Abstract
Retinitis pigmentosa (RP), the main cause of adult blindness, is a genetically heterogeneous disorder characterized by progressive loss of photoreceptors through apoptosis. Up to now, 39 genes and loci have been implicated in nonsyndromic RP, yet the genetic bases of >50% of the cases, particularly of the recessive forms, remain unknown. Previous linkage analysis in a Spanish consanguineous family allowed us to define a novel autosomal recessive RP (arRP) locus, RP26, within an 11-cM interval (17.4 Mb) on 2q31.2-q32.3. In the present study, we further refine the RP26 locus down to 2.5 Mb, by microsatellite and single-nucleotide polymorphism (SNP) homozygosity mapping. After unsuccessful mutational analysis of the nine genes initially reported in this region, a detailed gene search based on expressed-sequence-tag data was undertaken. We finally identified a novel gene encoding a ceramide kinase (CERKL), which encompassed 13 exons. All of the patients from the RP26 family bear a homozygous mutation in exon 5, which generates a premature termination codon. The same mutation was also characterized in another, unrelated, Spanish pedigree with arRP. Human CERKL is expressed in the retina, among other adult and fetal tissues. A more detailed analysis by in situ hybridization on adult murine retina sections shows expression of Cerkl in the ganglion cell layer. Ceramide kinases convert the sphingolipid metabolite ceramide into ceramide-1-phosphate, both key mediators of cellular apoptosis and survival. Ceramide metabolism plays an essential role in the viability of neuronal cells, the membranes of which are particularly rich in sphingolipids. Therefore, CERKL deficiency could shift the relative levels of the signaling sphingolipid metabolites and increase sensitivity of photoreceptor and other retinal cells to apoptotic stimuli. This is the first genetic report suggesting a direct link between retinal neurodegeneration in RP and sphingolipid-mediated apoptosis.
Introduction
Inherited retinal degenerative diseases are characterized by the progressive loss of mature photoreceptor cells through apoptosis. Among them, retinitis pigmentosa (RP [MIM 268000]), the most common hereditary cause of blindness, comprises a clinically and genetically heterogeneous group of retinal disorders that affects ∼1.5 million people worldwide (Sullivan and Daiger 1996). Patients with RP typically show night blindness, gradual visual impairment, and bone spicule–like pigment deposits in the retina.
RP is a paradigm of monogenic diseases with extremely high genetic heterogeneity (Hims et al. 2003). This complexity has clearly hindered the identification of causative genes, particularly in the recessive forms, since large nuclear families suitable for linkage studies are scarce. In fact, although 39 genes and loci have been described, many others, still unknown, could account for the remaining unassigned cases. At present, the reported RP causative genes fall into four categories: (a) genes directly involved in the phototransduction cascade, (b) genes encoding proteins responsible for the structure and polarity of the photoreceptors, (c) genes encoding proteins of the visual cycle, and (d) regulatory genes (such as transcription and splicing factors). In the study of autosomal recessive RP (arRP), mutations on 16 genes and linkage to five chromosomal regions have been reported (Wang et al. 2001; RetNet Web site). Therefore, RP gene identification is still a priority in this field, since it not only is needed for diagnosis but also is crucial for giving insights into the molecular basis of the disease.
RP26, one of the remaining uncharacterized loci, was previously mapped to an 11-cM interval on 2q31.2-q32 in a consanguineous family with arRP (Bayés et al. 1998). Later, this 11-cM interval, between markers D2S148 and D2S117, was ascribed to a physical distance of 17.4 Mb that comprised more than 50 genes. As the incoming human genome data were made available in the public databases, new markers—either microsatellites or, mainly, SNPs—were retrieved and analyzed, first for cosegregation and finally for homozygosity mapping. All of this new information allowed us to narrow the candidate region to 2.5 Mb, and mutational screening was subsequently performed to identify the RP26 gene.
Material and Methods
Families and DNA
The two families with arRP we analyzed, P2 and E1, have been described elsewhere (Bayés et al. 1996, 1998). Blood samples were collected from family members, and DNA was extracted using a commercial kit (Wizard Genomic DNA Purification Kit [Promega]). Informed consent was gathered from all family members. This research followed the tenets of the Declaration of Helsinki.
Cosegregation and Homozygosity Mapping
The RP26 locus was further refined using three flanking markers: D2S2978, D2S2261, and D2S273 (table 1). For subsequent homozygosity mapping, additional markers (13 microsatellites and 101 SNPs) were chosen on the basis of their physical position according to the National Center for Biotechnology Information (NCBI) Human Builds 29–33 at the University of California Santa Cruz Genome Bioinformatics Web site (table 1). The SNP sequences were retrieved from the NCBI dbSNP Home Page and The SNP Consortium database. New SNPs observed during this study were submitted and deposited in the NCBI dbSNP database (see table 1). SNP analyses involved PCR assays with flanking primers and subsequent sequencing to verify the genotype. Three amplification steps (30 s each), with the annealing temperature ranging from 48°C to 61°C, were performed. The reaction mixture (50 μl) contained 10 μM of each primer, 2 μM of dNTPs, 1.5 mM MgCl2, and 1 U of Taq polymerase (Innogenetics).
Table 1.
Microsatellite and SNP Markers Analyzed on 2q31.2-q32.3
| Microsatellite or SNP IDa | Locationb | Locus | Genotype inRP26 Patientsc |
| D2S148; AFM200WA11 | chr2: 176902551–176902745 | Recombinant | |
| D2S2978; ATA44H04 | chr2: 179788236–179788348 | Recombinant | |
| D2S2261; AFMB072WG1 | chr2: 180180111–180180442 | Recombinant | |
| rs1002207; TSC0036660 | chr2: 180237706 | Noninformative | |
| rs1949453; TSC1026876 | chr2: 180594170 | UBE2E3 | Homozygous |
| *rs2077783; TSC0046543 | chr2: 180834855 | Homozygous | |
| *rs720453; TSC0046544 | chr2: 180834909 | Homozygous | |
| *D2S2310; AFMB355XD5 | chr2: 180849531–180849858 | Noninformative | |
| *rs155100; TSC0531809 | chr2: 181033165 | ITGA4 | Homozygous |
| *rs155101; TSC0531810 | chr2: 181033223 | ITGA4 | Homozygous |
| *rs155102 | chr2: 181033525 | ITGA4 | Homozygous |
| *rs155103 | chr2: 181033821 | ITGA4 | Homozygous |
| rs1801262 | chr2: 181226533 | NEUROD1 | Homozygous |
| rs2583016 | chr2: 181228296 | NEUROD1 | Homozygous |
| *rs1157595; TSC0326375 | chr2: 181432646 | Homozygous | |
| *D2S364; AFM303YA9 | chr2: 181717611–181718002 | PDE1A | Homozygous |
| *rs3754929 | chr2: 181771662 | PDE1A | Homozygous |
| *rs1438065; TSC0655111 | chr2: 181902071 | PDE1A | Homozygous |
| *rs833152; TSC0655110 | chr2: 181902179 | PDE1A | Noninformative |
| rs288254; TSC0681111 | chr2: 182291705 | ERdj5 | Noninformative |
| rs7510 | chr2: 182472727 | NCKAP1 | Heterozygous |
| ss12676100 | chr2: 182476461 | NCKAP1 | Heterozygous |
| ss12676101 | chr2: 182476476 | NCKAP1 | Heterozygous |
| ss12676102 | chr2: 182482636 | NCKAP1 | Heterozygous |
| rs2271671 | chr2: 182510096 | NCKAP1 | Heterozygous |
| D2S350; AFM292WD1 | chr2: 182532022–182532389 | NCKAP1 | Heterozygous |
| ss12676103 | chr2: 182678153 | LOC129401 | Heterozygous |
| ss12676104 | chr2: 182678197 | LOC129401 | Heterozygous |
| ss12676105 | chr2: 182678220 | LOC129401 | Heterozygous |
| D2S2273; AFMB297XC1 | chr2: 182825486–182825797 | Heterozygous | |
| D2S2281; AFMB310XF5 | chr2: 182863973–182864335 | Heterozygous | |
| D2S2366; AFMA057VG9 | chr2: 183175061–183175459 | Noninformative | |
| rs826135; TSC1254035 | chr2: 183459257 | Noninformative | |
| rs826134; TSC0476562 | chr2: 183459267 | Noninformative | |
| ss12676106 | chr2: 183459509 | Heterozygous | |
| rs826133; TSC0658245 | chr2: 183459526 | Noninformative | |
| ss12676107 | chr2: 183459659 | Heterozygous | |
| ss12676108 | chr2: 183459776 | Heterozygous | |
| rs826132; TSC0658244 | chr2: 183459802 | Heterozygous | |
| D2S1391; GATA65C03 | chr2: 183675508–183675835 | Homozygous | |
| rs1443021; TSC0664031 | chr2: 183910062 | Heterozygous | |
| rs1443022; TSC0664032 | chr2: 183910149 | Heterozygous | |
| rs768352; TSC0076040 | chr2: 184012769 | Homozygous | |
| rs994653; TSC0317578 | chr2: 184193063 | Homozygous | |
| rs728534; TSC0064305 | chr2: 184484995 | Homozygous | |
| rs3046266; TSC1530131 | chr2: 184485293 | Noninformative | |
| rs1366842; TSC0514415 | chr2: 184485321 | Noninformative | |
| rs3731834 | chr2: 184486442 | Homozygous | |
| D2S1361; GATA14E05 | chr2: 184899775–184900087 | Heterozygous | |
| rs1016410; TSC0098979 | chr2: 185334459 | Heterozygous | |
| rs1019430; TSC0223209 | chr2: 185334589 | Heterozygous | |
| rs878845; TSC0212635 | chr2: 185580725 | Heterozygous | |
| ss12676109 | chr2: 186138356 | ITGAV | Heterozygous |
| ss12676110 | chr2: 186170238 | ITGAV | Heterozygous |
| ss12676111 | chr2: 186181185 | ITGAV | Heterozygous |
| ss12676112 | chr2: 186186304 | ITGAV | Heterozygous |
| ss12676113 | chr2: 186202416 | ITGAV | Heterozygous |
| rs3816386 | chr2: 186211713 | ITGAV | Heterozygous |
| rs2018302; TSC0091486 | chr2: 186467891 | Heterozygous | |
| rs1467990; TSC0384843 | chr2: 186468012 | Noninformative | |
| rs2018314; TSC0085681 | chr2: 186468088 | Heterozygous | |
| rs1000623; TSC0033486 | chr2: 186468142 | Heterozygous | |
| rs840570; TSC1028023 | chr2: 186855755 | Noninformative | |
| rs2308091 | chr2: 186856059 | Noninformative | |
| rs84069 | chr2: 186856203 | Noninformative | |
| rs84068 | chr2: 186856285 | Noninformative | |
| D2S152; AFM207XG1 | chr2: 186914674–186915071 | CALCRL | Noninformative |
| rs1398061; TSC0576737 | chr2: 186941063 | CALCRL | Noninformative |
| rs1464338; TSC0378776 | chr2: 187177069 | Noninformative | |
| rs1464337; TSC0378775 | chr2: 187177070 | Noninformative | |
| rs2176858; TSC1217605 | chr2: 187177146 | Noninformative | |
| rs2033838; TSC1047282 | chr2: 187551834 | Heterozygous | |
| rs2028374; TSC1091521 | chr2: 187666577 | Heterozygous | |
| rs1354906; TSC0490640 | chr2: 188129892 | CED-6 | Homozygous |
| rs1007120: TSC0084553 | chr2: 188175289 | Noninformative | |
| rs925825; TSC0241755 | chr2: 188431720 | Heterozygous | |
| rs893407; TSC0165376 | chr2: 189033193 | Heterozygous | |
| rs2289404 | chr2: 189318673 | ORMDL1 | Noninformative |
| rs6942; TSC0023616 | chr2: 189319070 | ORMDL1 | Homozygous |
| rs3791767 | chr2: 189322574 | ORMDL1 | Noninformative |
| rs288817; TSC1078112 | chr2: 189529648 | Noninformative | |
| ss12676114 | chr2: 189529690 | Homozygous | |
| rs998173; TSC0011271 | chr2: 189694293 | Homozygous | |
| rs3791789 | chr2: 189787488 | HIBCH | Heterozygous |
| D2S2262; AFMB082YE1 | chr2: 189985176–189985493 | Heterozygous | |
| rs7721 | chr2: 190069246 | Heterozygous | |
| rs1128723 | chr2: 190069374 | Heterozygous | |
| rs8962 | chr2: 190074316 | Noninformative | |
| rs1468685; TSC0385949 | chr2: 190268436 | Heterozygous | |
| rs1263136; TSC1488116 | chr2: 190298357 | Noninformative | |
| rs1263125; TSC0446273 | chr2: 190303089 | Homozygous | |
| D2S118; AFM066XC1 | chr2: 190309134–190309312 | Homozygous | |
| rs1263100; TSC0398872 | chr2: 190327138 | Heterozygous | |
| rs1476896; TSC0398871 | chr2: 190327184 | Homozygous | |
| D2S389; AFM333WF9 | chr2: 190347305–190347628 | Homozygous | |
| rs1882395; TSC0896111 | chr2: 190387668 | Homozygous | |
| D2S1775; GATA71C07 | chr2: 190402485–190402614 | Homozygous | |
| rs3217036 | chr2: 190462343 | GLS | Noninformative |
| rs2355571 | chr2: 190477428 | GLS | Heterozygous |
| rs2883713 | chr2: 190491082 | GLS | Heterozygous |
| rs3199237 | chr2: 190497863 | GLS | Noninformative |
| rs3207595 | chr2: 190497884 | GLS | Noninformative |
| rs3207596 | chr2: 190497887 | GLS | Noninformative |
| rs3199238 | chr2: 190497893 | GLS | Noninformative |
| rs3207597 | chr2: 190497917 | GLS | Noninformative |
| rs1801893 | chr2: 190498994 | GLS | Noninformative |
| rs3215259 | chr2: 190521768 | GLS | Noninformative |
| rs1058589 | chr2: 190522002 | GLS | Noninformative |
| rs1058590 | chr2: 190522011 | GLS | Noninformative |
| rs1058591 | chr2: 190522017 | GLS | Noninformative |
| rs1058592 | chr2: 190522020 | GLS | Noninformative |
| rs1547550; TSC0429185 | chr2: 190548390 | STAT1 | Heterozygous |
| rs925847; TSC0241788 | chr2: 190600205 | STAT4 | Noninformative |
| rs3024891 | chr2: 190601614 | STAT4 | Heterozygous |
| D2S2246; AFMB007WC1 | chr2: 191085514–191085746 | Heterozygous | |
| D2S318; AFM105XC1 | chr2: 191278428–191278834 | Homozygous | |
| D2S161; AFM224ZF4 | chr2: 191431730–191432052 | Heterozygous | |
| D2S280; AFM155YE1 | chr2: 191557728–191557932 | TMEFF2 | Homozygous |
| rs3738882 | chr2: 191751560 | TMEFF2 | Homozygous |
| rs935367; TSC0352562 | chr2: 191842107 | Noninformative | |
| D2S315; AFM081YG5 | chr2: 192076101–192076267 | Homozygous | |
| rs717621; TSC0040380 | chr2: 192153621 | Heterozygous | |
| D2S273; UT5048 | chr2: 192288946–192289538 | Recombinant | |
| D2S117; AFM065YF11 | chr2: 194331362–194331699 | Recombinant |
Microsatellite IDs are those that begin with “D,” and SNP IDs are those that begin with “rs” or “ss.” SNPs IDs that begin with “ss” are those identified in the present study and are deposited in the NCBI dbSNP database. Microsatellite and SNP IDs preceded by an asterisk (*) are the flanking CERKL markers used for haplotype analysis and comparison in families P2 and E1.
Location in NCBI Build 32.
Boldface italic type indicates candidate homozygous region.
Mutation Screening
Primers located at the flanking intron regions were designed to amplify each coding exon for all the analyzed genes in all samples. Three amplification steps (30 s each), with the annealing temperature ranging from 45°C to 60°C, were performed. Sequencing was performed using the BigDye Terminator Cycle Sequencing v3.1 kit and the automatic sequencers ABI 3700 and ABI 3730 (Applied Biosystems).
Mutation Restriction Analysis
A forward primer (5′-GAAGAATGCTGGGATGGAAACGGAC-3′) containing a mismatch nucleotide (underlined) that created an AvaII restriction site in the exon 5 wild-type sequence was designed. A three-step PCR (annealing temperature 50°C) was performed on the genomic DNA of the P2 and E1 family members, through use of the mismatch forward primer and exon 5 reverse primer (5′-GTTGTGCTGTCTAGATTAGC-3′), under the conditions mentioned above. The 157-bp PCR fragments were digested with AvaII and were resolved in a 4% low-melting-temperature agarose gel.
Human and Murine CERKL cDNA Characterization
Database searches from the available human genomic sequence allowed the identification of a truncated EST (GenBank accession number BE797822) and a partial cDNA (GenBank accession number BC020465), which overlapped 18 bp. To characterize the full-length cDNA, a human retina cDNA library (BD Biosciences) was screened (1.2×106 plaque-forming units) with a mixture of all the CERKL coding exons as a probe. The hybridization was performed overnight under highly stringent conditions (42°C in 50% formamide, 5× Denhardt’s, 5× SSPE, 0.1% sodium dodecyl sulfate, and 100 μg/ml salmon sperm DNA), and several washes were performed at 55°C in 2× SSC and 1× SSC before autoradiography. Only a partial CERKL cDNA was obtained. For the full-length coding sequence, we devised a PCR strategy using two specific primers flanking the conceptual ATG and STOP codons on the Marathon-Ready retina cDNA (BD Biosciences) under the following conditions: 94°C for 30 s, 60°C for 30 s, and 72°C for 2 min for 40 cycles. The reaction mixture (50 μl) contained 10 μM of each primer, 2 μM of dNTPs, 1.5 mM MgCl2, 1 U of Taq polymerase (Innogenetics), and 5% DMSO.
TBLASTN database searches at the NCBI BLAST server revealed two murine sequences homologous to human CERKL, one EST (GenBank accession number BY742285) and one partial cDNA (GenBank accession number BC046474), which had 377 nt of overlap with one another. Protein alignment was performed with CLUSTALW 1.8 at the BCM Search Launcher Web site and was depicted with BOXSHADE 3.21 at the EMBnet BoxShade Server.
Phylogenetic Analysis
Protein sequences from several members of the diacylglycerol, sphingosine, and ceramide kinase families were retrieved from public databases and aligned with human and murine CERKL through use of CLUSTALX version 1.64 (Thompson et al. 1997). The resulting phylogenetic tree was constructed using the neighbor-joining algorithm and considering the diacylglycerol kinase sequences as outgroup.
RT-PCRs
Two specific primers on exons 8 (5′-CCATTTAACAGCTCTGATGATGTGCAAG-3′) and 12 (5′-GACCTCTGATGCAACTTCCATTAAGTCAC-3′), which amplified 429 bp, were used to detect CERKL expression on a panel of first-strand cDNAs of several human adult and fetal tissues (BD Biosciences). Specific CERKL expression was confirmed by sequencing the amplified products. Amplification of GAPD was used to compare and normalize the samples, as suggested by the manufacturer. PCR conditions were as follows: 35 cycles of 94°C for 30 s and 65°C for 60 s. The reaction mixture (50 μl) contained 10 μM of each primer, 2 μM of dNTPs, 1.5 mM MgCl2, and 1 U Taq pol (Innogenetics).
In Situ Hybridizations
For in situ hybridization, adult mouse eye slides from the C57BL/6J strain were obtained from Novagen. Eyes were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned at 7 μm. Sections were washed for 7 min three times with xylene to remove paraffin, for 3 min twice with ethanol 100%, for 3 min with ethanol 90%, for 3 min with ethanol 70%, and for 3 min with diethyl pyrocarbonate–treated water, and then were fixed with 4% paraformaldehyde for 20 min. Sections were treated with 2 μg/ml proteinase K for 30 min, washed for 5 min twice with phosphate-buffered saline, and postfixed with 4% paraformaldehyde. Acetylation with 0.1 M triethanolamine-HCl (pH 8.0) containing 0.25% acetic anhydride was performed for 10 min. Slides were subsequently washed, ethanol dehydrated, and air dried. Hybridization was performed overnight at 55°C with 2 μg/ml digoxigenin-labeled riboprobes in 50% formamide, 1 × Denhardt’s solution, 10% dextran sulfate, 0.3 M NaCl, 10 mM Tris-HCl (pH 8.0), 5 mM EDTA (pH 8.0), 10 mM NaH2PO4, and 200 μg/ml salmon sperm DNA.
The riboprobes were generated from the T7 and T3 promoters of a pBluescript II KS vector containing either a 201-bp HindIII-PstI fragment, a 350-bp HindIII fragment, a 513-bp PstI-SphI fragment of the murine Cerkl cDNA IMAGE 4504238, or a 198-bp fragment from coding exon 4 of murine rhodopsin.
After hybridization, the slides were washed in 2× SSC for 20 min; washed for 5 min twice in a solution of 50% formamide and 2× SSC (washes at 50°C); equilibrated in NTE (0.5 M NaCl, 10 mM Tris-HCl pH 8.0, 5 mM EDTA) at 37°C; and then treated with 10 μg/ml RNase A in NTE at 37°C for 30 min. Subsequently, the sections were washed in NTE, 2× SSC, and 0.1× SSC (15 min each), equilibrated in buffer 1 (100 mM Tris-HCl pH 7.5, 150 mM NaCl), and blocked in blocking buffer (1% BSA and 0.1% Triton X-100 in buffer 1) for 30 min. An anti-digoxigenin-AP conjugate antibody (1:500; Roche Applied Science) in blocking buffer was incubated overnight at 4°C. The sections were washed twice in buffer 1 for 15 min and in buffer 2 (100 mM Tris-HCl pH 9.5, 150 mM NaCl, and 50 mM MgCl2) for 10 min prior to exposure to the alkaline phosphatase substrate, nitroblue tetrazolium–5-bromo-4-chloro-3-indoyl phosphate (NBT-BCIP; Roche Applied Science). The reaction was stopped by several washes in distilled water. The sections were cover-slipped with 70% glycerol in PBS and were photographed using a Nomarski optics microscope (Axioplan, Carl Zeiss) equipped with a Nikon Coolpix digital camera.
Results
Refinement of the Cosegregation Region and Candidate Gene Analysis
The analysis of two proximal (D2S2978 and D2S2261) and one distal (D2S273) flanking markers (table 1) allowed further refinement of the RP26 locus to a 12.5-Mb interval (fig. 1A). As a first attempt to identify the causative gene, a mutational screening of candidate genes selected on the basis of their known retinal function or the phenotype of Drosophila mutants or knockout mice was performed. The analysis of five candidates (ORMDL1, ITGAV, GLS, NEUROD1, and FRZB) within this interval did not reveal any pathogenic mutation.
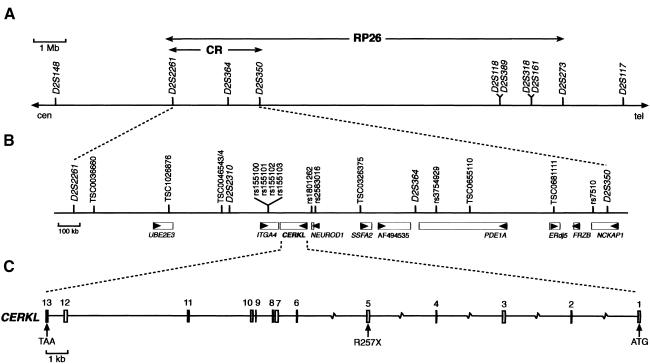
Figure 1.
The RP26 locus and the CERKL gene and protein. A, RP26 physical map of the 12.5-Mb cosegregation interval between markers D2S2261 and D2S273. B, Localization of CERKL within the candidate region (“CR”) defined by homozygosity mapping. Genes (rectangles) and the transcription direction (arrowheads) are indicated. C, CERKL exon/intron structure. The location of the R275X null mutation in exon 5 is shown.
Homozygosity Mapping and Mutation Screening
The reported consanguinity of the RP26 family and the availability of new markers after the sequencing of the human genome made homozygosity mapping feasible as a means to reduce the candidate region. Sixteen microsatellite markers and 101 SNPs located within the 12.5-Mb interval (table 1) were analyzed in all P2 family members. Eventually, only one homozygous segment spanning >1 Mb, between markers D2S2261 and D2S350 (fig. 1A), was identified. This candidate region, spanning 2.5 Mb, harbored five annotated genes and four partially characterized mRNAs (fig. 1B), which were prioritized for mutational analysis on the basis of (a) retinal expression, as assessed by PCR on a retina cDNA library; and (b) availability of functional data. All of them were eventually sequenced (in fact, two genes, NEUROD1 and FRZB, were analyzed in the previous section), but none showed any pathogenic variant.
Identification of a Novel Gene, CERKL
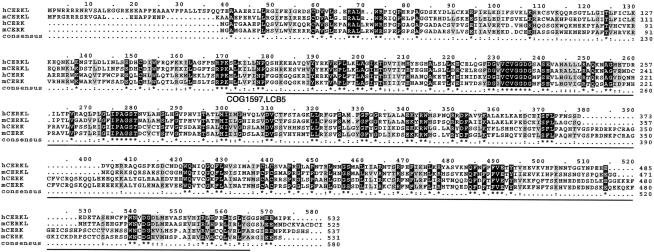
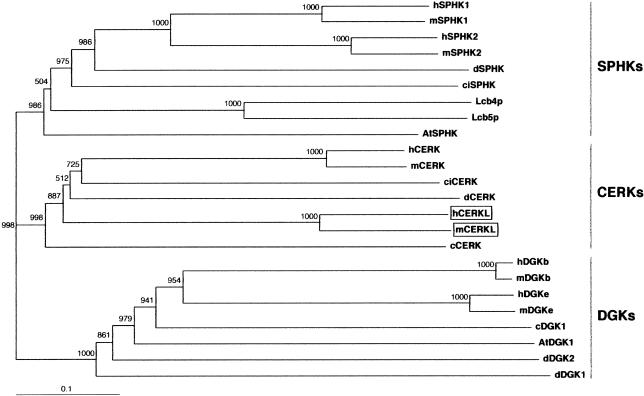
A more exhaustive database search for uncharacterized cDNAs and spliced ESTs within the candidate 2.5-Mb region was undertaken and revealed two close sequences, one EST (GenBank accession number BE797822) and one eye/retinoblastoma incomplete cDNA (GenBank accession number BC020465), located between the ITGA4 and the NEUROD1 genes. The conceptual protein from the in silico assembly of both sequences showed homology with eukaryotic ceramide, sphingosine, and diacylglycerol kinases and strongly suggested that the sequences analyzed corresponded to a novel, unreported gene. After screening a human retina cDNA library and performing serial PCR assays on retina cDNA, we obtained the full-length 1,596-nt coding sequence, which spanned 13 exons (fig. 1C). The predicted 532-aa protein showed the highest similarity (29% identity; 50% similarity) with the human ceramide kinase (CERK [Sugiura et al. 2002]) (fig. 2) and, therefore, the gene was named “human ceramide kinase–like” (CERKL [HUGO-approved nomenclature]). In addition, database searches revealed one murine EST (GenBank accession number BY742285) and one partial cDNA (GenBank accession number BC046474), which, upon assembly and conceptual translation, produced a protein that was highly homologous to human CERKL (75% identity; 85.6% similarity) (fig. 2). To verify that CERKL belonged to the ceramide kinase subfamily of lipid kinases, a phylogenetic tree was constructed using 24 protein sequences from different eukaryotic phyla. Human and murine CERKL sequences clearly clustered within the branch of ceramide kinases (fig. 3).
Figure 2.
Protein alignment of human (“h”) and murine (“m”) CERKL and CERK. Identical residues are highlighted in black, and conservative positions are in gray. The solid black line underlines the conserved COG1597 (LCB5) domain detected after comparison with the NCBI Conserved Domain Database. Murine CERKL sequence was assembled from GenBank sequences BY742285 and BC046474, human CERK sequence from AB079066, and murine CERK sequence from AB79067.
Figure 3.
Unrooted phylogenetic tree of several members of the diacylglycerol, sphingosine, and ceramide kinases, including the human and murine CERKL sequences described in the present study (boxed). The tree was obtained with the neighbor-joining algorithm of the program ClustalX version 1.64b. Numbers show the values for the bootstrap analysis. SPHKs = sphingosine kinases; CERKs = ceramide kinases; and DGKs = diacylglycerol kinases. Abbreviations and GenBank accession numbers are as follows: human sphingosine kinase 1 (hSPHK1), AAF73423; murine sphingosine kinase 1 (mSPHK1), AAC61697; human sphingosine kinase 2 (hSPHK2), AAF74124; murine sphingosine kinase 2 (mSPHK2), AAF74125; Drosophila sphingosine kinase (dSPHK), AAF48045; Ciona intestinalis sphingosine kinase (ciSPHK), AK112588; yeast sphingosine kinase Lcb4 (Lcb4p), NP_014814; yeast sphingosine kinase Lcb5 (Lcb5p), NP_013361; Arabidopsis thaliana sphingosine kinase (AtSPHK), AY128394; human ceramide kinase (hCERK), AB079066; murine ceramide kinase (mCERK), AB079067; Ciona intestinalis ceramide kinase (ciCERK), AK112750; Drosophila ceramide kinase (dCERK), AAF52040; human ceramide kinase-like (hCERKL), AY357073; murine ceramide kinase-like (mCERKL), BY742285 and BC046474; Caenorhabditis elegans ceramide kinase (cCERK), AAC67466; human diacylglycerol kinase β subunit (hDGKb), Q9Y6T7; murine diacylglycerol kinase β subunit (mDGKb), XP_147651; human diacylglycerol kinase epsilon subunit (hDGKe), NP_003638; murine diacylglycerol kinase epsilon subunit (mDGKe), NP_062378); Caenorhabditis elegans diacylglycerol kinase 1 (cDGK1), NP_508190; Arabidopsis thaliana diacylglycerol kinase 1 (AtDGK1), Q39017; Drosophila diacylglycerol kinase 2 (dDGK2), Q09103; and Drosophila diacylglycerol kinase 1 (dDGK1), Q01583.
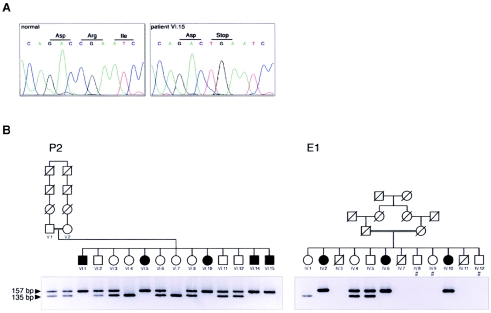
Premature Truncation of CERKL Causes arRP
The CERKL coding exons were sequenced in all members of the RP26 family. All patients were homozygous for a nonsense mutation (R257X; CGA→TGA) in exon 5, which prematurely truncates the protein within the predicted catalytic domain (fig. 4A and 4B). This mutation was not observed in 170 unrelated, ethnically matched control individuals. We subsequently evaluated cosegregation of RP with two CERKL flanking markers (D2S2310 and D2S364) in a panel of small nuclear Spanish families with RP whose causative gene was still unknown. An additional cosegregating pedigree, unrelated to the RP26 family, was identified. The CERKL sequence analysis showed that the affected members were also homozygous for the R257X mutation (fig. 4B), thus reinforcing the pathogenicity of this CERKL variant. Although both families share the same mutation, the haplotype analysis of the 12 nearest flanking markers (see table 1), stretching 600 kb around CERKL, does not support a common ancestry (data not shown).
Figure 4.
A, DNA sequence of exon 5 in an unaffected individual and patient VI.15 of the P2 family, showing the homozygous nonsense R257X mutation. B, Pedigrees of P2 and E1 families, showing the ARMS analysis of R257X mutation. A specific primer was designed to generate an AvaII restriction site in the amplified wild-type allele. All RP-affected individuals are homozygous for the mutant, nonrestricted allele. Individuals whose DNA samples were not available are indicated by a number sign (#).
CERKL Shows a Tissue-Specific Pattern of Expression
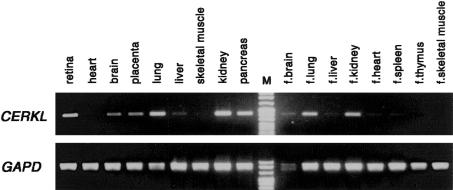
Northern expression analysis of CERKL did not reveal any transcript on two panels of several human tissues, even after long exposure times (data not shown). This suggested either specific expression in nontested tissues or very low transcriptional levels, in contrast to the higher and more ubiquitous expression of the CERK gene (Sugiura et al. 2002). The more sensitive RT-PCR assays revealed moderate expression in adult human retina, kidney, lung, and pancreas, and low levels in brain, placenta, and liver (fig. 5). The RT-PCRs on human fetal tissues also showed expression in lung, kidney, and brain (fig. 5).
Figure 5.
CERKL and GAPD (control) RT-PCR expression analysis on several human tissues, including retina
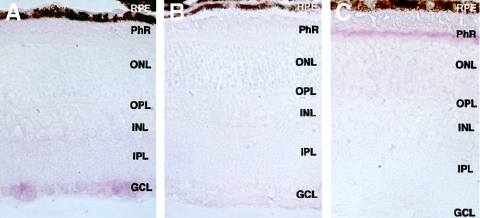
To assess CERKL expression in the retina more accurately, we performed in situ hybridization on mouse eye sections. The mouse CERKL ortholog is predominantly expressed in the retina ganglion cell layer, although a faint signal is also detected in the inner nuclear and photoreceptor cell layers (fig. 6A and 6B). The axons from the ganglion cells merge in the optic nerve and, therefore, constitute the last cellular component of phototransduction in the retina.
Figure 6.
A, Cerkl in situ hybridization on murine eye sections with the antisense riboprobe. B, Cerkl in situ hybridization on murine eye sections with the sense riboprobe (negative control). C, Rhodopsin in situ hybridization on murine eye sections (positive control). RPE = retinal pigment epithelium; PhR = photoreceptor cell layer; ONL = outer nuclear layer; OPL = outer plexiform layer; INL = inner nuclear layer; IPL = inner plexiform layer; GCL = ganglion cell layer.
As a positive control, we assayed rhodopsin, which is highly expressed in the photoreceptor cells (fig. 6C). It is remarkable that, at a comparable specific labeling and concentration probe, rhodopsin hybridization signal was much more intense and was detected much earlier than Cerkl expression, again supporting the low transcriptional levels of the latter.
Discussion
The identification of CERKL and its characterization as an RP causative gene opens new avenues for evaluating its contribution—as well as that of other sphingolipid (SL) metabolism enzymes—to retinal function and disease. The pathogenicity of the R257X mutation is supported not only by the absence of this variant in the control population (the test on 340 chromosomes allows the detection of a polymorphism of 1% with 95% statistical confidence [Collins and Schwartz 2002]) but also, and with more relevance to function, by the premature truncation of the protein (position 257 out of 532 aa), well within the strictly conserved catalytic domain of ceramide, sphingosine, and diacylglycerol kinases (fig. 2).
During the last decade and after exhaustive cosegregation analyses using internal and flanking markers of all the reported arRP genes and loci (including RP26), we have identified the causative gene in only 8 (∼15%) families out of 52 Spanish pedigrees with arRP. When we consider all our data, CERKL (responsible for the disease in two families [present study]) appears to contribute to arRP to a similar extent as other well-known arRP genes, such as PDE6B (three families [Bayés et al. 1996]), TULP1 (one family [Paloma et al. 2000]), ABCA4 (one family [Martínez-Mir et al. 1998]), and CNGA1 (one family [Paloma et al. 2002]). Although it is too early to assess the real contribution of CERKL to retinal disorders, we surmise that, after this first study, additional families and new mutations will be readily described.
When we consider CERKL function, we should remember that SLs are structural membrane components, particularly abundant in neurons. SL accumulation is associated with severe neurodegenerative disorders (Gaucher, Tay-Sachs, and Niemann-Pick A and B diseases [Buccoliero and Futerman 2003]). Moreover, increasing evidence points to both nonphosphorylated and phosphorylated sphingosine and ceramide as essential second messengers in cellular stress, senescence, growth, and apoptosis (Luberto et al. 2002). SLs and their metabolic products act as biosensors of the cellular state, and the enzymes that regulate SL metabolism appear to be fine-tuned cellular switches, connecting several pathways with antagonistic properties (Hannun and Obeid 2002). Thus, ceramide and sphingosine lead to cell growth arrest and apoptosis, whereas sphingosine-1-phosphate and ceramide-1-phosphate have antiapoptotic and neuroprotective effects (Frago et al. 1998, 2003; Hannun and Obeid 2002; Spiegel and Milstien 2002, 2003). In this context, sensory neuropathies (hereditary sensory neuropathy type 1 [Dawkins et al. 2001]) and other neurodegenerative diseases (Batten disease [Puranam et al. 1999]) have recently been associated with ceramide metabolism enzyme deficiencies. It is puzzling that some key enzymes regulating intracellular ceramide levels have remained elusive (Hannun and Obeid 2002)—for example, the first ceramide kinase to be characterized was reported only recently (Sugiura et al. 2002). However, despite the short lapse of time, data on the function of ceramide-1-phosphate and ceramide kinase in several scenarios have increased, stressing the roles of the relative levels of ceramide and ceramide-1-phosphate as intracellular indicators for cell death/survival decisions (Frago et al. 2003) and adding a prominent role in the regulation/modulation of inflammatory responses (Pettus et al. 2003).
Photoreceptor neurodegeneration in RP has been reported to proceed by apoptosis triggered by cellular stress. However, none of the described genetic defects underlying inherited retinal dystrophies has provided a direct link to apoptosis. It is interesting that a very recent report showed that a decrease of the intracellular ceramide pools rescued photoreceptor degeneration in Drosophila mutants (Acharya et al. 2003). Ceramide may be produced in the photoreceptors in response to stress. In this case, deficiency of CERKL, a specific retinal ceramide kinase, would increase the ceramide pool or decrease the ceramide-1-phosphate levels, rendering the photoreceptor cells more susceptible to stress and, thus, more sensitive to apoptotic signals. The cumulative effects of this enhanced susceptibility could lead to progressive depletion of photoreceptors and, eventually, to blindness. Failure to process the ceramide to ceramide-1-phosphate may trigger cell death not only by increasing the endogenous ceramide pool but also by failing to activate the corresponding downstream signalling pathway. Whether CERKL is transcriptionally modulated in response to cellular stress stimuli remains an open question.
This study—to our knowledge, the first genetic report linking RP to ceramide-induced apoptosis—highlights the SL metabolism enzymes as new functional candidates and establishes novel targets for therapeutic intervention of retinal diseases.
Acknowledgments
We are indebted to the families with arRP who generously contributed to this study, particularly to all members of the P2 family and to Isabel Tejada from the Hospital General de Basurto (Bilbao, Spain) for providing the E1 family samples. This research followed the tenets of the Convention of Helsinki. We are grateful to Rebeca Valero and Olga Gonzàlez-Angulo, for technical help; to Susana Balcells, for valuable suggestions; and to Robin Rycroft, for revising the English. We thank the Serveis Científico-Tècnics (Universitat de Barcelona) for the sequencing and in situ hybridization facilities. This work was financed by Fundaluce (2001) and by Ministerio de Ciencia y Tecnología grant PM99-0168 (to R.G.-D.). M.T. was in receipt of a Formació d'Investigadors fellowship from the Generalitat de Catalunya.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- BCM Search Launcher, http://searchlauncher.bcm.tmc.edu/
- dbSNP Home Page, http://www.ncbi.nlm.nih.gov/SNP/ (for the new SNPs observed in the present study, which are deposited under the following identification numbers: ss12676100, ss12676101, ss12676102, ss12676103, ss12676104, ss12676105, ss12676106, ss12676107, ss12676108, ss12676109, ss12676110, ss12676111, ss12676112, ss12676113, and ss12676114)
- EMBnet BoxShade Server, http://www.ch.embnet.org/software/BOX_form.html
- GenBank, http://www.ncbi.nih.gov/Genbank/ (for truncated EST [accession number BE797822], partial cDNA [accession number BC020465], human SPHK1 [accession number AAF73423], murine SPHK1 [accession number AAC61697], human SPHK2 [accession number AAF74124], murine SPHK2 [accession number AAF74125], Drosophila SPHK [accession number AAF48045], C. intestinalis SPHK [accession number AK112588], yeast Lcb4p [accession number NP_014814], yeast Lcb5p [NP_013361], A. thaliana SPHK [accession number AY128394], human CERK [accession number AB079066], murine CERK [accession number AB079067], C. intestinalis CERK [accession number AK112750], Drosophila CERK [accession number AAF52040], human CERKL [accession number AY357073], murine CERKL [accession numbers BY742285 and BC046474], C. elegans CERK [accession number AAC67466], human DGKb [accession number Q9Y6T7], murine DGKb [accession number XP_147651], human DGKe [accession number NP_003638], murine DGKe [accession number NP_062378], C. elegans DGK1 [accession number NP_508190], A. thaliana DGK1 [accession number Q39017], Drosophila DGK2 [accession number Q09103], and Drosophila DGK1 [accession number Q01583])
- NCBI BLAST, http://www.ncbi.nlm.nih.gov/BLAST/
- NCBI Conserved Domain Database, http://www.ncbi.nih.gov/Structure/cdd/cdd.shtml
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for RP) [PubMed]
- RetNet, http://www.sph.uth.tmc.edu/RetNet/
- SNP Consortium, The, http://snp.cshl.org/
- UCSC Genome Bioinformatics Web Site, http://genome.ucsc.edu/
References
- Acharya U, Patel S, Koundakjian E, Nagashima K, Han X, Acharya JK (2003) Modulating sphingolipid biosynthetic pathway rescues photoreceptor degeneration. Science 299:1740–1743 10.1126/science.1080549 [DOI] [PubMed] [Google Scholar]
- Bayés M, Goldaracena B, Martínez-Mir A, Iragui-Madoz MI, Solans T, Chivelet P, Bussaglia E, Ramos-Arroyo MA, Baiget M, Vilageliu L, Balcells S, González-Duarte R, Grinberg D (1998) A new autosomal recessive retinitis pigmentosa locus maps on chromosome 2q31-q33. J Med Genet 35:141–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayés M, Martínez-Mir A, Valverde D, del Rio E, Vilageliu L, Grinberg D, Balcells S, Ayuso C, Baiget M, Gonzàlez-Duarte R (1996) Autosomal recessive retinitis pigmentosa in Spain: evaluation of four genes and two loci involved in the disease. Clin Genet 50:380–387 [DOI] [PubMed] [Google Scholar]
- Buccoliero R, Futerman AH (2003) The roles of ceramide and complex sphingolipids in neuronal cell function. Pharmacol Res 47:409–419 10.1016/S1043-6618(03)00049-5 [DOI] [PubMed] [Google Scholar]
- Collins JS, Schwartz CE (2002) Detecting polymorphisms and mutations in candidate genes. Am J Hum Genet 71:1251–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins JL, Hulme DJ, Brahmbhatt SB, Auer-Grumbach M, Nicholson GA (2001) Mutations in SPTLC1, encoding serine palmitoyltransferase, long chain base subunit-1, cause hereditary sensory neuropathy type I. Nat Genet 27:309–312 10.1038/85879 [DOI] [PubMed] [Google Scholar]
- Frago LM, Cañón S, de la Rosa EJ, León Y, Varela-Nieto I (2003) Programmed cell death in the developing inner ear is balanced by nerve growth factor and insulin-like growth factor I. J Cell Sci 116:475–486 10.1242/jcs.00223 [DOI] [PubMed] [Google Scholar]
- Frago LM, León Y, de la Rosa EJ, Gómez-Munoz A, Varela-Nieto I (1998) Nerve growth factor and ceramides modulate cell death in the early developing inner ear. J Cell Sci 111:549–556 [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM (2002) The ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem 277:25847–25850 10.1074/jbc.R200008200 [DOI] [PubMed] [Google Scholar]
- Hims MM, Diager SP, Inglehearn CF (2003) Retinitis pigmentosa: genes proteins and prospects. Dev Ophthalmol 37:109–125 [DOI] [PubMed] [Google Scholar]
- Luberto C, Kraveka JM, Hannun YA (2002) Ceramide regulation of apoptosis versus differentiation: a walk on a fine line. Lessons from neurobiology. Neurochem Res 27:609–617 10.1023/A:1020267831851 [DOI] [PubMed] [Google Scholar]
- Martínez-Mir A, Paloma E, Allikmets R, Ayuso C, del Rio T, Dean M, Vilageliu L, Gonzàlez-Duarte R, Balcells S (1998) Retinitis pigmentosa caused by a homozygous mutation in the Stargardt disease gene ABCR. Nat Genet 18:11–12 [DOI] [PubMed] [Google Scholar]
- Paloma E, Hjelmqvist L, Bayés M, García-Sandoval B, Ayuso C, Balcells S, Gonzàlez-Duarte R (2000) Novel mutations in the TULP1 gene causing autosomal recessive retinitis pigmentosa. Invest Ophthalmol Vis Sci 41:656–659 [PubMed] [Google Scholar]
- Paloma E, Martínez-Mir A, García-Sandoval B, Ayuso C, Vilageliu L, González-Duarte R, Balcells S (2002) Novel homozygous mutation in the alpha subunit of the rod cGMP gated channel (CNGA1) in two Spanish sibs affected with autosomal recessive retinitis pigmentosa. J Med Genet 39:E66 10.1136/jmg.39.10.e66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettus BJ, Bielawska A, Spiegel S, Roddy P, Hannun YA, Chalfant CE (2003) Ceramide kinase mediates cytokine and calcium ionophore-induced arachidonic acid release. J Biol Chem 278:38206–38213 10.1074/jbc.M304816200 [DOI] [PubMed] [Google Scholar]
- Puranam KL, Guo WX, Qian WH, Nikbakht K, Boustany RM (1999) CLN3 defines a novel antiapoptotic pathway operative in neurodegeneration and mediated by ceramide. Mol Genet Metab 66:294–308 10.1006/mgme.1999.2834 [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S (2002) Sphingosine 1-phosphate, a key cell signaling molecule. J Biol Chem 277:25851–25854 10.1074/jbc.R200007200 [DOI] [PubMed] [Google Scholar]
- ——— (2003) Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol 4:397–407 10.1038/nrm1103 [DOI] [PubMed] [Google Scholar]
- Sugiura M, Kono K, Liu H, Shimizugawa T, Minekura H, Spiegel S, Kohama T (2002) Ceramide kinase, a novel lipid kinase: molecular cloning and functional characterization. J Biol Chem 277:23294–23300 10.1074/jbc.M201535200 [DOI] [PubMed] [Google Scholar]
- Sullivan LS, Daiger SP (1996) Inherited retinal degeneration: exceptional genetic and clinical heterogeneity. Mol Med Today 2:380–386 10.1016/S1357-4310(96)10037-X [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniha F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882 10.1093/nar/25.24.4876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Chen Q, Zhao K, Wang L, Wang L, Traboulsi EI (2001) Update on the molecular genetics of retinitis pigmentosa. Ophthalmic Genet 22:133–154 10.1076/opge.22.3.133.2224 [DOI] [PubMed] [Google Scholar]