Abstract
Background
Long non-coding RNAs (lncRNAs) have been shown to have crucial regulatory roles in human cancer biology. LncRNA PANDAR is a novel identified lncRNA that was previously reported to be increased in various cancers; however, its effect in colorectal cancer (CRC) remains unknown. The aim of this study was to explore the expression and role of lncRNA PANDAR in CRC.
Methods
The expression of lncRNA PANDAR was examined in CRC samples and cell lines by qRT-PCR. Kaplan–Meier survival analysis and univariate and multivariate Cox proportional hazards model were performed to evaluate the clinical and prognostic significance of lncRNA PANDAR in CRC patients. Furthermore, the biological function of lncRNA PANDAR on tumor cell growth, apoptosis and mobility was investigated through CCK-8, soft agar colony formation, flow cytometry, transwell migration and invasion assays in vitro. The potential mechanism of lncRNA PANDAR was demonstrated by Western blot and qRT-PCR.
Results
The expression level of PANDAR was higher in CRC tissues and cells compared to adjacent non-tumor tissues and normal colonic epithelial cells. Patients with high PANDAR expression level had poorer overall survival than those with low PANDAR expression. Moreover, multivariate analysis showed that the status of PANDAR expression was an independent prognostic indicator for CRC. Knockdown of PANDAR could inhibit cell growth, migration and invasion, arrest cell cycle as well as induce apoptosis of CRC cells in vitro study. In addition, PANDAR could affect epithelial–mesenchymal transition through inhibiting N-cadherin, vimentin, β-catenin, Snail and Twist expression and increasing the expression levels of E-cadherin.
Conclusion
Our data suggested that lncRNA PANDAR was a novel molecule involved in CRC progression, which provided a potential prognostic biomarker and therapeutic target for new therapies in patients with CRC.
Keywords: Long non-coding RNA, PANDAR, Colorectal cancer, Prognosis
Introduction
Colorectal cancer (CRC) is one of the most commonly diagnosed and lethal malignancy among men and women in most parts of the world (Chen et al. 2016; Siegel et al. 2016). Although chemotherapy and radiation therapy in combination with colectomy or rectectomy improves overall survival of 5 years, metastasis of CRC is associated closely with poor prognosis (Ferlay et al. 2015; Global Burden of Disease Cancer C et al. 2015). Therefore, it is emergently necessary for understanding of molecular mechanisms of CRC progression to identify new therapeutic strategies or diagnostic and prognostic biomarkers.
LncRNAs, which are currently defined as transcripts comprising more than 200 nt in length without evident protein coding function, were once considered to be transcriptional “noise” (Kunej et al. 2014). But more and more studies have revealed that lncRNAs play significant roles in a large range of biological processes, including cell differentiation, proliferation and apoptosis (Sun and Kraus 2015). Moreover, some lncRNAs are also important factors involving in cancer development and progression (Huarte 2015). PANDAR (promoter of CDKN 1A antisense DNA damage activated RNA), a novel non-coding RNA mapping to 6p21.2, underlies metastatic progression and chromosomal instability in multiple cancer. Recently, Zhang et al. (2016) found that long non-coding RNA PANDAR functions as an oncogene in bladder cancer, promoting cellular proliferation, migration and apoptosis. Moreover, lncRNA PANDAR regulates the G1/S transition of breast cancer cells by suppressing p16 (INK4A) expression (Sang et al. 2016). The long non-coding RNA PANDAR is up-regulated and associated with poor prognosis in gastric cancer (Ma et al. 2016) and hepatocellular carcinoma (Peng and Fan 2015). Further, low expression of long non-coding RNA PANDAR predicts a poor prognosis of non-small cell lung cancer and affects cell apoptosis by regulating Bcl-2 (Han et al. 2015).
However, the clinical significance and biological role of PANDAR and its mechanism in colorectal cancer remain largely unclear. In the present study, we characterized the expression of PANDAR in colorectal cancer tissues and explored its effects on two colorectal cancer cell lines (SW480 and LoVo). To the best of our knowledge, these data establish for the first time that PANDAR plays a great role in the genesis and development of colorectal cancer.
Materials and methods
Patients and sample collection
Fresh cancer tissues and pair-matched adjacent normal tissues were obtained from 124 patients with colorectal cancer between January 2010 and February 2011 at department of colorectal surgery, Zhejiang cancer hospital. All specimens from surgery were frozen and stored in liquid nitrogen until required. All patients did not receive preoperative treatment, such as radiation or chemotherapy before collecting specimens. This study was performed with the approval of the Research Ethics Committee of Zhejiang cancer hospital. Written informed consents were taken from all subjects. The clinical characteristics of all the patients are summarized in Table 1.
Table 1.
The relationship between PANDAR expression and clinicopathological factors in colorectal cancer
| Characteristics | Number | Expression of PANDAR | P value | |
|---|---|---|---|---|
| Low expression | High expression | |||
| Age (years) | ||||
| <60 | 51 | 29 | 22 | 0.201 |
| ≥60 | 73 | 33 | 40 | |
| Gender | ||||
| Male | 75 | 36 | 39 | 0.582 |
| Female | 49 | 26 | 23 | |
| Tumor diameter (cm) | ||||
| <5 | 82 | 42 | 33 | 0.009 |
| ≥5 | 42 | 13 | 29 | |
| Histological differentiation | ||||
| Well | 83 | 48 | 35 | 0.013 |
| Poorly | 41 | 14 | 27 | |
| TNM stage | ||||
| I/II | 46 | 32 | 14 | 0.001 |
| III/IV | 78 | 30 | 48 | |
| Lymph node metastasis | ||||
| Positive | 72 | 30 | 42 | 0.029 |
| Negative | 52 | 32 | 20 | |
| Depth of invasion | ||||
| T 1 + T 2 | 50 | 33 | 17 | 0.003 |
| T 3 + T 4 | 74 | 29 | 45 | |
| Distant metastasis | ||||
| Positive | 28 | 11 | 17 | 0.198 |
| Negative | 96 | 51 | 45 | |
| Primary tumor site | ||||
| Colon | 71 | 35 | 36 | 0.979 |
| Rectum | 53 | 26 | 27 | |
P value when expression levels were compared using Pearson Chi-square test
Cell lines and culture conditions
Human colorectal cancer cell lines SW480, LoVo, HCT-116, SW620, HT29 and normal colonic epithelial cell line were purchased from American type culture collection (ATCC) (Maryland, USA). Cancer cells were cultured in L-15 or DMEM (GIBCO-BRL, Carlsbad, CA, USA) supplemented with 10 % fetal bovine serum 100 U/mL penicillin, and 100 mg/mL streptomycin. Normal human colonic epithelial cells were cultured in RPMI1640 supplemented with 10 % fetal bovine serum and 2 mM l-glutamine (Gibco). All cell lines were cultured in humidified incubator at 37 °C with 5 % CO2.
RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
The total RNA of the tissue samples were extracted using the Trizol reagent (Invitrogen, Shanghai, China) according to the manufacturer’s instructions. The amount of RNA was quantitated by Nanodrop spectrophotometer (Thermo Scientific, USA). cDNA was converted from total RNA by using a Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) according to the instructions. Quantitative real-time PCR was performed with SYBR Green (Takara), and the data collection was carried out on the StepOnePlus™ Real-Time PCR Systems (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. The primers were synthesized by Invitrogen (Shanghai, China). Their sequences were as follows: PANDAR primers, forward: 5′-CTGTTAAGGTGGTGGCATTG-3′, reverse: 5′-GGAGGCTCATACTGGCTGAT-3′; E-cadherin primers forward: 5′-TGTAGTTACGTATTTATTTTTAGTGGCGTC-3′, reverse: 5′-CGAATACGATCGAATCGAACCG-3′; N-cadherin primers forward: 5′-CACTGCTCAGGACCCAGAT-3′, reverse: 5′-TAAGCCGAGTGATGGTCC-3′; β-catenin primers forward: 5′-TGCAGTTCGCCTTCACTATG-3′, reverse: 5′-ACTAGTCGTGGAATGGCACC-3′; Vimentin primers forward: 5′-TGGATTCACTCCCTCTGGTT-3′, reverse: 5′-GGTCATCGTGATGCTGAGAA-3′; Snail primers forward: 5′-GAGGCGGTGGCAGACTAG-3′, reverse: 5′-GACACATCGGTCAGACCAG-3′; Twist primers forward: 5′-TGAATCTTGCTCAGCTTGTC-3′, reverse: 5′-CGGGAGTCCGCAGTCTTA-3′; GAPDH primers, forward: 5′-CGCTCTCTGCTCCTCCTGTTC-3′, reverse: 5′-ATCCGTTGACTCCGACCTTCAC-3′. The average value in each triplicate was used to calculate the relative amount of PANDAR using methods. Experiments were repeated at least three times.
Small interfering RNA transfection
The siRNA specifically targeting PANDAR (Si-PANDAR) and the scrambled nucleotide was commercially constructed by Shanghai GenePharma Co. Ltd (Shanghai, China). The target sequences for Si-PANDAR were Si-PANDAR-1: 5′-GCAAUCUACAACCUGUCUU-3′, Si-PANDAR-2: 5′-AAUGUGUGCACGUAACAGAUU-3′. The scrambled nucleotide was used as the negative control (Si-NC). Cells were plated and cultured in growth media until cell density reached 70 % prior to siRNA transfection using lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. Cells were harvested after 48 h for qRT-PCR and Western blot analyses.
Cell proliferation assay
Cell proliferation was assayed using a cell proliferation kit, Cell Counting Kit-8 (CCK-8; Dojindo Molecular technologies, Inc., Kyushu, Japan) according to the manufacturer’s instructions. Cells were seeded into 96-well tissue culture plates at a density of 2 × 103 cells/well the day before transfection. At 24, 48, 72, 96 h after transfection, 20 μL of CCK8 reagent was added to each well and incubated at 37 °C for 2 h. The absorbance value was detected at a wave length of 450 nm at the indicated time point using Envision (PerkinElmer). Experiments were performed in triplicate.
Soft agar assay
The soft agar assay was performed using the CytoSelect™ 96-Well Cell Transformation Assay, Standard Soft Agar kit from Cell Biolabs according to the protocol provided by the manufacturer. Five thousand cells after transfection were plated in each well of a 96-well plate. Cells are incubated in a semisolid agar medium for 7–8 days. The cells are then solubilized, lysed and detected using the CyQuant, and analyzed using a 1420 Victor Multilabel Counter (PerkinElmer). The experiment was repeated twice with triplicates of each condition in each experiment.
Determination of cell apoptosis and cell cycle by flow cytometry
After transfection for 48 h, apoptosis was detected using the Annexin V-FITC Apoptosis Detection Kit (BD, USA). The SW480 and LoVo cells were then treated with Annexin V-FITC and propidium iodide (PI) in the dark at room temperature according to the manufacturer’s instructions. The cells were kept on ice in the dark and immediately analyzed by flow cytometry (FACSCalibur, BD Biosciences). The data were analyzed using the Cell Quest software. The experiment was repeated three times. The SW480 and LoVo cells transfected with Si-NC or Si-CCAT2 were harvested for 48 h for cell cycle analysis. Cells were then fixed with 70 % ethanol at −20 °C overnight and stained with propidium iodide (PI) (Sigma) in the presence of Ribonuclease A (Takara biotechnology, Dalian, China) for 30 min at room temperature. The cell cycle distribution was analyzed by flow cytometry (FACSCalibur, BD Biosciences).
Migration and invasion assay
This assay was performed as described previously (Wang et al. 2015a). BD 24-well transwell chamber (Costar, Massachusetts, USA) with or without Matrigel coating was used to measure cell migration or invasion according to manufacture’s guide. Cells (1 × 105 cells per well) suspended in 0.5 mL serum-free medium were added to the upper compartment of inserts in the 24-well plate and medium supplemented with 10 % FBS was added to the lower compartment. After incubating 18–24 h at 37 °C, 5 % CO2, the cells that invaded through membrane were fixed with 4 % formaldehyde and stained with 1 % crystal violet. The number of cells was determined in three randomly selected high-power fields across the center and the periphery of the membrane. Experiments were performed in triplicate.
Western blot analysis
Cells were lysed in RIPA buffer (150 mM NaCl, 50 mM Tris-base, 5 mM EDTA, 1 % NP-40, 0.25 % deoxycholate, pH 7.4) with protease and phosphatase inhibitors (Roche, Complete Mini). Protein concentrations were measured by the BCA protein assay (23227; Thermo Fisher Scientific, Waltham, MA, USA). Equal amounts of the protein were electrophoresed by SDS-PAGE, transferred to PVDF membranes and incubated with the following primary antibodies: anti-β-actin antibody, (Cell Signaling Technology); rabbit polyclonal anti-E-cadherin (A01589, GenScript, Edison, NJ), mouse monoclonal anti-N-cadherin (BD, Transduction, San Jose, CA), rabbit polyclonal anti-vimentin (A01189, GenScript, Edison, NJ), rabbit monoclonal anti-β-catenin (8480, Cell Signaling Technology), rabbit monoclonal anti-Snail (3879, Cell Signaling Technology), rabbit monoclonal anti-Twist (ab50581, Abcam). The primary antibody incubation was followed by incubation with an HRP-conjugated secondary antibody. The bound antibodies were detected using enhanced chemiluminescence reagent (32109; Thermo Fisher Scientific).
Statistical analysis
All experimental data from three independent experiments were analyzed by GraphPad Prism 5.0 and results were expressed as mean ± SD (standard deviation, SD). The Chi-square tests were performed to explore the associations between PANDAR level and clinicopathological factors. Comparisons between two groups were made by Student’s t test. One-way ANOVA was used when multiple comparisons were made. Survival analysis was performed using the Kaplan–Meier method, and the log-rank test was used to compare the differences between patient groups. Survival data were evaluated using univariate and multivariate Cox proportional hazards model. P values of <0.05 were considered to be statistically significant.
Results
Expression of PANDAR was up-regulated in colorectal cancer and associated with the clinicopathological factors in patients with colorectal cancer
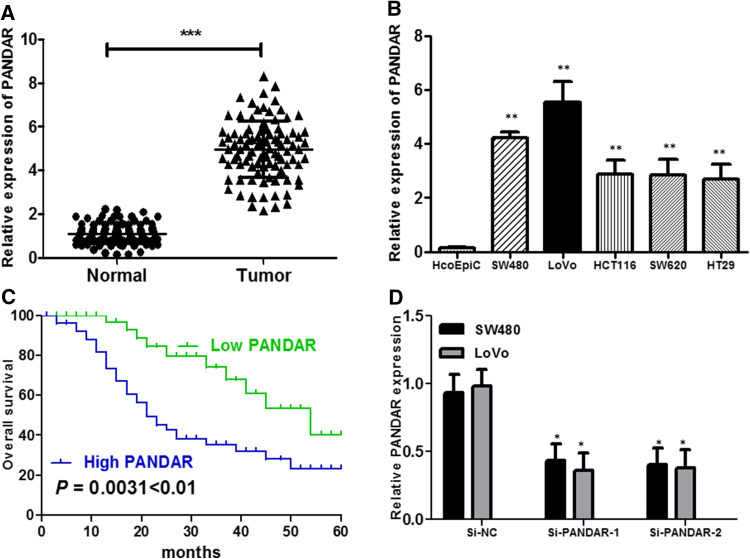
We firstly explored the relative expression level of PANDAR in colorectal cancer tissues (n = 124) compared with corresponding non-tumor tissues (n = 124) by qRT-PCR, and normalized to GAPDH. As shown in Fig. 1a, the PANDAR level was remarkably increased in colorectal cancer tissues compared with corresponding adjacent non-tumorous tissues (P < 0.001, Fig. 1a). Then, we examined the expression of PANDAR in a panel of colorectal cancer cells (SW480, LoVo, HCT116, SW620 and HT29) and human colonic epithelial cells (HcoEpiC) by qRT-PCR. As shown in Fig. 1b, the result of qRT-PCR demonstrated that the colorectal cancer cells showed high expression of PANDAR compared with the normal human colonic epithelial cells (P < 0.01, Fig. 1b). These results implied that abnormal PANDAR expression may be related with colorectal cancer pathogenesis. According to the median value of relative PANDAR expression in cancer tissues, colorectal cancer tissues were divided into two groups: relative high-PANDAR expression group (n = 62) and relative low-PANDAR expression group (n = 62). This classification was based on published study (Kogo et al. 2011; Li et al. 2015). The association of PANDAR expression level with the clinicopathological factors in colorectal cancer was analyzed (Table 1). PANDAR level was correlated to tumor diameter (P = 0.009), histological differentiation (P = 0.013), TNM stage (P = 0.001), lymph node metastasis (P = 0.029), depth of invasion (P = 0.003). No association between PANDAR expression and other factors, e.g., age (P = 0.201), gender (P = 0.582), distant metastasis (P = 0.198), primary tumor site (P = 0.979) were found in our study.
Fig. 1.
LncRNA PANDAR expression is increased in colorectal cancer and associates with overall survival in colorectal cancer patients. a Relative expression of PANDAR in 124 pairs of colorectal cancer tissues and adjacent non-tumor tissues by qRT-PCR analysis. ***P < 0.001 compared with non-tumor control. b The expression levels of PANDAR in a panel of colorectal cancer cell lines were determined by qRT-PCR and compared with that in human colonic epithelial cells (HcoEpiC). **P < 0.01 compared with the HcoEpiC cell. c Kaplan–Meier overall survival curves for two groups defined by low and high expression of PANDAR in patients with colorectal cancer (P = 0.0031 < 0.01). d qPCR analysis of PANDAR expression levels following the treatment of SW480 and LoVo cells with siRNAs against PANDAR. Data represent the mean ± SD from three independent experiments. *P < 0.05 compared with Si-NC; **P < 0.01 compared with Si-NC
High expression of PANDAR predicts poor prognosis in patients with colorectal cancer
To determine the relationship between PANDAR expression and colorectal cancer patients’ prognosis, Kaplan–Meier analysis and log-rank test were used to evaluate the correlation between PANDAR expression and the clinicopathological characteristics on overall survival (OS). As shown in Fig. 1c, patients with high PANDAR expression level had poorer overall survival (P = 0.0031) than those with low PANDAR expression. To further confirm the prognostic role of PANDAR in colorectal cancer patients, the univariate and multivariate survival analysis were performed for OS in colorectal cancer patients. As shown in Table 2, univariate analysis indicated that three prognosis factors (TNM stage, depth of invasion and PANDAR expression) were statistically significant risk factors influencing OS of patients. Multivariate analysis further confirmed that PANDAR expression could be regarded as a significant independent predictor of OS in colorectal cancer patients (Table 2). These results together suggested up-regulated expression of PANDAR dramatically shortened patients’ survival time and might play an important role in the prognosis of patients with colorectal cancer.
Table 2.
Univariate and multivariate analysis of clinic-pathologic factors for OS in colorectal cancer
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95 % CI | P value | HR | 95 % CI | P value | |
| Age (≥60 vs. <60 years) | 0.813 | 0.525–1.317 | 0.653 | |||
| Gender (male vs. female) | 0.671 | 0.358–1.125 | 0.783 | |||
| Tumor diameter (≥5 vs. <5 cm) | 1.246 | 1.174 –2.313 | 0.632 | |||
| Histological differentiation (poorly vs. well) | 0.975 | 0.782 –1.626 | 0.556 | |||
| Lymph node metastasis (N vs. P) | 1.164 | 0.736 –1.267 | 0.672 | |||
| Primary tumor size (colon vs. rectum) | 1.351 | 0.892 –1.892 | 0.531 | |||
| Distant metastasis (N vs. P) | 1.354 | 0.764 –1.902 | 0.481 | |||
| Depth of invasion (T 1 + T 2 vs. T 3 + T 4) | 2.378 | 1.127 –3.587 | 0.032* | 2.523 | 1.354 –3.087 | 0.014* |
| TNM stage (III/IV vs. I/II) | 2.958 | 1.256 –3.893 | 0.015* | 2.698 | 1.570 –3.242 | 0.001** |
| PANDAR expression (high vs. low) | 3.641 | 1.772 –4.956 | 0.006** | 3.532 | 1.413 –4.445 | 0.004** |
PFS progression-free survival, OS overall survival, HR hazard ratio, N negative, P positive
* P < 0.05; ** P < 0.01
Knockdown of PANDAR impairs proliferation of SW480 and LoVo cells in vitro
We detected that lncRNA PANDAR expression was comparatively higher in SW480 and LoVo cell lines than that in HCT116, SW620 and HT29 cancer cell lines (Fig. 1b). Therefore, we selected SW480 and LoVo cell lines for the following biological function studies. To further examine the role of PANDAR in colorectal cancer cells, the lncRNA PANDAR-specific Si-PANDAR was designed and transfected into SW480 and LoVo cells. As shown in Fig. 1d, cells transfected with Si-PANDAR presented a significantly decreased mRNA expression level of PANDAR compared with the Si-NC group in both cells by qRT-PCR (P < 0.05; Fig. 1d). To determine the effect of PANDAR on the proliferation of colorectal cancer cell in vitro, CCK-8 assays showed that the knockdown of PANDAR obviously suppressed the proliferation rate of SW480 and LoVo cells (P < 0.05; Fig. 2a, b). In addition, a soft agar colony formation assay was used to further probe the effect of PANDAR on anchor-independent growth of SW480 cells and LoVo cells. Consistently, the results showed that colorectal cancer cells transfected with siRNA significantly abrogated the colony numbers (P < 0.01; Fig. 2c). Additionally, flow cytometry was used following transfection to evaluate cell cycle distribution. The results indicated that the cell population in the G0/G1 phase was augmented, but the S phase population was reduced after the knockdown of PANDAR compared with the results detected for the Si-NC cells (Fig. 2d), further suggesting that knockdown of PANDAR expression may suppress cancer cell proliferation. Take together, these results illuminated that PANDAR may function as an oncogene involved in the stimulating of colorectal cancer cell proliferation.
Fig. 2.
The effect of PANDAR expression on cell viability and cell cycle. a, b CCK-8 assay showed that knockdown of PANDAR inhibited cell proliferation of SW480 and LoVo cells. c Soft agar assay measuring colony formation of PANDAR knockdown cells. Colony number was normalized to that obtained with cells transfected with Si-NC, which was set to 100 %. Silencing of PANDAR significantly decreased the colony-forming ability of SW480 and LoVo cells. d Effect of PANDAR knockdown on the SW480 and LoVo cell cycle. Cell cycle distribution was measured by propidium iodide staining followed by flow cytometry. The two cells had cell cycle arrest at the G0–G1 phase compared with cells transfected with Si-NC. Each assay was performed in triplicate. Data are mean ± SD. *P < 0.05 compared with Si-NC; **P < 0.01 compared with Si-NC
Knockdown of PANDAR increases apoptosis of colorectal cancer cells
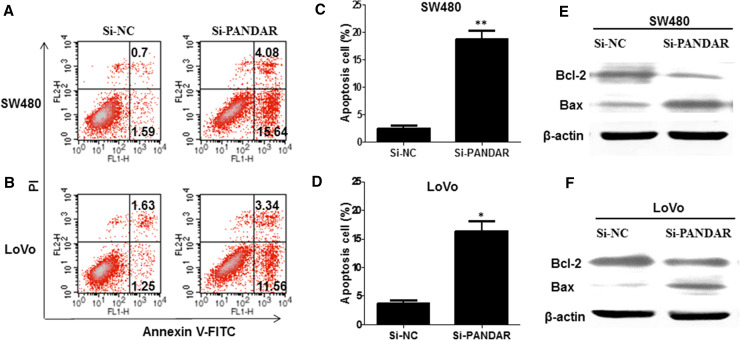
To investigate whether knockdown of PANDAR interfere cell apoptosis, flow cytometry was performed to dissect the cell apoptosis of colorectal cancer cells when transfected with Si-PANDAR. The data clarified that knockdown of PANDAR obviously induced apoptosis of SW480 (Fig. 3a) and LoVo cells (Fig. 3b), especially early apoptosis. Compared with the cells transfected with Si-NC, cell apoptosis increased approximately 17.43 % in SW480 cells when treated with Si-PANDAR (Fig. 3c), and 12.02 % in LoVo cells (Fig. 3d). Next, we examined Bcl-2 and Bax expression in colorectal cancer cells in response to PANDAR knockdown. Bcl-2 is recognized to inhibit apoptosis and facilitate resistance to multifarious apoptosis-inducing factors. However, Bax, a homologous gene of the Bcl-2 family, could inhibit the antiapoptotic effect of Bcl-2 by forming a heterodimer with Bcl-2 (Barclay et al. 2015; O’Neill et al. 2016). Western blotting analysis indicated that PANDAR knockdown suppressed the protein expression of Bcl-2 and, in contrast, enhanced the protein expression of Bax in SW480 (Fig. 3e) and LoVo cells (Fig. 3f).
Fig. 3.
Down-regulated lncRNA PANDAR increased the apoptosis of colorectal cancer cells. a, b Apoptosis of SW480 and LoVo cell lines was determined by flow cytometry. c, d Histogram of percentage of apoptotic cells, according to a, b. e, f Western blotting was used to detect the protein expression of Bcl-2 and Bax; GAPDH was used as control. Each assay was performed in triplicate. Data are mean ± SD. *P < 0.05 compared with Si-NC; **P < 0.01 compared with Si-NC
Knockdown of PANDAR inhibits migration and invasion of colorectal cancer cells
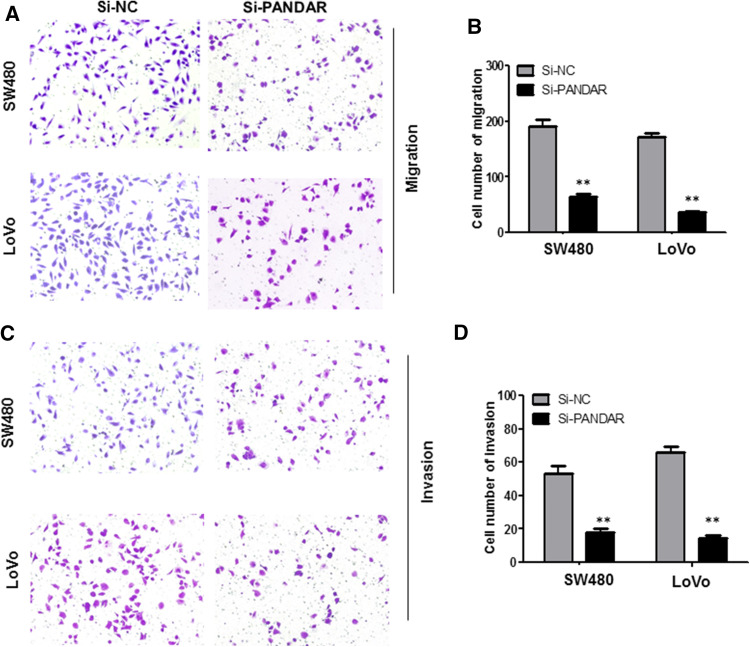
To further investigate the role of PANDAR in cell migration and invasion, we performed transwell assays in SW480 cells and LoVo cells. Knockdown of PANDAR strikingly inhibited SW480 and LoVo cell migration ability compared with the Si-NC group (P < 0.01, Fig. 4a, b). Next, transwell invasion assay was performed to evaluate the effect of PANDAR on the invasiveness of colorectal cancer cells. The results demonstrated that knockdown of PANDAR induced significant inhibition in ability of cell invasion compared with the Si-NC group (P < 0.01, Fig. 4c, d).
Fig. 4.
Knock-down PANDAR suppressed migration and invasion of colorectal cancer cells. Cell migration and invasion was determined by transwell assay. a, b Inhibition of migration of SW480 and LoVo cells by PANDAR siRNA. c, d inhibition of invasion of SW480 and LoVo cells by PANDAR siRNA. Data are shown as mean ± SD. The experiments were all repeated at least three times. **P < 0.01 compared with Si-NC
PANDAR facilitates epithelial–mesenchymal transition (EMT) in colorectal cancer
EMT is a main mechanism involved in cell migration and invasion (Ye and Weinberg 2015). Therefore, we next explored the expression of EMT-associated gene in colorectal cancer cells after transfection. The expression level of E-cadherin, N-cadherin, vimentin, β-catenin, Snail and Twist was examined by qRT-PCR and Western blot. By data, we found that the expression of N-cadherin, vimentin, β-catenin, Snail and Twist was markedly decreased, while E-cadherin expression was significantly boosted when PANDAR was knocked down in SW480 and LoVo cells (Fig. 5a–c). These data suggested that PANDAR involves mechanisms relevant to the promotion of colorectal cancer cell migration and invasion and that the underlying mechanisms may act by regulating the epithelial–mesenchymal transition (EMT) pathway.
Fig. 5.
Effect of PANDAR on EMT-related gene expression in colorectal cancer cells. SW480 and LoVo cells were transfected with Si-PANDAR and Si-NC for 48 h. The expression of EMT- associated genes including E-cadherin, N-cadherin, vimentin, β-catenin, Snail and Twist were analyzed by a, b qRT-PCR and c western blotting. Each assay was performed in triplicate. Data are mean ± SD. *P < 0.05 compared with Si-NC; **P < 0.01 compared with Si-NC
Discussion
Recently, the identification of cancer-associated lncRNAs and investigation of their clinical significance and biological functions in cancers have has been more and more intense (Li and Chen 2013). To date, many lncRNAs have been discovered, and their role in colorectal cancer has been identified. For instance, the long non-coding RNA CASC2 leads to the de-repression of genes downstream of STAT3 and consequentially inhibition of CRC cell proliferation and tumor growth in vitro and in vivo by sponging miR-18a in colorectal cancer (Huang et al. 2016). Also, long non-coding RNA TUG1 indicates a poor prognosis for colorectal cancer and affects cell proliferation, migration and invasion of CRC cell lines (Sun et al. 2016). Besides, long non-coding RNA HULC promotes colorectal carcinoma progression through epigenetically repressing NKD2 expression (Yang et al. 2016). Other well-studied lncRNAs, such as HOTAIR (Svoboda et al. 2014), H19 (Han et al. 2016), GAS5 (Krell et al. 2014), MEG3 (Yin et al. 2015) and MALAT1 (Ji et al. 2014), have demonstrated tumor suppressive or oncogenic roles in the development and progression of colorectal cancers. These findings suggested that lncRNAs could serve as diagnostic and prognostic biomarkers in colorectal cancer. However, the overall pathophysiological roles of lncRNAs to colorectal cancer remain largely unknown.
PANDAR has been shown to be up-regulated and knockdown of PANDAR expression led to cell growth arrest, invasion inhibition and elevated rates of apoptosis in bladder cancers, breast cancer, gastric cancer and hepatocellular carcinoma (Ma et al. 2016; Peng and Fan 2015; Sang et al. 2016; Zhan et al. 2016). Moreover, Han et al. (2015) found that PANDAR is down-regulated in non-small cell lung cancer and affects cell apoptosis by regulating Bcl-2. However, to our knowledge, the clinical significance and biological function of lncRNA PANDAR in colorectal cancer remains largely unknown. Our data in the present study showed remarkable up-regulation in PANDAR expression in colorectal cancer tissues compared with adjacent non-cancerous colorectal tissues. Further analysis showed that the up-regulation of PANDAR was positively associated with the tumor diameter, histological differentiation, TNM stage, lymph node metastasis, depth of invasion.
Furthermore, the prognostic value of PANDAR by Kaplan–Meier and Cox regression analysis was assessed. Our results suggested that high PANDAR expression had poor overall survival. The multivariate analyses implied that the high PANDAR expression was a potential independent prognostic factor for overall survival of colorectal cancer patients. These data indicated that high PANDAR expression may represent a novel indicator of poor prognosis in colorectal cancer and may be a potential therapeutic target for diagnosis and gene therapy.
Recent reports suggest that lncRNAs play crucial role in cancer growth and apoptosis. The long non-coding RNA HOTAIR increases tumor growth and limits apoptosis in cervical cancer by targeting the Notch pathway (Lee et al. 2016). Long non-coding RNA CRNDE promotes glioma cell growth and reduces apoptosis through mTOR signaling (Wang et al. 2015b). We then evaluated the effect of PANDAR in SW480 and LoVo cells on the proliferation and apoptosis. Knockdown of PANDAR significantly suppressed the proliferation of colorectal cancer cells by CCK-8 assay and soft agar assay, arrested cell cycle well as boosted the colorectal cancer cell apoptosis, implying that knockdown of PANDAR could abrogate the development of colorectal cancer.
In the present study, we clarified that knockdown of PANDAR expression can reduce migration and invasion of SW480 cells and LoVo cells by transwell assay. Meanwhile, Wang et al. (2016) reported that lncRNA-ROR plays an important role in the development of gallbladder cancer and mediates the EMT in gallbladder cancer. Long non-coding RNA LINC01133 inhibits epithelial–mesenchymal transition and metastasis in colorectal cancer by interacting with SRSF6 (Kong et al. 2016). Xiao et al. (2016) showed that UCA1 can modulate epithelial–mesenchymal transition (EMT) of MDA-MB-231 cells and knockdown of UCA1 impaired the mesenchymal properties. Additionally, MALAT1 induces tongue cancer cells’ EMT and inhibits apoptosis through Wnt/β-catenin signaling pathway (Liang et al. 2016). Thus, to further seek the molecular mechanism through which PANDAR promotes the metastasis of colorectal cancer cell, we determined the potential target proteins levels associated with these EMT-induced markers following knockdown of PANDAR. Our results showed that knockdown of endogenous PANDAR expression obviously weaken N-cadherin, vimentin, β-catenin, Twist, Snail expression and increased the expression levels of E-cadherin. These results exhibited that PANDAR might influence colorectal cancer metastasis by regulating EMT-related gene expression.
In summary, we firstly discovered that the PANDAR expression is strikingly up-regulated underlying colorectal cancer compared with paired-adjacent non-tumorous tissues, indicating that PANDAR may play a key oncogenic role as an indicator of poor survival rate and a negative prognostic factor for patients with colorectal cancer. We also illuminated that knockdown of PANDAR may inhibit proliferation, arrest cell cycle, facilitate apoptosis, suppress migration and invasion and EMT of colorectal cancer cells. These new findings suggest that PANDAR may be used as a potential prognostic and therapeutic target of colorectal cancer.
Acknowledgments
This study was funded by the Natural Science Foundation of Zhejiang Province (Grant No. Y15H160027) and Zhejiang Medical Technology Education Project (Grant No. 2013KYB046).
Compliance with ethical standards
Conflict of interest
All the authors declare that they have no competing interests.
Human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Research Ethics Committee of Zhejiang cancer hospital. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Min Lu and Zhuo Liu have contributed equally to this work.
References
- Barclay LA et al (2015) Inhibition of Pro-apoptotic BAX by a noncanonical interaction mechanism. Mol Cell 57:873–886. doi:10.1016/j.molcel.2015.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zheng R, Zuo T, Zeng H, Zhang S, He J (2016) National cancer incidence and mortality in China, 2012. Chin J Cancer Res 28:1–11. doi:10.3978/j.issn.1000-9604.2016.02.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386. doi:10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- Global Burden of Disease Cancer C et al (2015) The global burden of cancer 2013. JAMA Oncol 1:505–527. doi:10.1001/jamaoncol.2015.0735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L et al (2015) Low expression of long noncoding RNA PANDAR predicts a poor prognosis of non-small cell lung cancer and affects cell apoptosis by regulating Bcl-2. Cell Death Dis 6:e1665. doi:10.1038/cddis.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D et al (2016) Long noncoding RNA H19 indicates a poor prognosis of colorectal cancer and promotes tumor growth by recruiting and binding to eIF4A3. Oncotarget 7:22159–22173. doi:10.18632/oncotarget.8063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Wu X, Li S, Xu X, Zhu H, Chen X (2016) The long noncoding RNA CASC2 functions as a competing endogenous RNA by sponging miR-18a in colorectal cancer. Sci Rep 6:26524. doi:10.1038/srep26524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huarte M (2015) The emerging role of lncRNAs in cancer. Nat Med 21:1253–1261. doi:10.1038/nm.3981 [DOI] [PubMed] [Google Scholar]
- Ji Q et al (2014) Long non-coding RNA MALAT1 promotes tumour growth and metastasis in colorectal cancer through binding to SFPQ and releasing oncogene PTBP2 from SFPQ/PTBP2 complex. Br J Cancer 111:736–748. doi:10.1038/bjc.2014.383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogo R et al (2011) Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res 71:6320–6326. doi:10.1158/0008-5472.CAN-11-1021 [DOI] [PubMed] [Google Scholar]
- Kong J et al (2016) Long non-coding RNA LINC01133 inhibits epithelial–mesenchymal transition and metastasis in colorectal cancer by interacting with SRSF6. Cancer Lett 380:476–484. doi:10.1016/j.canlet.2016.07.015 [DOI] [PubMed] [Google Scholar]
- Krell J et al (2014) Growth arrest-specific transcript 5 associated snoRNA levels are related to p53 expression and DNA damage in colorectal cancer. PLoS One 9:e98561. doi:10.1371/journal.pone.0098561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunej T, Obsteter J, Pogacar Z, Horvat S, Calin GA (2014) The decalog of long non-coding RNA involvement in cancer diagnosis and monitoring. Crit Rev Clin Lab Sci 51:344–357. doi:10.3109/10408363.2014.944299 [DOI] [PubMed] [Google Scholar]
- Lee M et al (2016) The long non-coding RNA HOTAIR increases tumour growth and invasion in cervical cancer by targeting the Notch pathway. Oncotarget. doi:10.18632/oncotarget.10065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CH, Chen Y (2013) Targeting long non-coding RNAs in cancers: progress and prospects. Int J Biochem Cell Biol 45:1895–1910. doi:10.1016/j.biocel.2013.05.030 [DOI] [PubMed] [Google Scholar]
- Li Y et al (2015) NEAT expression is associated with tumor recurrence and unfavorable prognosis in colorectal cancer. Oncotarget 6:27641–27650. doi:10.18632/oncotarget.4737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Liang L, Ouyang K, Li Z, Yi X (2016) MALAT1 induces tongue cancer cells’ EMT and inhibits apoptosis through Wnt/beta-catenin signaling pathway. J Oral Pathol Med. doi:10.1111/jop.12466 [DOI] [PubMed] [Google Scholar]
- Ma P, Xu T, Huang M, Shu Y (2016) Increased expression of LncRNA PANDAR predicts a poor prognosis in gastric cancer. Biomed Pharmacother. 78:172–176. doi:10.1016/j.biopha.2016.01.025 [DOI] [PubMed] [Google Scholar]
- O’Neill KL, Huang K, Zhang J, Chen Y, Luo X (2016) Inactivation of prosurvival Bcl-2 proteins activates Bax/Bak through the outer mitochondrial membrane. Genes Dev 30:973–988. doi:10.1101/gad.276725.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W, Fan H (2015) Long non-coding RNA PANDAR correlates with poor prognosis and promotes tumorigenesis in hepatocellular carcinoma. Biomed Pharmacother. 72:113–118. doi:10.1016/j.biopha.2015.04.014 [DOI] [PubMed] [Google Scholar]
- Sang Y et al (2016) LncRNA PANDAR regulates the G1/S transition of breast cancer cells by suppressing p16(INK4A) expression. Sci Rep 6:22366. doi:10.1038/srep22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66:7–30. doi:10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- Sun M, Kraus WL (2015) From discovery to function: the expanding roles of long noncoding RNAs in physiology and disease. Endocr Rev 36:25–64. doi:10.1210/er.2014-1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Ding C, Yang Z, Liu T, Zhang X, Zhao C, Wang J (2016) The long non-coding RNA TUG1 indicates a poor prognosis for colorectal cancer and promotes metastasis by affecting epithelial–mesenchymal transition. J Transl Med 14:42. doi:10.1186/s12967-016-0786-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda M et al (2014) HOTAIR long non-coding RNA is a negative prognostic factor not only in primary tumors, but also in the blood of colorectal cancer patients. Carcinogenesis 35:1510–1515. doi:10.1093/carcin/bgu055 [DOI] [PubMed] [Google Scholar]
- Wang X, Yang J, Qian J, Liu Z, Chen H, Cui Z (2015a) S100A14, a mediator of epithelial–mesenchymal transition, regulates proliferation, migration and invasion of human cervical cancer cells Am. J Cancer Res 5:1484–1495 [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang Y, Li J, Zhang Y, Yin H, Han B (2015b) CRNDE, a long-noncoding RNA, promotes glioma cell growth and invasion through mTOR signaling. Cancer Lett 367:122–128. doi:10.1016/j.canlet.2015.03.027 [DOI] [PubMed] [Google Scholar]
- Wang SH, Zhang MD, Wu XC, Weng MZ, Zhou D, Quan ZW (2016) Overexpression of LncRNA-ROR predicts a poor outcome in gallbladder cancer patients and promotes the tumor cells proliferation, migration, and invasion. Tumour Biol. doi:10.1007/s13277-016-5210-z [DOI] [PubMed] [Google Scholar]
- Xiao C, Wu CH, Hu HZ (2016) LncRNA UCA1 promotes epithelial–mesenchymal transition (EMT) of breast cancer cells via enhancing Wnt/beta-catenin signaling pathway. Eur Rev Med Pharmacol Sci 20:2819–2824 [PubMed] [Google Scholar]
- Yang XJ, Huang CQ, Peng CW, Hou JX, Liu JY (2016) Long noncoding RNA HULC promotes colorectal carcinoma progression through epigenetically repressing NKD2 expression. Gene. doi:10.1016/j.gene.2016.08.002 [DOI] [PubMed] [Google Scholar]
- Ye X, Weinberg RA (2015) Epithelial–mesenchymal plasticity: a central regulator of cancer progression. Trends Cell Biol 25:675–686. doi:10.1016/j.tcb.2015.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin DD, Liu ZJ, Zhang E, Kong R, Zhang ZH, Guo RH (2015) Decreased expression of long noncoding RNA MEG3 affects cell proliferation and predicts a poor prognosis in patients with colorectal cancer. Tumour Biol 36:4851–4859. doi:10.1007/s13277-015-3139-2 [DOI] [PubMed] [Google Scholar]
- Zhan Y et al (2016) Up-regulation of long non-coding RNA PANDAR is associated with poor prognosis and promotes tumorigenesis in bladder cancer. J Exp Clin Cancer Res CR 35:83. doi:10.1186/s13046-016-0354-7 [DOI] [PMC free article] [PubMed] [Google Scholar]