For end-stage liver disease and liver-based metabolic conditions the accepted treatment is liver transplantation. With developments in surgical techniques and immunosuppressive drug therapy the survival of patients and grafts is now good. In the conventional procedure the patient's whole liver is replaced with a liver obtained from a braindead or living donor. When the donor liver is too big it can be reduced to a compatible size; and, recently, split-liver procedures have been performed whereby the right lobe is transplanted into an adult and the smaller left lobe into a child,1 thus increasing the effective donor pool.
Another important advance in surgical technique is the use of auxiliary liver transplantation for patients with acute liver failure and certain liver-based metabolic defects such as Crigler–Najjar syndrome type I, urea cycle defects, and familial hypercholesterolaemia. In this procedure, part of the patient's liver, often the left lobe, is replaced with part of a donor liver. In a patient with acute liver failure this leaves open the possibility of regeneration of the native liver, in which case immunosuppression can be stopped and the graft allowed to atrophy; and in a patient with a metabolic disorder the native liver will be available for future gene therapy. The results of auxiliary liver transplantation in man2 have supported observations in animals that small amounts of liver tissue can provide sufficient function to correct an underlying metabolic defect. This finding was a spur to work on hepatocyte transplantation for such disorders. The aim is to repopulate the liver with donor hepatocytes, injected either directly into the liver or into the spleen, from which they migrate to the liver.
If the technique proves successful, hepatocyte transplantation offers several potential advantages. In terms of supply, there is the possibility of using cells from livers that are unsuitable for conventional transplantation because of steatosis or trauma. The patient does not have to undergo major surgery; moreover, in metabolic conditions the native liver provides a safety net in case of failure. One of the most important advantages is the availability of the liver as a target organ for gene therapy when this becomes a clinical reality. Experience of hepatocyte transplantation has been gained in patients with acute liver failure3,4 and metabolic liver diseases such as Crigler–Najjar syndrome type I,5 glycogen storage disease type 1a,6 and urea cycle defects.7 The background to this work has been described elsewhere.8–11 The current paper discusses the sources of hepatocytes, the isolation process, preclinical studies and clinical experience in the UK, especially in the treatment of liver-based inborn errors of metabolism.
SOURCES OF HEPATOCYTES
Normal hepatocytes do not divide in vitro, so that hepatocytes for transplantation must be isolated directly from liver tissue. The main source of these cells for transplantation in man is unused donor livers or segments of livers; from these, hepatocytes of high viability can be isolated.12
An additional possible source is the non-heart-beating donor; livers removed after the heart has stopped beating and respiration has ceased are already being assessed for orthotopic transplantation13 and might allow isolation of hepatocytes. To further increase the tissue pool our unit has pioneered the isolation of cells from segment IV of the liver dissected from the right lobe after the split-liver procedure,14 so that one liver can be used for three recipients.
ISOLATION OF HEPATOCYTES
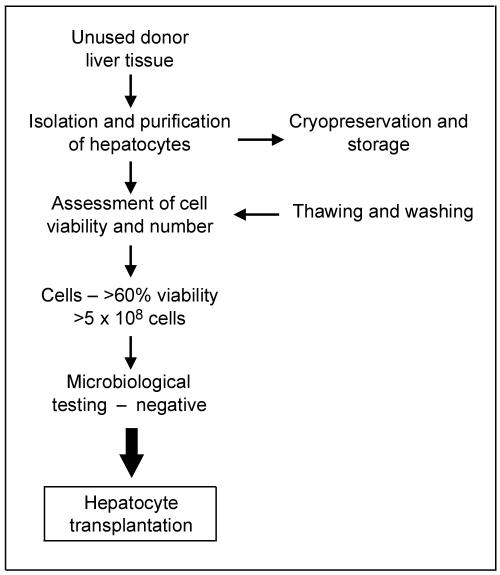
The well-established protocols for isolation of human hepatocytes15,16 employ collagenase digestion of perfused liver tissue at 37°C. Once the liver tissue is digested and cells are released, the hepatocytes are purified by centrifugation and assessed for cell viability and yield. Large numbers of cells can be obtained. Hepatocytes must be used as soon as possible for cell transplantation (within 24 h of isolation) since their function deteriorates even at 4°C. For longer-term storage cryopreservation methods are available, but the yield of viable hepatocytes on thawing tends to be insufficient. The best results are currently obtained by cryopreservation in a mixture of University of Wisconsin solution and 10% dimethyl sulfoxide by use of a controlled-rate cell freezer.17 The frozen hepatocytes can then be stored at -140°C until required. Cells from the hepatocyte bank can be thawed for immediate use.
An aseptic environment is required to prepare cells on a large scale in conditions of 'good manufacturing practice'. Air entering the laboratory passes through HEPA filters to remove any particles and an air-handling unit maintains a temperature-controlled environment. A gradient of air pressures provides the highest pressure in the aseptic room, where the tissue processing is performed. Standard operating procedures are followed for all aspects of work in the cell isolation unit. A comprehensive quality control system, which monitors all aspects of laboratory performance, is in operation.
All donated organs/tissues are screened for viral infection, including hepatitis and HIV, as with whole organ transplantation. The hepatocytes are released for transplantation only if the viability exceeds 60% and there is no microbial contamination (Figure 1).
Figure 1.
Preparation of hepatocytes for transplantation
PRECLINICAL STUDIES
Work in animals has explored hepatocyte transplantation to different sites including liver, spleen, pancreas, peritoneal cavity, and renal subcapsule, and in models of acute, chronic and metabolic liver disorders. (This paper concentrates on metabolic liver disease, but the results in acute liver injury, induced either surgically18 or by toxins,19 are encouraging.) For human metabolic disorders there are several animal models—e.g. Gunn rats for Crigler–Najjar disease,20 spf-ash mice for congenital ornithine transcarbamylase deficiency,21 Long–Evans cinnamon rats for Wilson's disease,22 Nagase analbuminaemic rats for hypoalbuminaemia,23 fumarylacetoacetate hydrolase —/— knockout mice for hereditary tyrosinaemia type I,24 Mdr2 —/— knockout mice lacking biliary phospholipid excretion for progressive familial intrahepatic cholestasis,25 dogs with hyperuricosuria,26 mice with histidinaemia,27 Watanabe rabbits with hypercholesterolaemia.28 In all these, hepatocyte transplantation has yielded long-term improvement of the biochemical abnormalities.
CLINICAL STUDIES
Clinically, hepatocyte transplantation was first tried in patients with liver failure, who have a high mortality and in whom liver transplantation is the only proven treatment. Experience in 30 patients with liver failure, from six centres in the USA, was reviewed by Strom et al. in 1999.29 Hepatocytes, either freshly isolated or thawed after cryopreservation, were infused into the splenic artery or portal vein of patients. Reductions in ammonia and bilirubin were recorded, with improvements in hepatic encephalopathy. The maximum amount of cells infused was 5% of normal liver mass; it is questionable whether this is sufficient to replace the lost function in acute liver failure.
In inherited metabolic liver diseases, where the aim is to replace a single deficient enzyme, fewer cells may be needed. One of the key early reports was from Fox and colleagues in 1998,5 who reported the case of a 10-year-old girl with Crigler–Najjar syndrome type I in whom liver expression of bilirubin UDP-glucuronosyltransferase activity continued for up to nine months after hepatocyte transplantation. The overall experience of hepatocyte transplantation in liver-based metabolic liver disorders, mainly in children and including that at King's College Hospital, London, is shown in Table 1.
Table 1.
Hepatocyte transplantation—clinical studies in liver-based metabolic diseases
| Disease | No. of patients | Effect/outcome | Reference |
|---|---|---|---|
| Familial hypercholesterolaemia | 5* | Some reduction in LDL in 3 patients | Grossman et al. (30) |
| Crigler–Najjar syndrome type I | 1 | 50% reduction in serum bilirubin; | Fox et al. (5) |
| 1 | 40% reduction in serum bilirubin | Dhawan et al. (unpublished) | |
| Urea cycle defect | 1 | Some clinical improvement; died after 42 days | Strom et al. (31) |
| 1 | Lowered blood ammonia and increased protein tolerance | Horslen et al. (7) | |
| 1 | No hyperammonaemia, increased urea synthesis | Mitry et al. (14) | |
| Infantile Refsum's disease | 1 | Partial correction of metabolic abnormality | Sokal et al. (32) |
| Glycogen storage disease type Ia | 1 | No hypoglycaemia on normal diet | Muraca et al. (6) |
| Factor VII deficiency | 2 | 80% reduction in recombinant factor VII requirement | Dhawan et al. (33) |
| PFIC2 | 2 | No clear benefit—fibrosis already present | Dhawan et al. (unpublished) |
LDL=low-density lipoprotein; PFIC2=progressive familial intrahepatic cholestasis
Ex-vivo gene therapy of autologous hepatocytes
Clinical experience in the UK
Only one centre in the UK, at King's College Hospital, London, is currently performing hepatocyte transplantation. Over the 4 years since the start of the project, 90 livers or liver segments have been processed for preparation of hepatocytes including, from conventional donors, 15 whole livers, 35 right lobes, 16 left lobes or left lateral segments and 6 liver segment IVs or caudate lobes. Mean viability of the hepatocytes was 65% (range 13–98%). In addition, isolations were made from 18 livers or segments obtained from non-heart-beating donors, perfused by the methods used for conventional donor livers; these gave a lower mean cell viability (50%, range 1–81%), but higher viability has lately been obtained by reducing both cold and warm ischaemia times before processing.
The methods for hepatocyte transplantation used at King's have been modified from those reported by Strom et al.29 in the USA. All the cell infusions were with ABO compatible hepatocytes. Up to 100 million cells per kg body weight are infused into the liver via the portal vein, with monitoring of portal pressure. Repeated infusions are performed until the donor hepatocyte cell mass is about 10% of recipient liver mass. At present there is no way to determine the proportion of administered cells that survive and engraft. The immunosuppression regimen used is similar to that given to whole-organ transplantation recipients, currently based on tacrolimus and prednisolone. Probably this needs to be modified for cell transplantation; also there are no laboratory tests to monitor cell allograft rejection.
Once the cell isolation methods had been established and validated, the first patient was treated. In September 2002 a boy with an antenatal diagnosis of severe ornithine transcarbamylase deficiency had hepatocytes infused via an umbilical vein catheter (the umbilical vein extends into the left portal vein). After transplantation of 1.9 billion hepatocytes he improved in terms of blood ammonia (maintained at low levels to prevent neurotoxicity) and urea (increased) while he was on normal protein intake.14 Longterm uncertainties about the efficacy of hepatocyte transplantation prompted auxiliary left lobe orthotopic liver transplantation at seven months of age. The patient was well at 2½ years of age with normal neurodevelopment and growth.
The next patients were two brothers with severe inherited coagulation factor VII deficiency—the first to have hepatocyte transplantation in such a condition. 1.1 and 2.2 billion hepatocytes were infused through a Hickman line. The coagulation defect improved, such that the requirement for exogenous factor VII (rFVIIa) became less than 20% of that before cell transplantation.33 Importantly, one of the patients received exclusively cryopreserved hepatocytes—so it seems that function related to clotting factors, at least, is maintained after cryopreservation. Six months after transplantation, both patients were needing higher rFVIIa doses, suggesting gradual loss of transplanted hepatocyte function. These two brothers later had successful orthotopic liver transplantation.
Two of the other children treated in 2003 had progressive familial intrahepatic cholestasis (PFIC2), a genetic disease34 in which the liver lacks the bile salt export pump. As a result of this defect, bile flow is severely impaired and cirrhosis develops rapidly. These children with PFIC2 each received 300 million fresh hepatocytes through a portal vein catheter, the rationale being that the injected hepatocytes would have a selective advantage over the native hepatocytes to repopulate the recipient liver (as shown in the mouse model of progressive familial intrahepatic cholestasis,25 where up to 70% of host hepatocytes were replaced with donor cells after nine months). However, no benefit was seen and both patients had whole-liver transplants five and fourteen months later. A possible explanation is that fibrosis in the hepatic sinusoids impaired engraftment, in which case earlier treatment, before the onset of fibrosis, might improve engraftment.
The most recently completed treatment was in a child with Crigler–Najjar syndrome type I who received a total of 4.3 billion fresh and cryopreserved hepatocytes with nine infusions over two weeks and a further infusion three months later. There was an encouraging reduction in serum bilirubin, still present nine months later. Overall 6 patients have been treated by hepatocyte transplantation without serious complications. The unit plans to extend the technique to children with acute liver failure.
THE FUTURE
Although considerable progress has been made in hepatocyte transplantation, the promise from animal experiments has not yet been fully borne out. There are several areas for improvement and development.
In terms of the limited supply of organs for isolation of hepatocytes, one potential source is livers rejected for clinical transplantation because of steatosis; we need better ways to isolate and purify hepatocytes from fatty livers so that their viability is good enough for transplantation. We also need to improve methods of hepatocyte storage, both for longer periods in the cold so that they can be used fresh after a few days and also cryopreservation for the longer term. Some progress has been made in our laboratories in preventing loss of hepatocyte function after cryopreservation by means of cryo/cytoprotectant agents.35 It is clear, also, that many injected cells do not engraft into the recipient liver and are either cleared by the reticuloendothelial system or lose viability during this early phase. The outcome of hepatocyte transplantation would benefit from methods to enhance engraftment and subsequent repopulation of the liver, although the options for this in man will be limited.
Clearly, one way to overcome the difficulties with hepatocyte supply and engraftment would be to use stem cells or stem-cell-derived hepatocytes.36 Possible sources are fetal liver, cord blood, embryos and bone marrow. Liver stem cell biology is under scrutiny worldwide but there are many hurdles to be jumped before clinical application. In theory, stem cells could be made to differentiate into all types of liver cell, could be frozen and thawed without harm and would be less immunogenic than donor cells (or non-immunogenic if autologous). As another approach, hepatocytes could be genetically manipulated in vitro to upregulate gene expression to enhance enzymatic activity and function—e.g. ornithine transcarbamylase, bilirubin glucuronosyltransferase—or render them immunotolerant. Methods to transfect hepatocytes are available and those employing non-viral vectors are of particular interest.
Acknowledgments
We thank the Children's Liver Disease Foundation and King's College Hospital Charitable Trust for financial support. This work would not have been possible without the contributions of Mr Nigel Heaton and Mr Mohamed Rela, transplant surgeons, Dr John Korani, consultant radiologist, and the other members of the liver transplant surgical team and staff at the Paediatric Liver Centre.
References
- 1.Rela M, Vougas V, Muiesan P, et al. Split liver transplantation: King's College Hospital experience. Ann Surg 1998;227: 282-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pereira SP, McCarthy M, Ellis AJ, et al. Auxiliary partial orthotopic liver transplantation for acute liver failure. J Hepatol 1997;26: 1010-17 [DOI] [PubMed] [Google Scholar]
- 3.Strom SC, Fisher RA, Thompson MT, et al. Hepatocyte transplantation as a bridge to orthotopic liver transplantation in terminal liver failure. Transplantation 1997;63: 559-69 [DOI] [PubMed] [Google Scholar]
- 4.Bilir BM, Guinette D, Karrer F, et al. Hepatocyte transplantation in acute liver failure. Liver Transplant 2000;6: 32-40 [DOI] [PubMed] [Google Scholar]
- 5.Fox IJ, Chowdhury JR, Kaufman SS, et al. Treatment of the Crigler–Najjar syndrome type I with hepatocyte transplantation. N Engl J Med 1998;338: 1422-6 [DOI] [PubMed] [Google Scholar]
- 6.Muraca M, Gerunda G, Neri D, et al. Hepatocyte transplantation as a treatment for glycogen storage disease type 1a. Lancet 2002;359: 317-18 [DOI] [PubMed] [Google Scholar]
- 7.Horslen SP, McCowan TC, Goertzen TC, et al. Isolated hepatocyte transplantation in an infant with a severe urea cycle disorder. Pediatrics 2003;111: 1262-7 [DOI] [PubMed] [Google Scholar]
- 8.Horslen SP, Fox IJ. Hepatocyte transplantation. Transplantation 2004;77: 1481-6 [DOI] [PubMed] [Google Scholar]
- 9.Fox IJ, Roy-Chowdhury J. Hepatocyte transplantation. J Hepatol 2004;40: 878-86 [DOI] [PubMed] [Google Scholar]
- 10.Fox IJ, Chowdhury JR. Hepatocyte transplantation. Am J Transplant 2004;4(suppl 6): 7-13 [DOI] [PubMed] [Google Scholar]
- 11.Najimi M, Sokal E. Update on liver cell transplantation. J Pediatr Gastroenterol Nutr 2004;39: 311-19 [DOI] [PubMed] [Google Scholar]
- 12.Mitry RR, Hughes RD, Aw MM, et al. Human hepatocyte isolation and relationship of cell viability to early graft function. Cell Transplant 2003;12: 69-74 [DOI] [PubMed] [Google Scholar]
- 13.Muiesan P. Can controlled non-heart-beating donors provide a solution to the organ shortage? Transplantation 2003;75: 1627-8 [DOI] [PubMed] [Google Scholar]
- 14.Mitry RR, Dhawan A, Hughes RD, et al. One liver, three recipients—segment IV from split liver procedures as a source of hepatocytes for cell transplantation. Transplantation 2004;77: 1614-16 [DOI] [PubMed] [Google Scholar]
- 15.Strom SC, Dorko K, Thompson MT, Pisarov LA, Nussler AK. Large scale isolation and culture of human hepatocytes. In: Franco D, Boudjema K, Varet B, eds. Îlots de Langerhans et hépatocytes. Paris: Editions INSERM, 1998: 195
- 16.Mitry RR, Hughes RD, Dhawan A. Progress in human hepatocytes: isolation, culture and cryopreservation. Semin Cell Dev Biol 2002;13: 463-7 [DOI] [PubMed] [Google Scholar]
- 17.Diener B, Utesch D, Beer N, Durk H, Oesch F. A method for the cryopreservation of liver parenchymal cells for studies of xenobiotics. Cryobiology 1993;30: 116-27 [DOI] [PubMed] [Google Scholar]
- 18.Demetriou AA, Reisner A, Sanchez J, Levenson SM, Moscioni AD, Chowdhury JR. Transplantation of microcarrier-attached hepatocytes into 90% partially hepatectomized rats. Hepatology 1988;8: 1006-9 [DOI] [PubMed] [Google Scholar]
- 19.Sutherland DE, Numata M, Matas AJ, Simmons RL, Najarian JS. Hepatocellular transplantation in acute liver failure. Surgery 1977;82: 124-32 [PubMed] [Google Scholar]
- 20.Matas AJ, Sutherland DE, Steffes MW, et al. Hepatocellular transplantation for metabolic deficiencies: decrease of plasma bilirubin in Gunn rats. Science 1976;192: 892-4 [DOI] [PubMed] [Google Scholar]
- 21.Michel JL, Rabier D, Rambaud C, et al. Intrasplenic transplantation of hepatocytes in spf-ash mice with congenital ornithine transcarbamylase deficiency. Chirurgie 1993. –94;119: 666-71 [PubMed] [Google Scholar]
- 22.Yoshida Y, Tokusashi Y, Lee GH, Ogawa K. Intrahepatic transplantation of normal hepatocytes prevents Wilson's disease in Long–Evans cinnamon rats. Gastroenterology 1996;111: 1654-60 [DOI] [PubMed] [Google Scholar]
- 23.Rozga J, Holzman M, Moscioni AD, Fujioka H, Morsiani E, Demetriou AA. Repeated intraportal hepatocyte transplantation in analbuminemic rats. Cell Transplant 1995;4: 237-43 [DOI] [PubMed] [Google Scholar]
- 24.Overturf K, Al-Dhalimy M, Ou CN, Finegold M, Grompe M. Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am J Pathol 1997;151: 1273-80 [PMC free article] [PubMed] [Google Scholar]
- 25.De Vree JM, Ottenhoff R, Bosma PJ, Smith AJ, Aten J, Oude Elferink RP. Correction of liver disease by hepatocyte transplantation in a mouse model of progressive familial intrahepatic cholestasis. Gastroenterology 2000;119: 1720-30 [DOI] [PubMed] [Google Scholar]
- 26.Kocken JM, Borel Rinkes IH, Bijma AM, et al. Correction of an inborn error of metabolism by intraportal hepatocyte transplantation in a dog model. Transplantation 1996;62: 358-64 [DOI] [PubMed] [Google Scholar]
- 27.Selden C, Calnan D, Morgan N, Wilcox H, Carr E, Hodgson HJ. Histidinemia in mice: a metabolic defect treated using a novel approach to hepatocellular transplantation. Hepatology 1995;21: 1405-12 [PubMed] [Google Scholar]
- 28.Wiederkehr JC, Kondos GT, Pollak R. Hepatoctye transplantation for the low-density lipoprotein receptor-deficient state. A study in the Watanabe rabbit. Transplantation 1990;50: 466-71 [DOI] [PubMed] [Google Scholar]
- 29.Strom SC, Chowdhury JR, Fox IJ. Hepatocyte transplantation for the treatment of human disease. Semin Liver Dis 1999;19: 39-48 [DOI] [PubMed] [Google Scholar]
- 30.Grossman M, Rader DJ, Muller DW, et al. A pilot study of ex vivo gene therapy for homozygous familial hypercholesterolaemia. Nat Med 1995;1: 1148-54 [DOI] [PubMed] [Google Scholar]
- 31.Strom SC, Fisher RA, Rubinstein WS, et al. Transplantation of human hepatocytes. Transplant Proc 1997;29: 2103-6 [DOI] [PubMed] [Google Scholar]
- 32.Sokal EM, Smets F, Bourgois A, et al. Hepatocyte transplantation in a 4-year-old girl with peroxisomal biogenesis disease: technique, safety, and metabolic follow-up. Transplantation 2003;76: 735-8 [DOI] [PubMed] [Google Scholar]
- 33.Dhawan A, Mitry RR, Hughes RD, et al. Hepatocyte transplantation for inherited factor VII deficiency. Transplantation 2004;78: 1812-14 [DOI] [PubMed] [Google Scholar]
- 34.Thompson R, Strautnieks S. BSEP: function and role in progressive familial intrahepatic cholestasis. Semin Liver Dis 2001;21: 545-50 [DOI] [PubMed] [Google Scholar]
- 35.Terry C, Dhawan A, Mitry RM, Lehec SC, Hughes RD. Preincubation of rat and human hepatocytes with cytoprotectants prior to cryopreservation can improve viability and function on thawing. Liver Transplant (in press) [DOI] [PubMed]
- 36.Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology 2004;39: 1477-87 [DOI] [PubMed] [Google Scholar]