Abstract
Purpose
This is a single-center uncontrolled retrospective study to evaluate the efficacy and safety of the biosimilar epoetin zeta after approval in chemotherapy-induced anemia (CIA).
Methods
Patients screened were >18 years old suffering from solid malignancies and CIA with Hg ≤10 or <11 g/dl if symptomatic anemia. Patients had measurable disease by TNM and Eastern Cooperative Oncology Group (ECOG). Patients were treated for at least 12 weeks and the primary endpoint was to determine the incidence of blood transfusions, and secondarily, the overall safety and efficacy defined as ≥1 g/dl rise in Hb concentration or ≥40,000 cells/μl rise in reticulocyte count. Quality of life was assessed with ECOG performance status (PS) and functional assessment of cancer therapy-anemia (FACT-An) score.
Results
1287 patients with median Hb 9.3 g/dl (range 8.3–10.6) were enrolled and included in the evaluation. Median age was 63 years (range 33–78). 74% of patients were stage III/IV. Patients received epoetin zeta subcutaneously at fixed 40,000-IU once weekly. Blood transfusions were given in 178 patients (13.8%; 95% CI 11.9–15.6%). Appropriate response was observed in 79% patients by week 4, 87% by week 8, and 91% by week 12. A mean Hb increase of 2.5 g/dl was observed by week 12 which correlated with an improvement in PS and Fact-An score. Thrombotic events occurred in 5.2% (95% CI 3.4–7.1%) of patients.
Conclusions
Epoetin zeta is effective in palliation and treatment of CIA in patients with solid tumors. Overall, it is well tolerated and safe even in patients with increased disease burden.
Keywords: Biosimilar, Epoetin zeta, Erythropoietin, Chemotherapy, Anemia, Quality of life
Introduction
Cancer-related anemia (CRA) can be associated with the myelosuppressive effects of the malignancy itself, with chronic kidney disease or with chemotherapy, and has a negative impact on both therapeutic outcome and quality of life (QoL). The prevalence of CRA varies between 30 and 90% of all patients and depends on tumor type, staging, chemotherapy regimen, the number of treatment cycles, and the cut-off value of anemia (Knight et al. 2004). In the European Cancer Anaemia Survey (ECAS), during a 6-month follow-up of a heterogenous group of patients, anemia of Hb <12 and of <10 g/dl was detected at least once in 67 and 39.3% of patients, respectively. For the patients treated with chemotherapy, anemia was detected in total at least once in 75% of patients and specifically in 83.3% of patients with lung cancer and 88.3% of patients with gynecological cancer. In ECAS, anemia was reported in 19.5% of patients during the first chemotherapy cycle and in 46.7% during the fourth or fifth cycle (Ludwig et al. 2004; Gilreath et al. 2014).
Management of chemotherapy-induced anemia (CIA) includes dose de-escalation of chemotherapy, iron replacement, erythropoiesis-stimulating agents (ESA) with or without iron supplementation, and red blood cell transfusions. Despite FDA black-box warnings for important side-effects during treatment, ESAs remain an important component of our arsenal for treatment of CIA. However, ECAS still reported that less than 40% with anemia received appropriate treatment for anemia and only 17% were treated with an ESA (Ludwig et al. 2004). Patent expiration of original erythropoietin analogues has led to the emergence of agents that are similar to the originals but complex molecules, and are equivalent in efficacy and safety, while they offer significant cost savings (Aapro et al. 2012). These are called follow-on-protein products; by the Food and drug administration (FDA) or ‘biosimilars’ by the European Medicines Agency (EMA) (2010). Epoetin zeta (ζ; SB309; Retacrit®, Hospira Enterprises B.V., Hoofddorp, The Netherlands; Silapo®, STADA Arzneimittel AG, Bad Vilbel, Germany), a biosimilar with an amino acid sequence identical to epoetin alpha, has successfully penetrated the European market and is, since 2007, an approved biosimilar for use in CIA as per EMA regulation (Gascon 2015).
In general, a competent volume of clinical data on the efficacy and safety of biosimilars is lacking. More specifically, data on the effectiveness and safety of epoetin zeta in CIA are limited in a previous phase III study (Tzekova et al. 2009) and a recent observational study (Michallet et al. 2014; Michallet and Losem 2016) of patients with either hematologic or solid malignancies. As part of pharmacovigilance in practice and post-marketing surveillance, in this study, we investigate the safety and efficacy of subcutaneous epoetin zeta in the treatment of CIA in patients with solid tumors.
Patients and methods
Eligibility criteria
In this study, inclusion criteria demanded patients male or female older than 18 years with an at least 6 months overall life expectancy who had: suffered from solid tumors; indications for receiving chemotherapy with an expected duration of at least 8 weeks; anemia defined as ≤10 g/dl (6.2 mmol/l) or <11 g/dl if the anemia was symptomatic; Eastern Cooperative Oncology Group (ECOG) performance status 0–4; otherwise, adequate hematologic, liver, and renal function. Exclusion criteria were diagnosis of hematologic, myeloid, or lymphoid malignancy; previous known adverse effects or hypersensitivity to epoetin or epoetin zeta inactive excipients; history of venous thromboembolism within 6 months; uncontrolled hypertension; unstable angina or congestive heart failure; history of pure red blood cell aplasia or other suspected clinical manifestations of anti-EPO antibodies; patients who could not receive adequate antithrombotic prophylaxis if indicated; history of epilepsy or seizures; history of chronic liver or renal failure; untreated other causes of anemia, including hemolysis, blood loss, or absolute deficiencies of iron (ferritin <30 ng/ml; transferrin saturation <20%), folate, and vitamin B12; had blood transfusion within 4 weeks or ESA within 8 weeks prior to chemotherapy; had received radiation treatment within 4 months prior to chemotherapy; had active infection or inflammatory disease; had malnutrition defined as loss of ≥20% of the original body weight; and non-compliance with the treatment protocol. Withdrawal from the trial was also considered if a patient experienced unacceptable or severe toxicities/adverse effects (AEs). Informed written consent was obtained from all individuals after a thorough risk to benefit discussion for epoetin zeta treatment.
Study design
This study was a retrospective open-label, non-controlled, single/multiple dose, single-center clinical study (phase IV) of subcutaneous epoetin zeta administered once weekly in CIA of patients with solid tumors. The clinical hypothesis put to test was real-life clinical practice experience in efficacy and safety of epoetin zeta in this setting. The study period was of 12-week duration and all patients received epoetin zeta until 1 month after completion of chemotherapy. As a consequence, all patients would receive at least three 4-week cycles of epoetin zeta. This study and its protocol were approved by local institutional review boards, and it was conducted in full compliance with the Declaration of Helsinki for studies on human subjects.
Study endpoints
Primary endpoint was to assess the proportion of patients receiving a peripheral red blood cell (PRBC) transfusion despite optimal therapy with epoetin zeta in patients with solid tumors suffering from CIA. Secondary endpoints included the evaluation of efficacy, safety, tolerability, and QoL. Efficacy was determined based on the previous experience (Tzekova et al. 2009; Michallet et al. 2014) and on manufacturer’s instructions as the rise of Hb ≥1 g/dl or the increase of reticulocyte count ≥40,000 cells/μl within the first 8 weeks of treatment. Furthermore, the incidence of Hb levels >12 g/dl, the incidence of Hb rise ≥2 g/dl in a 28-day cycle (rapid increase), the time to achieve an appropriate response, and the fraction of patients who successfully maintained the response were determined throughout the study. Safety was determined as the incidence of serious thrombotic events (venous thromboembolism, deep vein thrombosis, myocardial infarction or pulmonary embolism leading to left or right heart failure, cerebrovascular accidents, and thrombotic microangiopathy); mortality within the 12 weeks of the protocol; other significant AEs, such as the occurrence of uncontrollable hypertension and pure red aplasia or emergence of anti-EPO antibodies. All toxicities and responses were evaluated according to the Eastern Cooperative Oncology Group (ECOG) criteria (Oken et al. 1982). Regarding thrombotic events and since this was a “real-life” study, we did not incorporate any increased screening detection method. Thus, we examined the patients as we usually do at predetermined intervals, and depending on patient symptomatology and signs of disease, we used further confirmatory testing. Tolerability was determined objectively by the medical practitioner at each visit and by the patient using subjective measures. Regular QoL assessment and palliation of symptoms with treatment is included as a part of standard patient care at our institution. QoL assessments were made by the ECOG performance status (PS) scale as well as the functional assessment of cancer therapy-anemia (FACT-An v4.0) (Cella and Webster 1997). We did not use the European Organization for Research and Treatment of Cancer (EORTC) QoL questionnaires as we did in our previous studies (Trafalis et al. 2012), since FACT-An (total score 0–188) is more exhaustive and includes a General scale (score 0–108) plus 20 questions specific to anemia (score 0–80) of which a 13-question fatigue-specific subscale (score 0–52) (Cella 2002).
Treatment protocol
Target range for Hb was 10–12 g/dl according to FDA and EMA safety warning on ESA treatment (Hedenus et al. 2014; Michallet and Losem 2016; Gilreath et al. 2014). Dosing was based in the previous experience and on manufacturer’s recommendation. The initial dose was fixed at 40,000 IU given subcutaneously once weekly. An appropriate response was defined as an at least 1 g/dl rise in Hb concentration or an at least 40,000 cells/μl rise in reticulocyte count above baseline after a cycle (4 weeks) of treatment. If the increase was <1 g/dl for Hb and <40,000 cells/μl, then an epoetin zeta was given for an additional 4 weeks at 300 IU/kg three times per week. If the increase was still inappropriate or the patient had strong indications for PRBC transfusion, the treatment would be discontinued. If the therapeutic target had been achieved, then the dose would be adjusted to maintain Hb between 10 and 12 g/dl. Reductions of 25–50% would be made to maintain Hb at that level and dose titration was strongly considered. If the rise was rapid (e.g., more than 2 g/dl per 4-week cycles), then the dose would be reduced by 25–50%. If the Hb concentration exceeded 12 g/dl, a dose or more would be held and then reinstituted at a 25% lower dose than the one previously given. Patients would receive epoetin zeta up to 4 weeks after completion of chemotherapy. No other ESA treatment was given concurrently. However, all patients received adequate iron supplementation in the form of 200–300 mg elemental iron per os daily or intravenous iron 25–100 mg per week. PRBC transfusion was allowed at the discretion of the investigator. There were no determined criteria to guide transfusions other than caring physician decision based on clinical symptomatology and comorbidities. Thus, patients may have had an optimal response to treatment, but clinical symptoms could necessitate for PRBC transfusion. Treatment failure was considered when the therapeutic target had not been achieved at any point of the study period.
Statistical methods
All statistical analyses were performed on the intention to treat (ITT) population and using last observation carried forward. Results regarding efficacy and safety are presented with 95% confidence intervals and Hb changes as mean ± standard error of the mean (SEM). Statistical analysis and graphic presentation were performed with GraphPad Prism v. 6 (GraphPad Software, La Jolla, CA, USA).
Results
Baseline patient characteristics
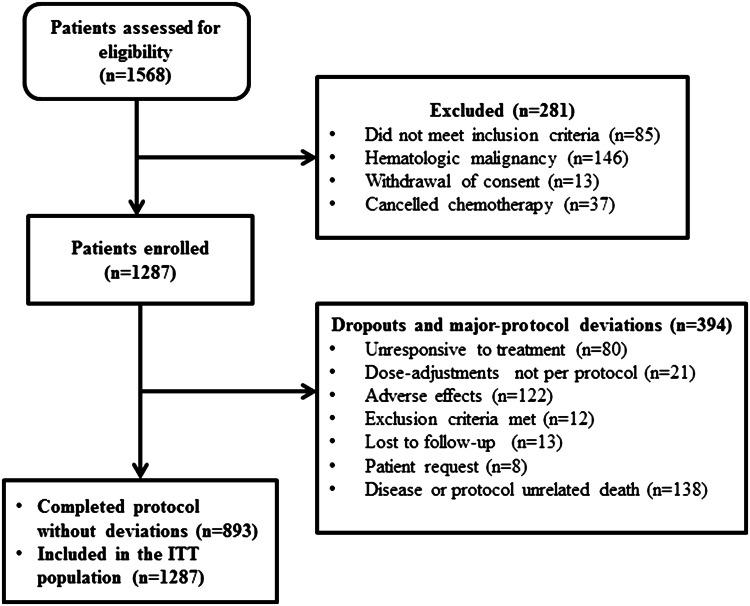
A total of 1568 patients were screened for the study between January 2010 and March 2015. Of those, 1287 were eligible for evaluation. All of the 1287 patients started treatment with epoetin zeta on intent to complete the 12 weeks of the protocol. Eight hundred and ninety three patients (69.4%) received at least 12 weeks of treatment. The reasons for patient withdrawal are depicted in Fig. 1. The rest of the patients’ baseline and demographic characteristics is shown in Table 1.
Fig. 1.
Patient flow chart. ITT intention to treat
Table 1.
Baseline patient characteristics
| Characteristics | Patients (n = 1287) |
|---|---|
| Age (mean), years | 67.9 |
| Median (range) | 63 (33–78) |
| Weight (kg) | |
| Median (range) | 72 (52–114) |
| Sex, n (%) | |
| Male | 807 (62.7) |
| Female | 480 (37.3) |
| Race/ethnicity (%) | |
| White | 100% |
| Other | – |
| ECOG PS, n (%) | |
| 0 | 377 (29.3) |
| 1 | 726 (56.4) |
| 2 | 170 (13.2) |
| 3 | 14 (1.1) |
| Smoking history, n (%) | |
| Former | 360 (28) |
| Current | 541 (42) |
| Never | 386 (30) |
| Time from the initial diagnosis (months) | |
| Median (range) | 3 (1–7) |
| Stage (%) | |
| I | 90 (7) |
| II | 245 (19) |
| III | 386 (30) |
| IV | 566 (44) |
| Prior treatment, n (%) | |
| Chemotherapy | 489 (38.1) |
| Radiotherapy | 293 (22.8) |
| Surgery | 958 (74.4) |
| Concomitant treatment besides chemotherapy, n (%) | |
| Radiotherapy | 282 (21.9) |
| Surgery | 0 |
| Other | – |
| Tumor | |
| Non-small cell lung carcinoma (NSCLC) | 438 (34) |
| Colorectal | 374 (29.1) |
| Breast | 308 (23.9) |
| Pancreatic, gastric, endometrial, cervical, ovarian, prostate, kidney, urothelial, H&N, esophageal, SCLC, neuroendocrine, sarcoma, other | 167 (<5) |
| Hb (g/dl) | |
| Median (range) | 9.3 (8.3–10.6) |
| Tf saturation (%) | |
| Median (range) | 41 (20–75) |
Efficacy
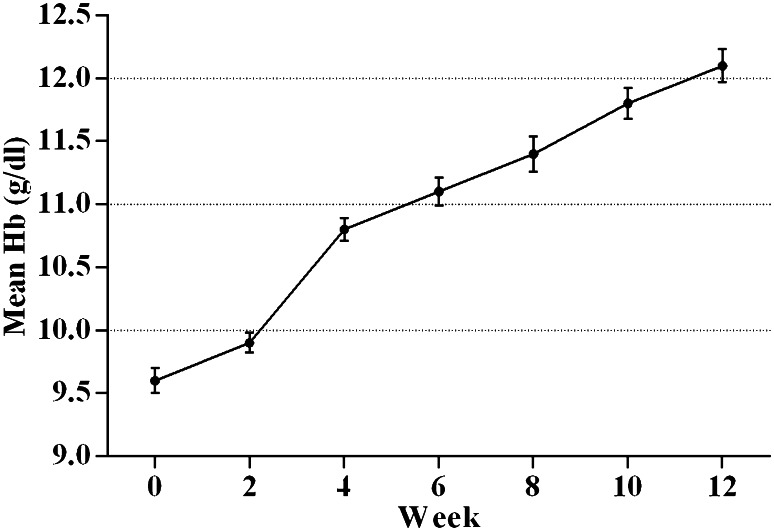
Between baseline and week 12, a statistically very significant increase of 2.5 ± 0.16 g/dl (mean ± SEM, p < 0.0001) in Hb was recorded in patients treated with epoetin zeta (Fig. 2). A total of 1016/1287 (79%) of patients achieved an appropriate Hb response >1.0 g/dl or an increase in reticulocyte count >40,000 cells/mL by week 4, and totally, 1120 out of 1287 (87%) had achieved response by week 8 and 93.8% by week 12. A ratio of 41% of patients achieved an Hb increase of >2.0 g/dl by week 8 and 87% by week 12. Epoetin zeta dose reductions occurred in 376 out of 1287 patients (29.2%) because of an excessive rise in Hb levels (>2 g/dl, or, in total >13.0 g/dl) within 4 weeks (95% CI 26.8–32.4%). For the majority of patients, 1107/1287 (86%), no blood transfusion was necessary. PRBC transfusions were performed in 178 (13.8%) of patients and more than one transfusions were given in 6.5% of those patients. The overall proportion of treatment failure that culminated in PRBC transfusion or other form of treatment was 6.2% between weeks 0 to 12 with a mean Hb level of 9.5 g/dl (range 7.5–10.3 g/dl).
Fig. 2.
Mean (±SEM) hemoglobin (Hb) levels from baseline (week 0) to week 12
Safety
All of the patients who received the study therapy (ITT) were included in the safety analysis. In total, 1248 AEs experienced by 452 patients (35.1%) of which 575 were severe AEs (SAEs, including thrombotic events) in 228 patients (17.8%), primarily during the first 8 weeks of treatment (72.4% of total AEs) (Table 2). Less frequent SAEs included pulmonary edema (0.02%) and acute heart failure (0.04%) all of which resolved without sequelae. Throughout the treatment period, 67/1287 patients (5.2%; 95% CI 3.4–7.1%) that received treatment with epoetin zeta experienced clinically significant or serious thrombotic events within the first 12 weeks of treatment (Table 3) Most of these AEs (1173/1248; 94%) were considered not to be treatment related. A possible causal relationship was reported for 14/1248 (1.1%) AEs and a probable causal relationship for 16/1248 (1.3%) AEs. 89% of SAEs were classified as “not related” to the study drug, 3.8% as “unlikely to be related”, and 7.1% as “possibly related”. SAEs that were linked to the epoetin zeta included the thrombotic events, an increase in blood pressure (1.8%), acute heart failure (0.02%), and pulmonary edema (0.01%) of which all resolved without any sequelae.
Table 2.
Adverse (AE) and serious adverse events (SAE) experienced during treatment with epoetin zeta (excluding thrombotic and vascular events)
| n, (%) | AE | SAE |
|---|---|---|
| Hematologic toxicities | ||
| Thrombocytosis | 1 (0.1) | – |
| Bleeding | 10 (0.8) | 4 (0.3) |
| Infections | 31 (2.4) | 17 (1.3) |
| General AEs | ||
| Fatigue/asthenia | 27 (2.1) | 4 (0.3) |
| Anorexia | 9 (0.7) | – |
| Headache | 33 (2.6) | 15 (1.2) |
| Dizziness | 48 (3.7) | 12 (0.9) |
| Arthralgias | 75 (5.8) | 23 (1.8) |
| Gastrointestinal AEs | ||
| Nausea/vomiting | 15 (1.2) | 1 (0.1) |
| Diarrhea/constipation | 19 (1.5) | 5 (0.4) |
| Cardiopulmonary AEs | ||
| Increase in blood pressure | 44 (3.4) | 40 (3.1) |
| Edema | 10 (0.8) | 4 (0.3) |
| Other | ||
| Non-specific rash | 6 (0.5) | – |
| Flu-like symptoms | 53 (4.1) | 4 (0.3) |
| Injection site reactions | 1 (0.1) | – |
| Total | 385 (29.9) | 162 (12.6) |
Table 3.
Clinically significant or serious thrombotic and vascular events that may be related to the treatment with epoetin zeta within the first 12 weeks of treatment
| Clinical adverse events | (N = 67) | Clinical outcomes |
|---|---|---|
| Myocardial ischemia | 4/65 (6.15%) | Resolved in 4/4 |
| Myocardial infarction | 4/65 (6.15%) | Resolved in 3/4; fatal in ¼ |
| Cerebral hemorrhage or cerebral infarction | 2/65 (3.0%) | Resolved in 2/2 |
| Transient ischemic attack | 6/65 (9.2%) | Resolved in 6/6 |
| Pulmonary embolism | 5/65 (7.7%) | Resolved in 5/5 |
| Aneurysms | 2/65 (3.0%) | Follow-up |
| Retinal thrombosis | 1/65 (1.5%) | Resolved in 1/1 |
| Deep vein thrombosis | 8/65 (12.3%) | Resolved in 6/8 |
| Inferior cava thrombosis | 4/65 (6.15%) | Resolved in 3/4 |
| Superior cava thrombosis | 2/65 (3.0%) | Resolved in 1/2 |
| Thrombophlebitis and other vascular disorders | 28/65 (43.1%) | Resolved in 23/28 |
Study treatment was withdrawn permanently from the 122/1287 patients (9.5%) owing to 426 AEs. No patients developed evidence of anti-erythropoietin antibodies at any point during the trial.
Tolerability
Subjective local administration tolerability as deemed by the patients at week 12 was excellent (85%) or very good (10%) or good (5%), and rated by the attending physicians as excellent (92%) or very good (4%) or good (4%). General tolerability was rated by patients as excellent (55%) or very good (20%) or good (10%), and was rated by the physicians as excellent (53%) or very good (8%) or good (22%).
Quality of life
Table 4 depicts the changes between weeks 0–12 for ECOG PS scores for the ITT group (last observation carried forward). ECOG score on week 12 was 0 in 46.8%, 1 in 34.3%, 2 in 8.9%, 3 in 2.3%, 4 in 1.6%, and 5 in 6.1% of patients, and specifically, it demonstrated a significant improvement in 23.8% of patients, remained stable in 60.6%, and exacerbated in 15.6% of patients during the study. Regarding the Fact-An score, the rate of completed questionnaires at baseline was 96% (1227/1287) and that in the end of the study was 94.5% (845/894). The mean baseline Fact-An score was 120.1 ± 25.3, and specifically, anemia subscale (ASS) and fatigue subscale (FSS) were 47.3 ± 15.1 and 29.2 ± 10.3, respectively. At the end of the study on week 12, mean Fact-An score was 131.2 ± 22.6 (p < 0.0001), ASS was 53.8 ± 11.2 (p < 0.0001), and FSS was 34.4 ± 9.7 (p < 0.0001). During the study period, 80 patients had died; however, none of the cases were associated with the study treatment.
Table 4.
ECOG PS scores in ITT group from baseline to week 12
| Baseline, n (%) | Week 12, n (%) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | ||
| 0 | 377 (29.3) | 333 (25.9) | 18 (1.4) | 9 (0.7) | 4 (0.3) | 1 (0.1) | 12 (0.9) |
| 1 | 726 (56.4) | 259 (20.1) | 395 (30.7) | 46 (3.6) | 9 (0.7) | 3 (0.2) | 14 (1.1) |
| 2 | 170 (13.2) | 10 (0.8) | 28 (2.2) | 50 (3.9) | 15 (1.2) | 17 (1.3) | 49 (3.8) |
| 3 | 14 (1.1) | 9 (0.7) | 1 (0.1) | 4 (0.3) | |||
Treatment protocol adjustments
Oral iron was supplemented in 100% of patients, IV iron was given in 9.7% of patients and folate or other vitamins were given in 11.3% of patients. In most instances, folate was given as supplementation to cytotoxic chemotherapy or as general nutritional supplements. Prophylactic antithrombotic treatment was given in 10.1% for other medical indications.
At week 0, the initial dose was 40,000 IU per week. Dose reductions were performed in 46.3% of patients due to satisfactory response, 9.5% patients were withdrawn due to AEs, and in 6.5% of patients due to inefficacy.
Discussion
In this study, we demonstrated that once-weekly epoetin zeta over 12 weeks significantly increased Hb levels in patients with solid tumors and CIA while significantly reducing the need for transfusion and with an acceptable safety profile. The dosing strategy was in line to the previous studies of epoetin zeta and in accord to the manufacturer’s recommendations. The previous studies of ESAs for CIA have produced significant positive results in treating anemia, reducing PRBC transfusion requirements and an improvement of QoL. In this study, the transfusion requirements were thus lower than expected. However, since 2007, there have been a number of concerning reports of severe side-effects regarding their use in patients.
Of the 1287 patients enrolled, 394 patients dropped out and did not complete the protocol, as shown in Fig. 1. This event is expected in patients dealing with advanced cancer where fatigue and weakness or rapid disease progression lead to a culminant phase which inhibits them from receiving further palliative treatment. Furthermore, ESA by its nature takes an important amount of time to take an effect over several weeks and has no effect in more than 30% of patients with CIA (Rizzo et al. 2010).
The reported incidence of thrombotic events with the use of darbopoetin, epoetin alfa, and epoetin beta in randomized controlled trials is estimated between 0 and 30% with a median of 4.5% and a relative risk of 1.67 (95% CI 1.35–2.06) (Bohlius et al. 2006; Rosenzweig et al. 2004). However, these studies were designed before a regulatory notification by FDA and EMA and there is not a post-hoc analysis available in accord to the revised target Hb levels. In this study, the incidence of thrombotic events was 5.2%. The definition of thrombotic event was as broad as possible to avoid bias in favor to the epoetin zeta. In the 2007 study by Tzekova et al., the incidence was 4.2% (95% CI 1.9–7.8%), suggesting that epoetin zeta may not be associated with a more increased thombotic rate when compared to ESAs. In another trial (441-54-04-46-0000) of SB309 in 216 patients with CIA, the incidence of significant thrombotic events was 3.9% within 12 weeks of treatment (Abraham and MacDonald 2012). In the recent ORHEO study (Michallet et al. 2014), the rate of thromboembolic events in 12 weeks was 2.4% and in 24 weeks 3.74% in patients with solid tumors. Therefore, we recorded similar thromboembolic events compared to historical control in this setting, even though the population constituted of 30% stage III and 44% stage IV disease. In the previous studies, epoetin alfa caused severe AEs in 48% of patients with CIA (Witzig et al. 2005). In this study of epoetin zeta, 457 (35.1%) of patients experienced an AE, 228 (17.7%) patients suffered from a SAE, and 122 (9.5%) had to drop out of the protocol due to SAEs. However, only 2.1% of SAEs were likely related to epoetin zeta. This compares favorably to the previous reports of Tzekova et al. (2009) which reported an AE in 91 patients (42%), SAEs in 42 patients where 2.9% probably related to epoetin zeta, and 32% did not complete the protocol due to AEs. In the 441-54-04-46-0000 trials, 84/208 patients experienced 163 AEs during the 12-week treatment of which 65 were severe; however, probably 2.5% and another 1.2% possibly were linked to epoetin zeta (EMA 2007). In the ORHEO study, 17.1% of patients developed at least one significant AE.
In this study, a significant continuous increase was observed during the 12-week treatment. The previous data on epoetin alfa demonstrated efficacy in Hb increase by 1.8 g/dl within 10 weeks (Cortesi et al. 2005) to 3.3 g/dl within 12–24 weeks (Littlewood et al. 2001). In the Tzekova et al. study (2009), a Hb response >11.5 g/dl by week 12 was seen in 65/100 (65%) of patients, an appropriate response was seen in 80% of patients by week 4 and in 96% of patients by week 8. 87% of patients achieved a Hb response >2 g/dl by week 8 in our study, whereas in Tzekova et al. (2009), this occurred in 82% of patients. In the ORHEO study, the mean increase in Hb from inclusion to week 12 was 1.44 g/dl in the subgroup of solid tumors, and overall, 81.6% of patients had appropriate response on week 12. In the Tzekova trial (2009), 23% of patients received a PRBC transfusion and it occurred once in 10.6% of patients, and more for 6% of patients. In the ORHEO study, in total, 9.4% had at least one transfusion and at least 9.7% for solid tumors which was lower to that of this study. The data in this study are not comparative to those of the ORHEO study, but their result was used as reference, since it is the first post-EMA approval study for epoetin zeta with post-hoc results on solid tumors. The relatively low percentage of PRBC transfusions observed can be attributed to the fact that there is reluctancy in prescribing transfusion, even though a liberal strategy was implemented. In general, there is a trend to observe anemia until symptoms become to affect the QoL.
Functional iron deficiency often arises after continued erythropoietin use. Thus, iron supplementation will eventually be required in most patients to maintain erythropoiesis (Rodgers et al. 1992). In our study, oral iron was, indeed, supplemented in 100% of patients, whereas IV iron was given in 9.7% of patients (almost 1 in 10). Although many studies show the superiority of intravenous over oral administration of iron in functional iron deficiency anemia (Henry et al. 2007; Steensma et al. 2011), we try to avoid intravenous administrations for multiple reasons. First, intravenous iron must be given in the hospital setting, and second, long-term increases in morbidity (Beare and Steward 1996), infection, cardiovascular morbidity, and thromboembolic episodes have not been thoroughly explored. Specifically, we prefer not to administer IV iron on the same day with anthracyclines to avoid possible cardiotoxicity (Minotti et al. 2004) or under low neutrophil cell counts to avoid increased infection rates (Bullen et al. 2005; Litton et al. 2013). Thus, we aim to administer oral iron in all outpatients and if needed intravenous iron can be given (in our study this occurred in about 10% of patients). It is unclear if oral iron supplementation necessarily decreased the need for intravenous iron supplementation or if it allowed a decrease in epoetin zeta requirements. However, oral iron supplementation when compared to intravenous, although it demonstrates less effective erythropoietic response, it may, however, decrease the need for transfusions (Mhaskar et al. 2016). Further studies are needed to elucidate this area of treatment.
Anemia in CIA and cancer patients in general is considered a main determinant of QoL decrement and is associated with comorbidities, such as perceived fatigue, pain, sleep, and depression, and it is suggested that Hb levels correlate with performance status (Narayanan and Koshy 2009). In the ORHEO study, in patients with solid tumors, around 34% of patients had improved and about 25% had exacerbated ECOG scores. In the Tzekova trial (2009), the ECOG score, along with the described CLAS scale, it demonstrated an improvement in the levels of energy, the daily activities’ performance, and overall QoL. However, in comparison to our study, the fatigue and anemia were not specifically assessed. As we described earlier, both the FSS and ASS improved which specifically underscore the palliative effect of the treatment of CIA.
Conclusion
Our study was not controlled and included patients from one medical center. The number of patients included was large and the design was uniform with patients of only solid malignancies. With this observational study, it is clear that subcutaneous weekly epoetin zeta is an effective and safe treatment for CIA in patients with solid tumors who are undergoing chemotherapy and are at risk of transfusion if a judicious use of epoetin zeta is made according to FDA and EMA regulations. The incidence of overall side-effects is clearly reduced and the cost: benefit ratio weighs in favor of ESA use. This was a “real-world” study that incorporated patients with a high percentage of stage III/IV and thus having significant underlying disease and tumor burden. Despite this fact, we noticed a comparable to historic data risk of AEs and thrombotic events. Furthermore, there was a significant efficacy of epoetin zeta in rapidly increasing and maintaining Hb which reduced unnecessary PRBC transfusions especially in these patients with advanced stage cancer. The QoL is improved in a significant number of patients which demonstrates the importance of palliation of symptoms in this population. Taking it all together, epoetin zeta may be a cost effective treatment in terms of clinical benefit and financial cost (Aapro et al. 2012; Nikolaidi et al. 2013). Further real-world controlled studies comparing epoetin zeta to epoetin alfa are required to better quantify the risk over benefit ratio in CIA and to approve its use for CIA by FDA.
Compliance with ethical standards
Conflict of interest
We declare that we have no conflict of interest. This study was not sponsored.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Aapro M, Cornes P, Sun S, Abraham I (2012) Comparative cost efficiency across the European G5 countries of originators and a biosimilar erythropoiesis-stimulating agent to manage chemotherapy-induced anemia in patients with cancer. Ther Adv Med Oncol 4:95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham I, MacDonald K (2012) Clinical safety of biosimilar recombinant human erythropoietins. Expert Opin Drug Saf 11:819–840 [DOI] [PubMed] [Google Scholar]
- Beare S, Steward WP (1996) Plasma free iron and chemotherapy toxicity. Lancet 347:342–343 [DOI] [PubMed] [Google Scholar]
- Bohlius J, Wilson J, Seidenfeld J (2006) Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst 98:708–714 [DOI] [PubMed] [Google Scholar]
- Bullen JJ, Rogers HJ, Spalding PB, Ward CG (2005) Iron and infection: the heart of the matter. FEMS Immunol Med Microbiol 43:325–330 [DOI] [PubMed] [Google Scholar]
- Cella D (2002) The effects of anemia and anemia treatment on the quality of life of people with cancer. Oncology (Williston Park) 16:125–132 [PubMed] [Google Scholar]
- Cella D, Webster K (1997) Linking outcomes management to quality-of-life measurement. Oncology (Williston Park) 11:232–235 [PubMed] [Google Scholar]
- Cortesi E, Gascón P, Henry D, Littlewood T, Milroy R, Pronzato P, Reinhardt U, Shasha D, Thatcher N, Wilkinson P (2005) Standard of care for cancer-related anemia: improving hemoglobin levels and quality of life. Int Soc Cell 68:22–32 [DOI] [PubMed] [Google Scholar]
- European Medicines Agency (EMA) (2007) EPAR for Silapo, Annex I: summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000760/WC500050914.pdf. Accessed 10 Jan 2017
- Gascon P (2015) The evolving role of biosimilars in hematology-oncology: a practical perspective. Ther Adv Hematol 6:267–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilreath JA, Stenehjem DD, Rodgers GM (2014) Diagnosis and treatment of cancer-related anemia. Am J Hematol 89:203–212 [DOI] [PubMed] [Google Scholar]
- Hedenus M, Ludwig H, Henry DH, Gasal E (2014) Pharmacovigilance in practice: erythropoiesis-stimulating agents. Cancer Med 3:1416–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry DH, Dahl NV, Auerbach M, Tchekmedyian S, Laufman LR (2007) Intravenous ferric gluconate significantly improves response to epoetin alfa versus oral iron or no iron in anemic patients with cancer receiving chemotherapy. Oncologist 12(2):231–242 [DOI] [PubMed] [Google Scholar]
- Knight K, Wade S, Balducci L (2004) Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med 116:11S-26S [DOI] [PubMed] [Google Scholar]
- Littlewood TJ, Bajetta E, Nortier JW, Vercammen E, Rapoport B, Epoetin Alfa Study Group (2001) Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: results of a randomized, double-blind, placebo controlled trial. J Clin Oncol 19:2865–2874 [DOI] [PubMed] [Google Scholar]
- Litton E, Xiao J, Ho KM (2013) Safety and efficacy of intravenous iron therapy in reducing requirement for allogeneic blood transfusion: systematic review and meta-analysis of randomized clinical trials. BMJ 347:f4822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig H, Van Belle S, Barrett-Lee P, Birgegård G, Bokemeyer C, Gascón P, Kosmidis P, Krzakowski M, Nortier J, Olmi P, Schneider M, Schrijvers D (2004) The European Cancer Anaemia survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer 40:2293–2306 [DOI] [PubMed] [Google Scholar]
- Mhaskar R, Wao H, Miladinovic B, Kumar A, Djulbegovic B (2016) The role of iron in the management of chemotherapy-induced anemia in cancer patients receiving erythropoiesis-stimulating agents. Cochrane Database Syst Rev 2:CD009624. doi:10.1002/14651858.CD009624.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michallet M, Losem C (2016) Biosimilar Epoetin Zeta in oncology and haematology: development and experience following 6 years of use. Acta Haematol 135:44–52 [DOI] [PubMed] [Google Scholar]
- Michallet M, Luporsi E, Soubeyran P, Amar NA, Boulanger V, Carreiro M, Dourthe LM, Labourey JL, Lepille D, Maloisel F, Mouysset JL, Nahon S, Narciso B, Nouyrigat P, Radji R, Sakek N, Albrand H, ORHEO Study Group (2014) BiOsimilaRs in the management of anaemia secondary to chemotherapy in HaEmatology and Oncology: results of the ORHEO observational study. BMC Cancer 10(14):503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L (2004) Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56(2):185–229 [DOI] [PubMed] [Google Scholar]
- Narayanan V, Koshy C (2009) Fatigue in cancer: a review of literature. Indian J Palliat Care 15:19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaidi E, Hatzikou M, Geitona M (2013) Budget impact analysis on erythropoiesis-stimulating agents use for the management of chemotherapy-induced anemia in Greece. Cost Eff Resour Alloc 11:16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655 [PubMed] [Google Scholar]
- Rizzo JD, Brouwers M, Hurley P, Seidenfeld J, Arcasoy MO, Spivak JL, Bennett CL, Bohlius J, Evanchuk D, Goode MJ, Jakubowski AA, Regan DH, Somerfield MR, American Society of Clinical Oncology, American Society of Hematology (2010) American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update on the use of epoetin and darbopoetin in adult patients with cancer. J Clin Oncol 28:4996–5010 [DOI] [PubMed] [Google Scholar]
- Rodgers GM 3rd, Becker PS, Blinder M, Cella D, Chanan-Khan A, Cleeland C, Coccia PF, Djulbegovic B, Gilreath JA, Kraut EH, Matulonis UA, Millenson MM, Reinke D, Rosenthal J, Schwartz RN, Soff G, Stein RS, Vlahovic G, Weir AB 3rd (1992) Cancer- and chemotherapy-induced anemia. J Natl Compr Canc Netw 10(5):628–653 [DOI] [PubMed] [Google Scholar]
- Rosenzweig MQ, Bender CM, Lucke JP, Yasko JM, Brufsky AM (2004) The decision to prematurely terminate a trial of R-HuEPO due to thrombotic events. J Pain Symptom Manag 27:185–189 [DOI] [PubMed] [Google Scholar]
- Steensma DP, Sloan JA, Dakhil SR, Dalton R, Kahanic SP, Prager DJ, Stella PJ, Rowland KM Jr, Novotny PJ, Loprinzi CL (2011) Phase III, randomized study of the effects of parenteral iron, oral iron, or no iron supplementation on the erythropoietic response to darbepoetin alfa for patients with chemotherapy-associated anemia. J Clin Oncol 29(1):97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafalis DT, Alifieris C, Krikelis D, Tzogkas N, Stathopoulos GP, Athanassiou AE, Sitaras NM (2012) Topotecan and pegylated liposomal doxorubicin combination as palliative treatment in patients with pretreated advanced malignant pleural mesothelioma. Int J Clin Pharmacol Ther 50:490–499 [DOI] [PubMed] [Google Scholar]
- Tzekova V, Mihaylov G, Elezovic I, Koytchev, Epoetin Zeta Oncology Study Group (2009) Therapeutic effects of epoietin zeta in the treatment of chemotherapy-induced anaemia. Curr Med Res Opin 25:1689–1697 [DOI] [PubMed] [Google Scholar]
- Witzig TE, Silberstein PT, Loprinzi CL (2005) Phase III, randomized, double-blind study of epoetin alfa compared with placebo in anemic patients receiving chemotherapy. J Clin Oncol 23:2606–2617 [DOI] [PubMed] [Google Scholar]