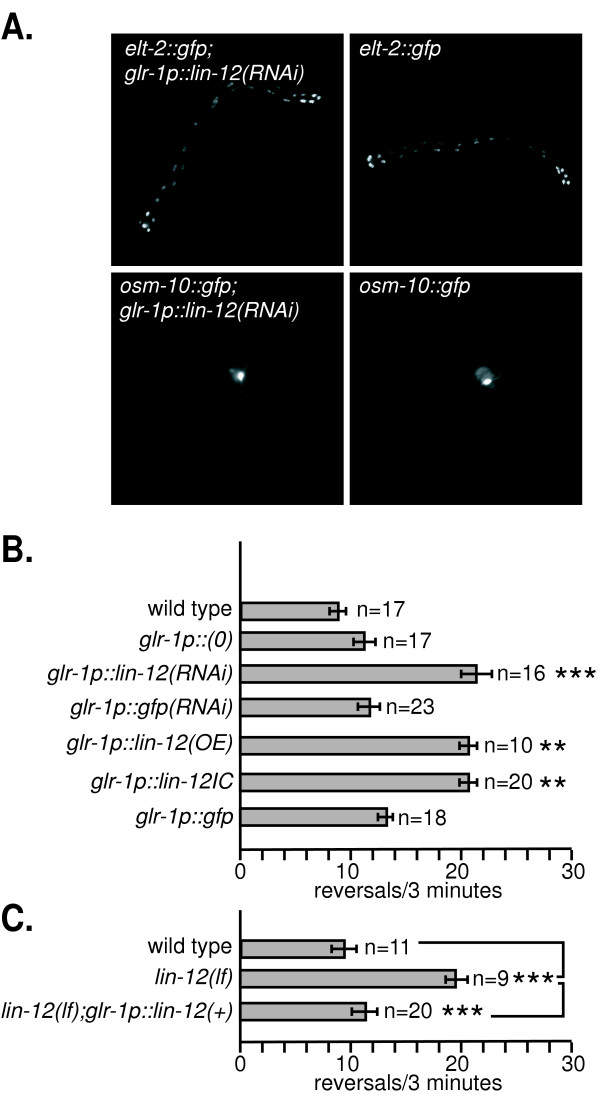
lin-12 likely acts in a subset of glr-1 expressing neurons to regulate reversals. A. RNAi driven by the glr-1 promoter expressing dsRNA does not spread to nearby tissues. The glr-1 promoter drives expression in the command interneurons AVA, AVB, AVD, AVE, and PVC, as well as AIB, AVG, AVJ, DVC, PVQ, RIG, RIM, RIS, RMD, RMEL/R, SMD, and URY, all of which (except DVC, PVQ, and PVC) are located in the head 48, 49. The lin-12 cDNA fragment used to express lin-12 dsRNA also contains GFP sequences in cis. The dsRNA expressing construct was introduced into strains expressing osm-10p::gfp in ASH neurons or elt-2p::gfp in intestinal cells, and compared to control strains for GFP expression levels. Adult animals from multiple transgenic lines were scored; representative images are shown. B. Effect of altering lin-12 activity in glr-1 expressing neurons. glr-1p indicates the glr-1 promoter used to drive expression of various transgenes, and glr-1p::(0) indicates the promoter only control. lin-12(RNAi) and gfp(RNAi) indicate lin-12 and gfp dsRNA, respectively. lin-12(OE) indicates transgenic animals injected with a rescuing lin-12 cDNA construct at a high concentration (see Methods). lin-12IC indicates a truncated, activated lin-12 allele that lacks the extracellular domain.**p<0.01 and ***p<0.001 vs. wild type, respectively. C. Expressing lin-12 cDNA in glr-1 expressing neurons rescues the lin-12(lf) reversal defect. *** p<0.001.
Altering lin-12 activity in adult animals is sufficient to increase reversal rates
lin-12 Notch plays well established roles in development. Therefore, we asked whether increased reversals in lin-12 mutant animals depended on lin-12 activity during development or in adults. lin-12 loss of function was induced by expressing an inverted repeat of a lin-12 cDNA fragment under the control of a heat shock promoter to knock down lin-12 activity by RNAi (hsp::lin-12(RNAi)) in otherwise normal adult animals (Figure 2A). Uninduced hsp::lin-12(RNAi) adult animals (filled triangles) raised at 25°C had normal reversal rates, while heat shock induction resulted in dramatically increased reversal rates within 4 hours. Reversals returned to near basal levels after overnight recovery (approx. 14 hours). Heat shock had no effect on wild type control animals (filled circles). As a control, we generated transgenic animals containing an inverted repeat of a cDNA fragment from the Gprotein coupled receptor kinase-2 grk-2) gene under control of the heat shock promoter (hsp::grk-2(RNAi)). grk-2 loss of function causes sensory defects [40], but had no effect on reversal rates (data not shown). Heat shock induction of hsp::grk-2(RNAi) did not alter reversal rates (open squares), indicating that neither the presence of the heat shock vector nor overexpression of an unrelated dsRNA influenced reversal rates. We conclude that loss of function of lin-12 in adult animals is sufficient to alter behavior.
Figure 2.
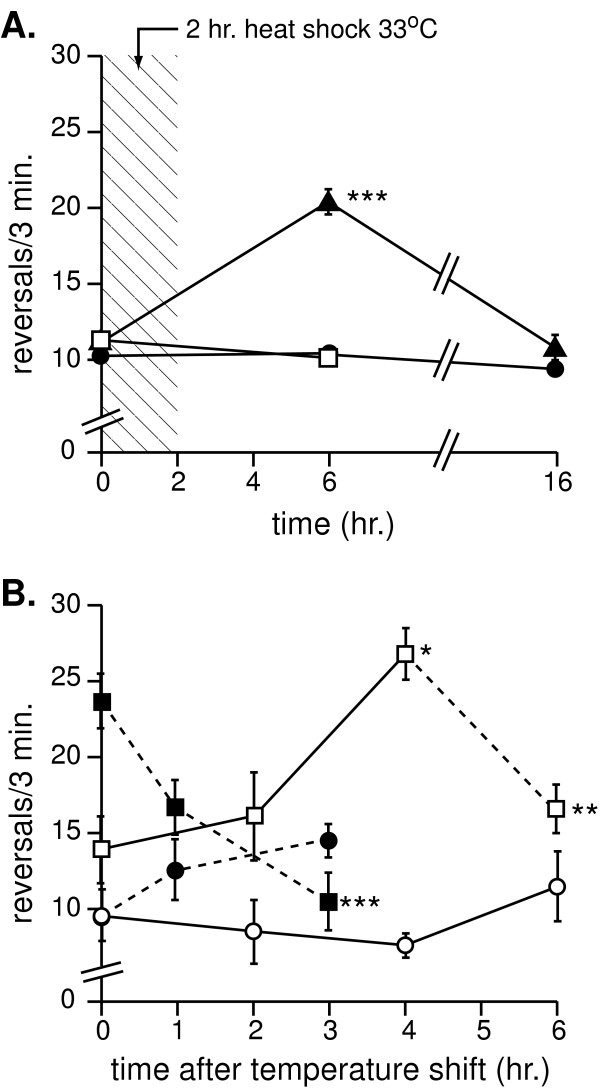
Changing lin-12 activity in adults alters spontaneous reversal rates. Changing lin-12 activity in adults alters spontaneous reversal rates. A. Reversal rate changes in hsp::lin-12(RNAi) animals. Filled triangles indicate hsp::lin-12(RNAi) animals; filled circles indicate wild type control animals; and open squares indicate hsp::grk-2(RNAi) control animals. Animals were heat shocked at 33°C for 2 hours, allowed to recover at 25°C then tested 4 hours later. After recovery at 25°C overnight (approx. 16 hours), animals were tested again. *** p<0.001 vs. wild type. B. Reversal rate changes in lin-12(gfcs) animals. lin-12(gfcs) animals raised at the permissive temperature (25°C) are indicated by open squares, and those raised at the restrictive temperature (15°C) by filled squares. Open and filled circles indicate wild type animals raised at 25 and 15°C, respectively. Temperature shifts from 25 to 15°C are indicated by solid lines, and those from 15 to 25°C are indicated by dotted lines. * p<0.01 vs. t = 0 hrs.; ** p<0.01 vs. t = 4 hrs.; *** p<0.001 vs. t = 0 hrs.
We examined lin-12(gfcs) animals in temperature shift experiments (Figure 2B). lin-12(gfcs) adults raised at the restrictive temperature 15°C (filled square, t = 0) initially had increased reversal rates. When these animals were moved to the permissive temperature of 25°C (dotted line with filled squares), reversal rates gradually decreased, and after 3 hours reversals decreased to wild type levels. In reciprocal experiments, lin-12(gfcs) adults raised at 25°C (open square, t = 0) initially had almost normal reversal rates. When they were moved to 15°C (solid line with open squares), reversal rates gradually increased until they reached levels comparable to those of lin-12(gfcs) animals raised at 15°C. When these animals were moved back to 25°C (dotted line with open square), reversal rates decreased to original levels within 2 hours. Temperature shifts and cultivation temperature had only minimal effects on control wild type animals (open and filled circles). Taken together, these data demonstrate that altering lin-12 Notch activity for a few hours in post-developmental adult animals is sufficient to change behavior and suggests that lin-12 activity is regulating a physiological, not a developmental, process.
lin-12 is not required in the vulval lineage to regulate reversals
Where does lin-12 function to regulate reversal rates? LIN-12 is expressed in the somatic gonad and vulval lineages, based on previous studies using a functional lin-12::gfp transgene [37]. To test if lin-12 activity in these tissues regulated reversal rates, we eliminated the somatic gonad and the vulva by killing the progenitor cells of these lineages using a laser and then determining the reversal rates of the operated animals. Vulval development depends on cell-cell signaling from the anchor cell to the vulval precursor cells [41]. The anchor cell (and somatic gonad) is derived from one of two equipotent cells called Z1 and Z4 in L1 larvae; thus, killing Z1 and Z4 results in animals that lack gonads and vulvae.
Wild type Z1-Z4-killed adult animals had normal reversal rates, indicating that these tissues play no role in regulating the reversal rate in wild type animals (Fig. 3). We tested the role of the vulval and gonadal lineages in regulating reversal rates in lin-12(RNAi) (see Methods for details) and lin-12p::lin-12(OE) animals. Z1-Z4-killed lin-12(RNAi) and lin-12p::lin-12(OE) animals maintained high reversal rates comparable to mock treated animals. These data indicate that lin-12 must function outside of the somatic gonad and vulva to regulate reversal rates.
Figure 3.
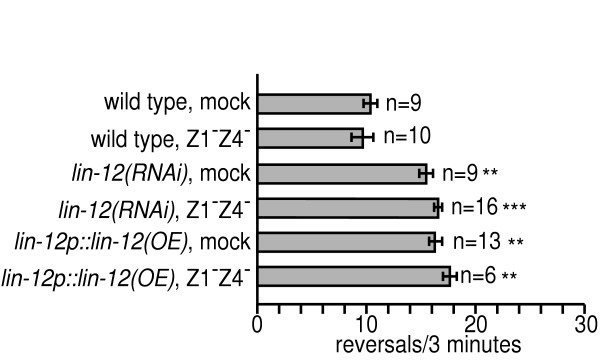
lin-12 is not required in the gonadal or vulval lineages to regulate reversal rates. The somatic gonads and vulval tissues were eliminated by killing the progenitor Z1 and Z4 cells in L1 larvae with a laser microbeam (see text for details). Successful ablation of Z1 and Z4 was scored visually as follows: animals in which both Z1 and Z4 were ablated lacked gonads and vulvae; animals in which only one of the two cells were killed results in a protruding vulva; and animals in which neither cell was killed resulted in fertile animals with normal vulvae. Only animals in which both Z1 and Z4 were killed were scored for behavior. The reversal rates of mock treated lin-12(RNAi) and lin-12p::lin-12(OE) animals were slightly lower compared to untreated animals, but they were still significantly higher than that of wild type. ** p<0.01, *** p<0.001. Statistical comparisons are to wild type.
We considered the possibility that the gross morphological vulval defects in lin-12 mutant animals, but not lin-12 signaling per se, might account for changes in reversal rates. lin-12(lf) animals have a large protruding vulva, while lin-12(gfcs) animals raised at the restrictive temperature 15°C have multiple pseudovulvae. We measured basal locomotion rates in lin-12(lf) and lin-12(gfcs) animals and found that they had slight but significant decreases in basal movement rate (Table 2). However, lin-12(RNAi) and lin-12p::lin-12(OE) animals, which phenocopy the reversal phenotypes but not the vulval phenotypes of the mutant animals, had normal basal movement rates. These data indicate that morphological defects of the vulva in lin-12 mutant animals cannot account for the altered behavior. Taken together, we conclude that lin-12 expressed in the vulva and somatic gonad does not contribute to the regulation of reversal rates during locomotion.
Table 2.
Basal locomotion rates of animals with altered lin-12 activity. Animals were tested under identical conditions as reversal assays in 10 second bins. A single body bend was scored as a complete dorsal to ventral oscillation. Only forward moving animals were scored; if an animal reversed direction during the assay, that data point was discarded.
| strain | body bends/10 sec. ± S.E.M. | n | p value |
| wild type | 5.2 ± 0.2 | 26 | |
| lin-12(lf) | 3.9 ± 0.2 | 43 | <10-5 vs. wild type |
| lin-12(gfcs) 25°C | 5.1 ± 0.2 | 20 | |
| lin-12(gfcs) 15°C | 3.8 ± 0.2 | 24 | <10-4 vs. wild type |
| lin-12(RNAi) | 5.3 ± 0.3 | 24 | |
| lin-12p::lin-12(OE) | 5.2 ± 0.2 | 24 |
lin-12 acts in a subset of glr-1 expressing neurons to regulate reversals
lin-12 might act in or upon neurons to control C. elegans behavior. Previous studies have implicated the command interneurons AVA, AVB, AVD, AVE and PVC in regulating normal forward and backward locomotion [42]. The intrinsic activation of these interneurons affects reversal rates [43]. Other neurons presynaptic to the command interneurons, such as ASH [43] and AIY [44-46], can affect reversal rates as well. We did not detect any overt cell fate changes or morphological defects in any of these or other neurons in lin-12(lf), lin-12(gfcs) or lin-12(n137gf) mutant animals (data not shown), consistent with a previous report [47] and supporting our conclusion that lin-12 mediated behavioral changes were not due to developmental defects. LIN-12 expression was not detected in any of these neurons either by immunohistochemical analysis or by GFP fluorescence (data not shown). However, occasional LIN-12::GFP expression was observed in the RIG neurons of young larvae (Fig. 4). These observations and the preceding temperature shift experiments suggested that LIN-12 may be expressed in adult neurons at levels too low to detect. Increasing LIN-12::GFP levels further caused lethality (data not shown); therefore, a functional approach was taken to determine whether lin-12 acts in the nervous system.
Figure 4.
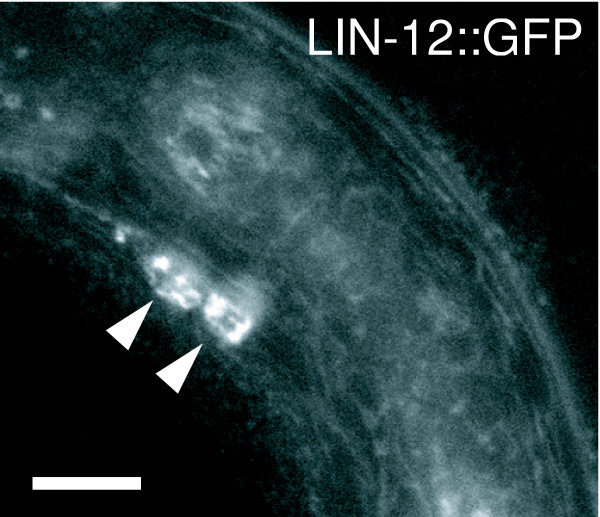
LIN-12::GFP is expressed in RIG neurons. RIG neurons are indicated by arrowheads. Expression of LIN-12::GFP was detected in approximately 25% of L1/L2 animals. The identity of RIG neurons was confirmed by using a nmr-1::dsRed reporter gene that labels the AVG neuron, which is located in between the RIG neurons (data not shown). Scale bar = 10 μm.
lin-12 activity was knocked down by RNAi in a subset of neurons by expressing lin-12 dsRNA under the control of the glr-1 promoter, which drives expression in the aforementioned command interneurons and twelve other classes of neurons including RIG [48,49] (glr-1p:::lin-12(RNAi)). Because RNAi effects can spread systemically [50], we first validated the cellular specificity of this approach. The lin-12 cDNA fragment used to generate the glr-1::lin-12(RNAi) constructs was derived from a lin-12::gfp fusion; thus, the dsRNA expressed in these transgenic animals contains both lin-12 and gfp sequences. When glr-1p::lin-12(RNAi) constructs were injected into strains that express GFP in the intestine or in ASH sensory neurons (which are physically close to glr-1 expressing neurons), no decreases in GFP fluorescence were observed (Figure 5A). Furthermore, these transgenic animals had grossly normal fertility and vulval morphology (data not shown). Thus, RNAi effects did not appear to spread from glr-1 expressing neurons to nearby neurons, the intestine, or to the vulva. Also, we found that transgenic animals injected with the glr-1 promoter fragment alone (glr-1p::(0)) or constructs expressing gfp only dsRNA under control of the glr-1 promoter had no effect on reversal rates (Fig. 5B). When we examined the behavior of glr-1p::lin-12(RNAi) animals, we found that reversal rates increased significantly. Thus, knocking down lin-12 activity in glr-1 expressing neurons was sufficient to recapitulate lin-12(lf) behavioral defects.
The requirement for lin-12 activity in the nervous system was also tested by driving lin-12 cDNA expression using the glr-1 promoter (Fig. 5B). Increasing lin-12 activity by overexpressing either a full length lin-12 cDNA or a truncated, activated form of lin-12 under the control of the glr-1 promoter (glr-1p::lin-12(OE) and glr-1p::lin-12IC, respectively) also increased reversal rates. Expression of GFP using the glr-1 promoter (glr-1p::gfp) as a control had no effect (Fig. 5B). Finally and most significantly, expression of the lin-12 cDNA under the control of the glr-1 promoter (glr-1p::lin-12(+)) rescued the behavioral defects of lin-12(lf) animals, restoring reversal rates to wild type levels (Fig. 5C). These results demonstrate that lin-12 activity in glr-1 expressing neurons is sufficient to regulate reversal rates.
Increased lin-12 activity affects reversal rates via RIG neurons
The RIG neurons, in which we observed weak LIN-12::GFP expression, express glr-1. Interestingly, the RIG neurons are presynaptic to command interneurons. The role of RIG neurons in spontaneous reversal rates was tested by laser ablation. Laser ablation of the RIG neurons did not dramatically affect reversal rates in wild type or lin-12(RNAi) animals. However, eliminating the RIG neurons of lin-12p::lin-12(OE) animals ameliorated reversal rate increases (Fig. 6), Thus, increased lin-12 function in the RIG neurons is likely responsible for increased reversal rates in lin-12p::lin-12(OE) animals.
Figure 6.
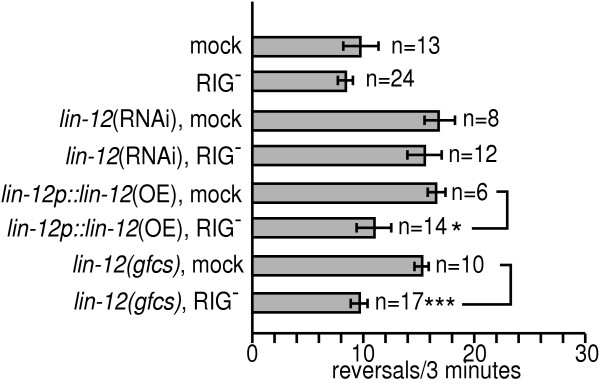
The RIG neurons are a likely site of lin-12 gain of function action. To facilitate RIG neuron identification, these experiments were carried out in a flp-18p::gfp background (see Methods). lin-12(gfcs) animals were raised at 25°C, then were shifted as young adults to 15°C 4 hours prior to behavioral assays. * p<0.05, *** p<0.0001.
Despite the fact that lin-12 overexpression recapitulated lin-12(gfcs) behavioral defects, we considered the possibility that the lin-12p::lin-12(OE) transgene might act ectopically or during development to alter reversal rates. Increasing lin-12 activity in adult lin-12(gfcs) animals in temperature shift experiments was sufficient to increase reversal rates (Fig. 2B). Therefore, we carried out RIG laser ablations in lin-12(gfcs) animals, using the same temperature shift paradigm described above (Fig. 2B). RIG killed, temperature shifted lin-12(gfcs) animals had normal reversal rates, while mock treated lin-12(gfcs) control animals retained high reversal rates. We conclude that increased lin-12 activity in the RIG neurons of adult animals increases reversal rates.
Genes that interact with lin-12 to regulate reversals
To determine if the canonical lin-12 signaling pathway regulates spontaneous reversal rates, we examined the reversal rates of animals that are defective in genes of the lin-12 pathway, specifically lag-1 (which encodes a transcriptional cofactor that is the major effector for lin-12 signaling) and lag-2 (which codes for a Notch ligand) (Fig. 7A). The reversal rates of partial loss-of-function lag-1 and lag-2 mutant animals were relatively normal. However, partial loss of lag-1 function suppressed increased reversal rates in glr-1p::lin-12IC animals that had constitutively activated lin-12 signaling, consistent with lag-1 functioning downstream of lin-12 regulating reversal rates. Also, a semidominant allele of lag-2 that suppresses the lin-12 gain-of-function multivulval phenotype caused increased reversal rates. Although strong loss of function alleles could not be tested due to embryonic lethality, our results suggest that lin-12, lag-2, and lag-1 likely act together in the nervous system to regulate reversals.
Figure 7.
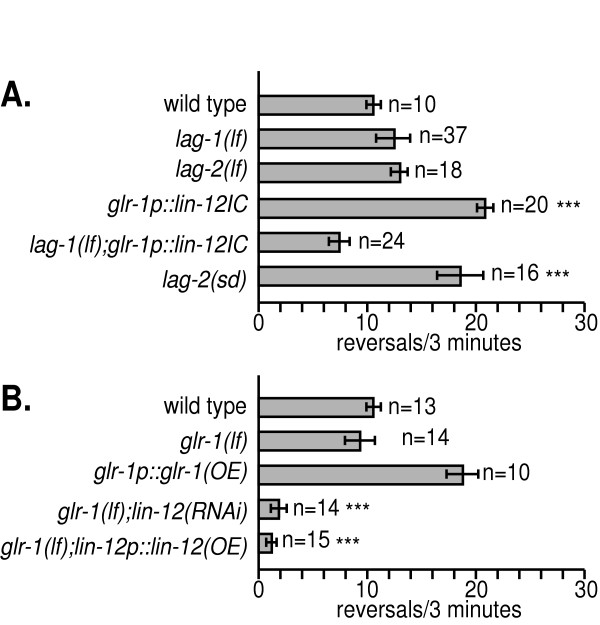
Genes that interact genetically with lin-12 to regulate reversals. A. lag-1 and lag-2 likely function with lin-12 to regulate reversals. Complete loss of function in lag-1 and lag-2 cause lethality; therefore, partial loss of function alleles were used (om13 and q420, respectively). lag-2(sd) is lag-2(sa37), a semidominant suppressor of lin-12 gain of function. B. The AMPA/kainate glutamate receptor glr-1 genetically interacts with lin-12. glr-1(lf) is glr-1(n2461), a genetic null allele. glr-1p::glr-1(OE) is nuIs25 (see Methods for details). *** p<0.001 vs. wild type.
Finally, given the previously described role of the glr-1 AMPA receptor in the command interneurons [43,48,49,51], we examined more closely the role of glr-1 in spontaneous reversals and lin-12 mediated behavioral changes (Fig. 7B). Consistent with a previous report, complete loss of glr-1 function (glr-1(lf)) alone had no effect on reversal rates [43]. However, we found that overexpression of glr-1 (glr-1p::glr-1(OE)) increased spontaneous reversal rates. We note that different constructs are used here than previous studies [43] (see Methods for details) and that reversal rates can be dependent on assay conditions. Both glr-1(lf);lin-12p::lin-12(OE) and glr-1(lf);glr-1p::lin-12(RNAi) animals had dramatically decreased reversal rates (below wild type levels). Yet, there were no dramatic changes in the expression of a glr-1p::gfp transcriptional reporter in lin-12 (gfcs) or (lf) animals (data not shown). Our results suggest that glr-1 AMPA receptor activity, but not levels, are modulated by lin-12 signaling to regulate reversals.
Discussion
In this study we demonstrate a non-developmental role for lin-12 Notch in the adult nervous system regulating C. elegans behavior. lin-12 mediated behavioral changes can be rapidly induced within a few hours in adult animals and are reversible. Knocking down lin-12 activity by RNAi or by activating lin-12 in glr-1 expressing neurons is sufficient to reproduce the behavioral defects of lin-12 mutant animals. The rapidity with which behavioral changes can be induced in post-developmental adult animals argues that neither lin-12 mediated cell fate changes nor de novo neurite outgrowth are the likely mechanisms for altering behavior. Rather, our results are consistent with a novel role for lin-12 signaling acutely regulating neuronal physiology via transcriptional activation, clearly distinct from previously described roles in cell fate specification.
Signaling pathways used to pattern the developing nervous system can also play important roles in the adult nervous system. For example, ephrins and Eph receptors function both in nervous system patterning during development and in synaptic plasticity in the adult nervous system (reviewed in [52]). Recent studies suggest that Notch signaling may also play a role in adult neurons. In Drosophila, adult animals harboring temperature sensitive, loss-of-function Notch alleles are defective for long term memory formation after one to two days at the restrictive temperature [27,28]. In mice, Notch1 and CBF1 heterozygous adult animals have specific defects in spatial learning and memory [26]. Similarly, adult mice in which Notch protein levels have been partially depleted by antisense RNA are defective in long term potentiation (LTP) [30]. Conditional knockout of both presenilin genes in the postnatal forebrain in mice results in defects in long-term contextual memory and LTP, when assayed in two month old animals [29]. Our heat shock and temperature shift experiments indicate that behavioral defects appear within hours, suggesting that Notch mediated alterations in neuronal function can occur on a much shorter timescale than days [27,28] or months [29] as previously reported.
The lin-12 allelic series for reversal rates is complex. In particular, lin-12(n137n460) gain-of-function hemizygotes, heterozygotes, and homozygotes all have high reversal rates, while stronger gain-of-function alleles (n427 and n137) have decreased reversals, raising the possibility that lin-12(n137n460) could be a neomorphic allele. Several lines of evidence argue against this hypothesis. First, based on vulval phenotypes, there is no evidence of any neomorphic activity. lin-12(n137n460), which is a recessive hypermorphic allele, is a revertant of lin-12(n137), a dominant hypermorphic allele; the n460 mutation confers a temperature sensitive, partial loss of function onto n137 [38,39]. Both the n137 and the n137n460 alleles cause multiple pseudovulvae, indicating that lin-12(n137n460) is simply a weaker hypermorph than lin-12(n137). Second, modestly increasing lin-12 activity through several other independent means also caused increased reversals. These include moderate overexpression of lin-12 (lin-12p::lin-12(OE)) at levels that do not affect fertility and vulval development, and placing the strong hypermorphic allele lin-12(n137) over the null allele (i.e., lin-12(n137/lin-12(n941) animals).
We favor the hypothesis that the unconventional lin-12 allelic series for reversal rates reflects the underlying complexity of Notch signaling and the neuronal signaling pathways that regulate behavior. lin-12 acts at multiple places during vulval cell fate specification, specifically the AC/VU decision and VPC lateral inhibition, resulting in a complex allelic series for vulval phenotypes. Similarly, lin-12 gain and loss of function may have different cellular foci for action in the nervous system, making it difficult to predict the behavioral output based on simple genetic rules. This is partially supported by the RIG ablation studies, wherein killing RIG neurons in lin-12 gain of function animals ameliorated reversal increases, but had no effect in lin-12 loss of function animals. Alternatively, lin-12 may act coordinately with other genes to regulate reversals. Further genetic studies may lead to a clearer picture. Consistent with this hypothesis, we have found that glp-1, another C. elegans Notch homolog, modulates reversal rates (in preparation). Our data suggest that lin-12 regulates reversal rates in a complex fashion.
The behavioral changes observed in lin-12 animals are dramatically dependent on GLR-1 AMPA receptor function. Taken together with our finding that lin-12 acts in glr-1 expressing neurons to regulate reversals, it suggests a possible relationship between AMPA receptors and Notch receptors in post-developmental synaptic plasticity. This is consistent with a recent study that demonstrated that altering Notch signaling caused defects in LTP in mice [29,30]. Based on our genetic analysis, glr-1 may be a target of lin-12 signaling or lin-12 signaling may act in parallel with glr-1. For example, lin-12 signaling may modulate other glutamate-gated currents to influence membrane excitability. Consistent with this hypothesis, loss of function in avr-15, one of several semi-redundant C. elegans genes encoding conserved glutamate-gated chloride channel subunits [53], results in increased reversals. avr-15 is expressed in the AVA command interneurons (data not shown) and chloride currents have been observed in these interneurons [51], making AVR-15 a candidate target for regulation by LIN-12 signaling. Similarly, loss of function of nmr-1, which encodes an NMDA glutamate receptor subunit, results in decreased spontaneous reversals [54], suggesting that nmr-1 activity could be influenced by lin-12. Additional behavioral and genetic analysis will be required to further delineate the targets of lin-12 signaling in adult neurons.
It should be noted that defects in Notch signaling can result in pleiotropic developmental disorders and nervous system dysfunction. CADASIL syndrome is associated with mutations in human Notch3 and is characterized by seizures, late onset neurodegeneration and vascular defects [32]. Mutations in Jagged1 (a DSL protein family member) are implicated in Alagille syndrome, which is characterized by defects in liver, cardiac, and skeletal tissues, and less frequently, neurovascular defects and mental retardation [34,35]. Familial, early onset Alzheimer's disease is often caused by mutations in presenilin 1 or presenilin 2 [33,36]. The developmental defects associated with CADASIL and Alagille syndromes make it difficult to establish a role for Notch signaling in neurons, but it may play a role in the defects observed in some of the late-onset symptoms. Given the emerging role for Notch signaling in the adult nervous system, a role for defective Notch signaling in these and other neurological disorders warrants further investigation.
Conclusion
We have demonstrated a novel role for lin-12 Notch in Caenorhabditis elegans in the adult nervous system. Changing lin-12 activity postdevelopmentally in adult animals alters the spontaneous reversal rates during locomotion. lin-12 activity in the vulva and somatic gonad, where lin-12 expression was previously reported, is not required to control reversal rates. In contrast, altering lin-12 activity in specific neurons is sufficient to alter behavior. lin-12 likely acts through the canonical Notch signaling pathway that includes the ligand lag-2 and the downstream effector lag-1. The neuronal function of lin-12 is clearly independent from cell fate specification during development.
Methods
Behavioral assays
Spontaneous reversals are modulated by sensory input, environmental conditions and feeding status [43,55]. To control these variables, animals were cultured on NGM agar plates containing OP50 E. coli at 25°C, except in temperature shift experiments, in which animals were cultured at 15°C and moved to room temperature 30 minutes prior to assays. Young adults (containing at least 4 eggs) were moved from the bacterial lawn of an uncrowded plate to an NGM plate lacking food, allowed to crawl around briefly to remove bacterial residue, then quickly transferred to another NGM plate lacking food for assays. Spontaneous initiation of backward locomotion was recorded over three minutes during the next 1.5 to 10.5 minutes with the lid on. Up to three animals per assay plate per trial were used; no effect on reversal rates was observed for up to three animals per plate. Freshly poured NGM agar plates were dried in a laminar flow hood for approx. 2 hours, sealed with Parafilm, then stored at 4°C at least overnight. Plates were allowed to warm up to room temperature for at least 30 minutes prior to use. Several assay plates were tested until a plate that resulted in an average of 10 reversals in 3 minutes was observed for N2 control animals; this plate was then used for all subsequent assays on that day. Each initiation of backward locomotion was scored as one reversal; omega turns without reversals were not scored. A subset of animals was scored blind as to genotype and/or transgene to confirm results. lin-12 mutants have defective vulvae, which are visually obvious; therefore, lin-12 mutant animals were scored independently by two observers. Statistical analysis was performed using the two tailed Student's t test.
Laser ablations
Laser ablations were performed as previously described [56] using a Micropoint ablation system (Photonic Instruments, St. Charles, IL). RIG ablations were undertaken in nyIs60 animals expressing flp-18p::GFP [57]. These animals are uncoordinated but have normal spontaneous reversal rates. lin-12(lf) mutant animals were not subjected to laser microsurgery because they rarely survived the procedure. lin-12(gfcs) mutant animals did not survive laser microsurgery as L1 larvae, but most survived when operated on as L2-L3 larvae. After laser microsurgery, lin-12(gfcs) animals were allowed to recover at the permissive temperature 25°C for 1–2 days, then were shifted to the restrictive temperature 15°C for 4 hours prior to behavioral assays. The flp-18p::gfp transgene did not affect the temperature dependence of lin-12(gfcs) phenotypes (data not shown). After behavioral assays were completed, successful ablation of the RIG neurons was scored by the lack of GFP labeled neuronal cell bodies in the retrovesicular ganglion. In nearly all laser ablation experiments, mock treated animals with altered lin-12 activity had slightly lower reversal rates than untreated animals. However, they still had significantly higher reversal rates than wild type mock treated animals.
Molecular biology
Plasmids used for transgenes are as follows: lin-12p::lin-12(OE), plin-12::gfp; hsp::lin-12(RNAi) and lin-12(RNAi), pHA#394; hsp::grk-2(RNAi), pHA#327; glr-1p::(0), pHA#421; glr-1p::glr-1(OE), pCR#3; glr-1p::gfp(RNAi), pKP#6 and pHA#424; glr-1p::lin-12(OE) and glr-1p::lin-12(+), pHA#444; glr-1p::lin-12IC, pHA#382; glr-1p::lin-12(RNAi), pHA#380 and pHA#381. Plasmid details are available upon request.
Genetics and strains
Strains used in this study: N2 Bristol wild type isolate, lin-12(n137n460gfcs), lin-12(n941lf)/unc-32, lin-12(n941lf)/eT1, lin-12(n941lf)/qC1, lin-12(n137)/unc-32, lin-12(n302), lin-12(n379), lin-12(n427), lin-12(n676), lag-1(om13), lag-2(sa37), lag-2(q420), glr-1(n2461) ncl-1(e1865), pha-1(e2123ts), nyIs60 [lin-15(+) flp-18p::gfp], mgIs18 [lin-15(+) ttx-3p::gfp], nuIs25 [lin-15(+) glr-1p::glr-1::gfp], nuIs1 [lin-15(+) glr-1p:::gfp], rtIs11 [osm-10p::gfp], and rtIs18 [elt-2p::gfp]. Transgenes were co-injected using pha-1(+) (pBX1), myo-2p::gfp (pPD48.33), and/or elt-2p::gfp (pJM67) as markers; details upon request. Heat shock induction occurred at 33°C for 2 hours. hsp::lin-12(RNAi) introduced at 8 ng/μl yielded inducible transgenic lines (hsp::lin-12(RNAi)); introduction at 50 ng/μl resulted in lines with increased reversal rates even in the absence of heat shock (26.8 ± 1.9 reversals/3 min., n = 11; see also Fig. 3); these lines are designated lin-12(RNAi) in the text to distinguish them from the inducible hsp::lin-12(RNAi) lines. Transgenic lines overexpressing lin-12p::lin-12 at very high levels (100 ng/μl) often had extra vulvae and were difficult to generate and maintain; these animals were used only for expression analysis. Moderate overexpression (50 ng/μl) of lin-12p::lin-12 was not overtly deleterious and vulval perturbations were infrequent; these animals were used for behavioral analysis. The integrated transgene nuIs25 that overexpresses a GFP tagged glr-1 rescue construct [58] increased reversals (shown in Fig. 7B). We also generated extrachromosomal arrays marked by pha-1 that overexpress a glr-1 rescue construct lacking GFP; animals carrying these arrays also had increased reversals (15.7 ± 0.8 reversals/3 min., n = 26, p<10-4 vs. wild type).
Authors' contributions
M.Y.C, J.L.-F., T.T., and A.C.H. all contributed to the genetic, molecular, and behavioral experiments. M.Y.C. and A.C.H. drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
We wish to thank Bob Horvitz, Iva Greenwald, Paul Sternberg, Stuart Kim, Villu Maricq, Josh Kaplan, Chris Rongo, Oliver Hobert, Andy Fire, Chris Li, Calum Macrae, Robert Nowak and Diane Levitan for strains, plasmids, and use of equipment, and members of the Hart, van den Heuvel, and Artavanis-Tsakonas laboratories and the C. elegans research community for helpful discussions. We acknowledge the assistance of Caenorhabditis Genetics Center for providing numerous strains and the help of Enrico Montana and Alex Ihring during the MBL Neurobiology course, 2002. This work was supported by an NIH NIGMS grant to A.C.H. and a MBRC Tosteson fellowship to M.Y.C.
Contributor Information
Michael Y Chao, Email: chao@helix.mgh.harvard.edu.
Jonah Larkins-Ford, Email: j_lford@yahoo.com.
Tim M Tucey, Email: ttucey@partners.org.
Anne C Hart, Email: hart@helix.mgh.harvard.edu.
References
- Fehon RG, Kooh PJ, Rebay I, Regan CL, Xu T, Muskavitch MA, Artavanis-Tsakonas S. Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF-homologous genes in Drosophila. Cell. 1990;61:523–534. doi: 10.1016/0092-8674(90)90534-L. [DOI] [PubMed] [Google Scholar]
- Fleming RJ, Scottgale TN, Diederich RJ, Artavanis-Tsakonas S. The gene Serrate encodes a putative EGF-like transmembrane protein essential for proper ectodermal development in Drosophila melanogaster. Genes Dev. 1990;4:2188–2201. doi: 10.1101/gad.4.12a.2188. [DOI] [PubMed] [Google Scholar]
- Johansen KM, Fehon RG, Artavanis-Tsakonas S. The notch gene product is a glycoprotein expressed on the cell surface of both epidermal and neuronal precursor cells during Drosophila development. J Cell Biol. 1989;109:2427–2440. doi: 10.1083/jcb.109.5.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klueg KM, Muskavitch MA. Ligand-receptor interactions and trans-endocytosis of Delta, Serrate and Notch: members of the Notch signalling pathway in Drosophila. J Cell Sci. 1999;112 ( Pt 19):3289–3297. doi: 10.1242/jcs.112.19.3289. [DOI] [PubMed] [Google Scholar]
- Kopczynski CC, Alton AK, Fechtel K, Kooh PJ, Muskavitch MA. Delta, a Drosophila neurogenic gene, is transcriptionally complex and encodes a protein related to blood coagulation factors and epidermal growth factor of vertebrates. Genes Dev. 1988;2:1723–1735. doi: 10.1101/gad.2.12b.1723. [DOI] [PubMed] [Google Scholar]
- Thomas U, Speicher SA, Knust E. The Drosophila gene Serrate encodes an EGF-like transmembrane protein with a complex expression pattern in embryos and wing discs. Development. 1991;111:749–761. doi: 10.1242/dev.111.3.749. [DOI] [PubMed] [Google Scholar]
- Chung HM, Struhl G. Nicastrin is required for Presenilin-mediated transmembrane cleavage in Drosophila. Nat Cell Biol. 2001;3:1129–1132. doi: 10.1038/ncb1201-1129. [DOI] [PubMed] [Google Scholar]
- Hu Y, Ye Y, Fortini ME. Nicastrin is required for gamma-secretase cleavage of the Drosophila Notch receptor. Dev Cell. 2002;2:69–78. doi: 10.1016/S1534-5807(01)00105-8. [DOI] [PubMed] [Google Scholar]
- Kopan R, Goate A. Aph-2/Nicastrin: an essential component of gamma-secretase and regulator of Notch signaling and Presenilin localization. Neuron. 2002;33:321–324. doi: 10.1016/S0896-6273(02)00585-8. [DOI] [PubMed] [Google Scholar]
- Struhl G, Adachi A. Requirements for presenilin-dependent cleavage of notch and other transmembrane proteins. Mol Cell. 2000;6:625–636. doi: 10.1016/S1097-2765(00)00061-7. [DOI] [PubMed] [Google Scholar]
- Lopez-Schier H, St Johnston D. Drosophila nicastrin is essential for the intramembranous cleavage of notch. Dev Cell. 2002;2:79–89. doi: 10.1016/S1534-5807(01)00109-5. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Kobayakawa Y, Tamura K, Kimura K, Kawaichi M, Tanimura T, Honjo T. Suppressor of hairless, the Drosophila homologue of RBP-J kappa, transactivates the neurogenic gene E(spl)m8. Jpn J Genet. 1995;70:505–524. doi: 10.1266/jjg.70.505. [DOI] [PubMed] [Google Scholar]
- Hsieh JJ, Henkel T, Salmon P, Robey E, Peterson MG, Hayward SD. Truncated mammalian Notch1 activates CBF1/RBPJk-repressed genes by a mechanism resembling that of Epstein-Barr virus EBNA2. Mol Cell Biol. 1996;16:952–959. doi: 10.1128/mcb.16.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourtois M, Schweisguth F. The neurogenic suppressor of hairless DNA-binding protein mediates the transcriptional activation of the enhancer of split complex genes triggered by Notch signaling. Genes Dev. 1995;9:2598–2608. doi: 10.1101/gad.9.21.2598. [DOI] [PubMed] [Google Scholar]
- Struhl G, Fitzgerald K, Greenwald I. Intrinsic activity of the Lin-12 and Notch intracellular domains in vivo. Cell. 1993;74:331–345. doi: 10.1016/0092-8674(93)90424-O. [DOI] [PubMed] [Google Scholar]
- Chen N, Greenwald I. The lateral signal for LIN-12/Notch in C. elegans vulval development comprises redundant secreted and transmembrane DSL proteins. Dev Cell. 2004;6:183–192. doi: 10.1016/S1534-5807(04)00021-8. [DOI] [PubMed] [Google Scholar]
- Henderson ST, Gao D, Lambie EJ, Kimble J. lag-2 may encode a signaling ligand for the GLP-1 and LIN-12 receptors of C. elegans. Development. 1994;120:2913–2924. doi: 10.1242/dev.120.10.2913. [DOI] [PubMed] [Google Scholar]
- Tax FE, Yeargers JJ, Thomas JH. Sequence of C. elegans lag-2 reveals a cell-signalling domain shared with Delta and Serrate of Drosophila. Nature. 1994;368:150–154. doi: 10.1038/368150a0. [DOI] [PubMed] [Google Scholar]
- Yochem J, Weston K, Greenwald I. The Caenorhabditis elegans lin-12 gene encodes a transmembrane protein with overall similarity to Drosophila Notch. Nature. 1988;335:547–550. doi: 10.1038/335547a0. [DOI] [PubMed] [Google Scholar]
- Levitan D, Greenwald I. Facilitation of lin-12-mediated signalling by sel-12, a Caenorhabditis elegans S182 Alzheimer's disease gene. Nature. 1995;377:351–354. doi: 10.1038/377351a0. [DOI] [PubMed] [Google Scholar]
- Li X, Greenwald I. HOP-1, a Caenorhabditis elegans presenilin, appears to be functionally redundant with SEL-12 presenilin and to facilitate LIN-12 and GLP-1 signaling. Proc Natl Acad Sci USA. 1997;94:12204–12209. doi: 10.1073/pnas.94.22.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlund B, Parry D, Clover R, Basson M, Johnson CD. Reverse genetic analysis of Caenorhabditis elegans presenilins reveals redundant but unequal roles for sel-12 and hop-1 in Notch-pathway signaling. Proc Natl Acad Sci USA. 1999;96:2497–2502. doi: 10.1073/pnas.96.5.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen S, Kodoyianni V, Bosenberg M, Friedman L, Kimble J. lag-1, a gene required for lin-12 and glp-1 signaling in Caenorhabditis elegans, is homologous to human CBF1 and Drosophila Su(H) Development. 1996;122:1373–1383. doi: 10.1242/dev.122.5.1373. [DOI] [PubMed] [Google Scholar]
- Berezovska O, Xia MQ, Hyman BT. Notch is expressed in adult brain, is coexpressed with presenilin-1, and is altered in Alzheimer disease. J Neuropathol Exp Neurol. 1998;57:738–745. doi: 10.1097/00005072-199808000-00003. [DOI] [PubMed] [Google Scholar]
- Stump G, Durrer A, Klein AL, Lutolf S, Suter U, Taylor V. Notch1 and its ligands Delta-like and Jagged are expressed and active in distinct cell populations in the postnatal mouse brain. Mech Dev. 2002;114:153–159. doi: 10.1016/S0925-4773(02)00043-6. [DOI] [PubMed] [Google Scholar]
- Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, Alamowitch S, Domenga V, Cecilion M, Marechal E, Maciazek J, Vayssiere C, Cruaud C, Cabanis EA, Ruchoux MM, Weissenbach J, Bach JF, Bousser MG, Tournier-Lasserve E. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–710. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Yu CE, Jondro PD, Schmidt SD, Wang K. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science. 1995;269:973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- Li L, Krantz ID, Deng Y, Genin A, Banta AB, Collins CC, Qi M, Trask BJ, Kuo WL, Cochran J, Costa T, Pierpont ME, Rand EB, Piccoli DA, Hood L, Spinner NB. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet. 1997;16:243–251. doi: 10.1038/ng0797-243. [DOI] [PubMed] [Google Scholar]
- Oda T, Elkahloun AG, Pike BL, Okajima K, Krantz ID, Genin A, Piccoli DA, Meltzer PS, Spinner NB, Collins FS, Chandrasekharappa SC. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet. 1997;16:235–242. doi: 10.1038/ng0797-235. [DOI] [PubMed] [Google Scholar]
- Rogaev EI, Sherrington R, Rogaeva EA, Levesque G, Ikeda M, Liang Y, Chi H, Lin C, Holman K, Tsuda T, Sorbi S. Familial Alzheimer's disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer's disease type 3 gene missense mutation of S182 gene in Italian families with early-onset Alzheimer's disease. Nature. 1995;376:775–778. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- Levitan D, Greenwald I. LIN-12 protein expression and localization during vulval development in C. elegans. Development. 1998;125:3599–3606. doi: 10.1242/dev.125.16.3101. [DOI] [PubMed] [Google Scholar]
- Ambros V. Cell cycle-dependent sequencing of cell fate decisions in Caenorhabditis elegans vulva precursor cells. Development. 1999;126:1947–1956. doi: 10.1242/dev.126.9.1947. [DOI] [PubMed] [Google Scholar]
- Greenwald IS, Sternberg PW, Horvitz HR. The lin-12 locus specifies cell fates in Caenorhabditis elegans. Cell. 1983;34:435–444. doi: 10.1016/0092-8674(83)90377-X. [DOI] [PubMed] [Google Scholar]
- Fukuto HS, Ferkey DM, Apicella AJ, Lans H, Sharmeen T, Chen W, Lefkowitz RJ, Jansen G, Schafer WR, Hart AC. G protein-coupled receptor kinase function is essential for chemosensation in C. elegans. Neuron. 2004;42:581–593. doi: 10.1016/S0896-6273(04)00252-1. [DOI] [PubMed] [Google Scholar]
- Sternberg PW, Horvitz HR. Pattern formation during vulval development in C. elegans. Cell. 1986;44:761–772. doi: 10.1016/0092-8674(86)90842-1. [DOI] [PubMed] [Google Scholar]
- Chalfie M, Sulston J, White JG, Southgate E, Thomson JN, Brenner S. The neural circuit for touch sensitivity in Caenorhabditis elegans. J Neurosci. 1985;5:956–964. doi: 10.1523/JNEUROSCI.05-04-00956.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Brockie PJ, Mellem JE, Madsen DM, Maricq AV. Neuronal control of locomotion in C. elegans is modified by a dominant mutation in the GLR-1 ionotropic glutamate receptor. Neuron. 1999;24:347–361. doi: 10.1016/S0896-6273(00)80849-1. [DOI] [PubMed] [Google Scholar]
- Gray JM, Hill JJ, Bargmann CI. A circuit for navigation in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2005;102:3184–3191. doi: 10.1073/pnas.0409009101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsalik EL, Hobert O. Functional mapping of neurons that control locomotory behavior in Caenorhabditis elegans. J Neurobiol. 2003;56:178–197. doi: 10.1002/neu.10245. [DOI] [PubMed] [Google Scholar]
- Wakabayashi T, Kitagawa I, Shingai R. Neurons regulating the duration of forward locomotion in Caenorhabditis elegans. Neurosci Res. 2004;50:103–111. doi: 10.1016/j.neures.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Altun-Gultekin Z, Andachi Y, Tsalik EL, Pilgrim D, Kohara Y, Hobert O. A regulatory cascade of three homeobox genes, ceh-10, ttx-3 and ceh-23, controls cell fate specification of a defined interneuron class in C. elegans. Development. 2001;128:1951–1969. doi: 10.1242/dev.128.11.1951. [DOI] [PubMed] [Google Scholar]
- Hart AC, Simms S, Kaplan JM. Synaptic code for sensory modalities revealed by C. elegans GLR-1 glutamate receptor. Nature. 1995;378:82–85. doi: 10.1038/378082a0. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Peckol E, Driscoll M, Bargmann CI. Mechanosensory signalling in C. elegans mediated by the GLR-1 glutamate receptor. Nature. 1995;378:78–81. doi: 10.1038/378078a0. [DOI] [PubMed] [Google Scholar]
- Feinberg EH, Hunter CP. Transport of dsRNA into cells by the transmembrane protein SID-1. Science. 2003;301:1545–1547. doi: 10.1126/science.1087117. [DOI] [PubMed] [Google Scholar]
- Mellem JE, Brockie PJ, Zheng Y, Madsen DM, Maricq AV. Decoding of Polymodal Sensory Stimuli by Postsynaptic Glutamate Receptors in C. elegans. Neuron. 2002;36:933–944. doi: 10.1016/S0896-6273(02)01088-7. [DOI] [PubMed] [Google Scholar]
- Gerlai R. Eph receptors and neural plasticity. Nat Rev Neurosci. 2001;2:205–209. doi: 10.1038/35058582. [DOI] [PubMed] [Google Scholar]
- Ge X, Hannan F, Xie Z, Feng C, Tully T, Zhou H, Zhong Y. Notch signaling in Drosophila long-term memory formation. Proc Natl Acad Sci USA. 2004;101:10172–10176. doi: 10.1073/pnas.0403497101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presente A, Boyles RS, Serway CN, de Belle JS, Andres AJ. Notch is required for long-term memory in Drosophila. Proc Natl Acad Sci USA. 2004;101:1764–1768. doi: 10.1073/pnas.0308259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa RM, Honjo T, Silva AJ. Learning and memory deficits in Notch mutant mice. Curr Biol. 2003;13:1348–1354. doi: 10.1016/S0960-9822(03)00492-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chan SL, Miele L, Yao PJ, Mackes J, Ingram DK, Mattson MP, Furukawa F. Involvement of Notch signaling in hippocampal synaptic plasticity. Proc Natl Acad Sci USA. 2004;101:9458–9462. doi: 10.1073/pnas.0308126101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura CA, Choi SY, Sun LD, Yang X, Handler M, Kawarabayashi T, Younkin L, Fedeles B, Wilson MA, Younkin S, Kandel ER, Kirkwood A, Shen J. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron. 2004;42:23–36. doi: 10.1016/S0896-6273(04)00182-5. [DOI] [PubMed] [Google Scholar]
- Dent JA, Davis MW, Avery L. avr-15 encodes a chloride channel subunit that mediates inhibitory glutamatergic neurotransmission and ivermectin sensitivity in Caenorhabditis elegans. EMBO J. 1997;16:5867–5879. doi: 10.1093/emboj/16.19.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockie PJ, Mellem JE, Hills T, Madsen DM, Maricq AV. The C. elegans glutamate receptor subunit NMR-1 is required for slow NMDA-activated currents that regulate reversal frequency during locomotion. Neuron. 2001;31:617–630. doi: 10.1016/S0896-6273(01)00394-4. [DOI] [PubMed] [Google Scholar]
- Zhao B, Khare P, Feldman L, Dent JA. Reversal frequency in Caenorhabditis elegans represents an integrated response to the state of the animal and its environment. J Neurosci. 2003;23:5319–5328. doi: 10.1523/JNEUROSCI.23-12-05319.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AC, Kass J, Shapiro JE, Kaplan JM. Distinct signaling pathways mediate touch and osmosensory responses in a polymodal sensory neuron. J Neurosci. 1999;19:1952–1958. doi: 10.1523/JNEUROSCI.19-06-01952.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C, Reale V, Kim K, Chatwin H, Li C, Evans P, de Bono M. Inhibition of Caenorhabditis elegans social feeding by FMRFamide-related peptide activation of NPR-1. Nat Neurosci. 2003;6:1178–1185. doi: 10.1038/nn1140. [DOI] [PubMed] [Google Scholar]
- Rongo C, Whitfield CW, Rodal A, Kim SK, Kaplan JM. LIN-10 is a shared component of the polarized protein localization pathways in neurons and epithelia. Cell. 1998;94:751–759. doi: 10.1016/S0092-8674(00)81734-1. [DOI] [PubMed] [Google Scholar]


