Abstract
Purpose
The homeobox B8 (HOXB8) functions as a sequence-specific transcription factor that is involved in development. Increased expression of this gene is associated with a wide variety of tumor; however, its function in gastric cancer has not been clarified. In the present study, the expression of HOXB8 in gastric cancer tissues and influence of HOXB8 on gastric cancer cellular were evaluated.
Methods
The expression levels of HOXB8 mRNA in human gastric cancer tissues were analyzed through quantitative RT-PCR. To test the role of HOXB8 in gastric cancer metastasis, the cell transwell assay was performed. Microarray, ChIP-qPCR, and Western blot were used to explore the possible mechanism that HOXB8 promotes gastric cancer cells metastasis.
Results
In this study, we found that HOXB8 showed higher expression in metastatic tissues than no-metastatic tissues. Overexpression of HOXB8 can promote gastric cancer cells migration and invasion, while silencing HOXB8 leads to the opposite results. Overexpression of HOXB8 also increases the rate of metastasis in NCI-N87 mice, while silencing HOXB8 has the opposite results. Furthermore, HOXB8 promotes epithelial–mesenchymal transformation of AGS cells. We also found that ZEB2 can interact with HOXB8 and may be a downstream factor of HOXB8 by using microarray. Knockdown of ZEB2 can inhibit HOXB8-induced migration and invasion capacity, as well as the epithelial–mesenchymal transformation in gastric cancer cells.
Conclusions
The results showed that HOXB8 plays an important role in the development and metastasis of gastric carcinoma.
Keywords: HOXB8, Gastric cancer, Epithelial–mesenchymal transition, ZEB2
Background
Gastric cancer is one of the most popular cancers in the world (Chen et al. 2016a; Siegel et al. 2015). In recent years, with increasing awareness and treatment of gastric cancer, the incidence and the mortality rate has declined, but gastric cancer is still third leading cause of cancer death in the world (Chen et al. 2016b; Siegel et al. 2013, 2015). And in China, the incidence and mortality of gastric cancer rank the third in all human tumors. The current opinion in development of gastric cancer is interactions between considered risk factors and the host.
HOX genes, also known as I type homeobox gene, are a kind of evolutionarily highly conserved multigene family, which widely present in almost all eukaryotic cells (Haria and Naora 2013). So far, 39 Human HOX genes have been identified, which is divided into Hoxa, Hoxb, Hoxc, Hoxd four clusters, located on 7p15, 17p21, 12q13, and 2q31 chromosomes in turn (Bhattacharjee et al. 2015; Haria and Naora 2013). HOX gene encoding protein is a class of homeotic transcription factor domain in vertebrate embryonic development, which plays an important role in controlling characteristics of physical qualities, regulating the central nervous system, and so on (Chen et al. 2009). HOX homology region expression proteins play roles through its DNA-binding activity and its downstream gene transcription activity (Yang et al. 2009). HOX gene abnormal expression may lead abnormal morphology in tissue and organ formation process and the formation of malignant tumor. In recent years, many studies have shown that HOX genes play a very important role in the occurrence development, incursions, and metastasis of many tumors, such as colorectal cancer, breast cancer, prostate cancer, lung cancer, glioblastoma, thyroid tumors, ovarian cancer, bladder cancer, kidney cancer, and melanoma (Haria and Naora 2013).
HOXB8 is a member of HOX family, which is located on 17 chromosomes (Fujino et al. 2001). Recent studies found the abnormal expression of HOXB8 in ovarian serous cancer, and HOXB8 is correlated with the occurrence and development of ovarian serous cancer (Stavnes et al. 2013). HOXB8 regulates expression of microRNAs to control cell death and differentiation (Salmanidis et al. 2013). However, the clinical role of HOXB8 in gastric cancers is to date unknown.
In this study, we found that HOXB8 showed higher expression in metastatic tissues than no-metastatic tissues. Overexpression of HOXB8 promotes gastric cancer cells migration and invasion, while silencing HOXB8 leads to the opposite results. Furthermore, HOXB8 promotes epithelial–mesenchymal transformation of AGS cells. We also found that HOXB8 can interact with ZEB2 and which may be a downstream factor of HOXB8 by using microarray. Knockdown of ZEB2 can inhibit HOXB8-induced migration and invasion capacity, as well as the epithelial–mesenchymal transformation in gastric cancer cells. The results showed that HOXB8 plays an important role in the development and metastasis of gastric carcinoma.
Materials and methods
Chemicals and antibodies
Lipofectamine 2000 transfection and TRIZOL LS Reagents were purchased from Invitrogen (Grand Island, NY, USA). Antibodies against HOXB8 and ZEB2 were purchased from Abcam (Cambridge, MA, USA). E-cadherin, fibronectin, N-cadherin, vimentin, and β-actin antibodies were from Cell Signaling technology (Danvers, MA, USA). Anti-α-catenin antibody was from BD (Franklin Lakes, NJ, USA). Unless otherwise noted, all other chemicals were from Sigma (St. Louis, MO, USA).
Patients and specimens
A total of 102 tumor tissues which were used for qRT-PCR and immunohistochemical analysis were randomly collected from AGS patients who underwent curative resection with informed consent between 2007 and 2010 at the Xin Hua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. All tissues were collected immediately upon resection of the tumors in the operation theater, transported in liquid nitrogen, and then stored at −80 °C. Tumor staging was based on the 6th edition of the tumor node metastasis (TNM) classification of the International Union Against Cancer. Study protocols were approved by the Xin Hua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, and written informed consent was obtained from patients based on the Declaration of Helsinki.
Histological analysis
The lungs dissected from mice were fixed in 4 % paraformaldehyde in phosphate-buffered saline (PBS) overnight and subsequently embedded in paraffin wax. Sections cut at a thickness of 4 μm were stained with hematoxylin and eosin for histological analysis.
Cell culture
AGS, MKN28, and NCI-N87 cell lines were got from ATCC (Manassas, VA, USA) and were cultured using 10 % fetal bovine serum (Cat#10099-141, Invitrogen, Carlsbad, CA) in RPMI-1640 (Cat#C11875, Invitrogen). GES-1 cell lines were cultured using 10 % fetal bovine serum (Invitrogen) in Dulbecco’s modified Eagle medium (Cat#C11965, Invitrogen). Cell culture was made according to the manufacturer’s protocol. All the cell lines were grown at 37 °C in a 5 % CO2/95 % air atmosphere and were revived every 3–4 months.
Establishment of stable expression and knockdown cell lines
Retroviral construct containing human pBabe-HOXB8 cDNA and pSuper with shRNA against human HOXB8 and siZEB2s was prepared as described previously (Wang et al. 2014). The generation of retrovirus supernatants and transfection of gastric carcinoma cells were conducted as described previously (Wang et al. 2014). The expression of HOXB8 and ZEB2 was confirmed by qRT-PCR and Western blotting analysis.
Cell invasion and motility assay
Invasion of cells was measured in Matrigel (BD, Franklin Lakes, NJ, USA)-coated Transwell inserts (6.5 mm, Costar, Manassas, VA, USA) containing polycarbonate filters with 8-μm pores as detailed previously (Fu et al. 2010). The inserts were coated with 50 μl of 1 mg/ml Matrigel matrix according to the manufacturer’s recommendations. 2 × 105 cells in 200 μl of serum-free medium were plated in the upper chamber, whereas 600 μl of medium with 10 % fatal bovine serum was added to lower well. After 24-h incubation, cells that migrated to the lower surface of the membrane were fixed and stained. For each membrane, five random fields were counted at 10× magnification. Motility assays were similar to Matrigel invasion assay except that the Transwell insert was not coated with Matrigel.
Western blotting
Cells were lysed in lysis buffer, and total protein contents were determined by the Bradford method. 30 μg of lysis was separated by reducing SDS-PAGE and probed with specific antibodies. Blots were washed and probed with respective secondary peroxidase-conjugated antibodies, and the bands visualized by chemoluminescence (Amersham Biosciences).
qRT-PCR
Total RNA was extracted using Trizol reagent, and cDNA was synthesized using SuperScript II Reverse Transcriptase (Invitrogen). qRT-PCR and data collection were performed with an ABI PRISM 7900HT sequence detection system. The primers used for the amplification of the indicated genes are available upon request.
Gene expression profiling
Total RNA quality and quantity were determined using Agilent 2100 Bioanalyzer and NanoDrop ND-1000. Affymetrix HU U133 plus 2.0 arrays were used according to the manufacturer’s protocol. The data were initially normalized by robust multiarray average (RMA) normalization algorithms in expression console software (Affymetrix). Significantly altered genes between HOXB8 knockdown and its control cells were considered by scatter plots, and the genes up- and downregulated ≥5-fold. Clustering analysis was done using gene list by Gene Cluster v3.0 software, and heat maps were visualized using Java TreeView v1.1.4r3 software. Gene set enrichment analysis was carried out using ConceptGen. Gene sets were either obtained from the ConceptGen or from published gene signatures.
Chromatin immunoprecipitation (ChIP)-qPCR
Chromatin Immunoprecipitation kit (Cat. 17-371) was purchased from Millipore, and ChIP experiments were carried out essentially as described (Ang et al. 2011). Immunoprecipitated DNA was analyzed on the ABI PRISM 7900HT sequence detection system. The primers used for detection of promoters after ChIP are available upon request.
In vivo tumor metastasis
Nude mice were purchased from the Shanghai Slac Laboratory Animal Co. Ltd and maintained in microisolator cages. All animals were used in accordance with institutional guidelines, and the current experiments were approved by the Use Committee for Animal Care. For metastasis assays, cells were suspended in PBS at a concentration of 1 × 107 cells ml−1. Cell suspension (0.1 ml) was injected into tail veins of nude mice. All of the mice were killed by CO2 60 days after inoculation.
Statistical analysis
Data were described as the mean ± SD. Association between ZEB2 and HOXB8 expression in AGS tissues was assessed using Spearman’s rank correlation test. Comparisons between different groups were undertaken using the Student’s two-tailed t test. The statistical significance of the differences between mean values was determined by P < 0.05. Statistical analysis was done with SPSS/Win11.0 software (SPSS, Inc., Chicago, IL, USA).
Results
HOXB8 is overexpressed in malignant cancers
Compared with normal gastric mucosa cells, HOXB8 showed significant high mRNA expression in gastric cancer organizations (Fig. 1a). And the mRNA expression level of HOXB8 was significantly correlated with distant metastasis (Fig. 1b). In gastric cancer cells, the protein and mRNA level of HOXB8 was trice higher than that in normal gastric cancer cells (Fig. 2a–c). Notably, the level of HOXB8 in invasive cancer cells is about 1.5 times higher than that in noninvasive cancer cells (Fig. 2b, c).
Fig. 1.
HOXB8 is overexpressed in gastric cancer tissues and correlated with metastasis. a HOXB8 expression in gastric cancer tissues was analyzed by qRT-PCR. b HOXB8 expression in no-metastatic and metastatic gastric cancer tissues was analyzed and compared. P < 0.001 in panel B based on the Student’s t test. Error bars SD
Fig. 2.
HOXD9 expression is correlated with metastasis of gastric cancer cells. a HOXB8 expression in gastric cancer cell lines was analyzed by Western blotting. b Quantitative chart of HOXB8 protein level in gastric cancer cell lines. c HOXB8 expression in gastric cancer cell lines was analyzed by qRT-PCR. P < 0.001 in b and c based on the Student’s t test. Error bars SD
HOXB8 promotes migration and invasion capacity of gastric cancer cells
Silencing of HOXB8 in gastric cancer cells was retroviral established using NCI-N87 cell line, designated as NCI-N87-shHOXB8 #1, #2, #3. The levels of HOXB8 in these cells were verified on protein and mRNA levels (Fig. 3a, b). To evaluate the effects of HOXB8 on migration and invasion in gastric cancer cells, transwell assay and Matrigel assay were carried out. Silencing HOXB8 can significantly reduce the numbers of cells migrating through the membrane to the bottom of the aperture (Fig. 3c). To detect the function of HOXB8 in distant metastasis in vivo, NCI-N87-shHOXB8#2 and its corresponding control cells were injected into nude mice through the tail vein. Silencing HOXB8 significantly decreased the number of mice with distant metastasis (Fig. 4a). In addition, less metastasis foci in gastric cancer cells (Fig. 4b, c) were counted in each mouse injected with gastric cancer cells silencing HOXB8.
Fig. 3.
Silencing HOXB8 in gastric cancer cells decreases the migration and invasion capacity of gastric cancer cells. a Western Blot analysis of HOXB8 levels in the NCI-N87 established cell lines. b qRT-PCR analysis of HOXB8 levels in the NCI-N87 established cell lines. c Gastric cancer cells with silent expression of HOXB8 possessed less invaded abilities in transwell and Matrigel assay. P < 0.01 is based on the Student’s t test. Error bars SD
Fig. 4.
Silencing HOXB8 in NCI-N87 cell decreases the rate of tumor metastasis in vivo. a Less number of mice with distant metastasis was found in mouse injected with shHOX8-NCI-N87 cells. b, c Less metastasis foci in gastric cancer cells were counted in each mouse injected with shHOX8-NCI-N87 cells. P < 0.01 is based on the Student’s t test. Error bars SD
Stable overexpression of HOXB8 in gastric cancer cells was retroviral established using AGS cell line. The levels of HOXB8 in these cells were verified on protein and mRNA levels (Fig. 5a, b). To evaluate the effects of HOXB8 on migration and invasion in gastric cancer cells, transwell assay and Matrigel assay were carried out. The numbers of cell with high-expression HOXB8 was 3 times than cells with empty vector (Fig. 5c). To detect the function of HOXB8 in distant metastasis in vivo, AGS-HOXB8 and its corresponding control cells were injected into nude mice through the tail vein. Overexpression of HOXB8 increased the number of metastatic mice (Fig. 6a) and metastatic foci in gastric cancer cells (Fig. 6b, c). These results revealed that HOXB8 promotes migration and invasion of gastric cancer cells.
Fig. 5.
Overexpression of HOXB8 in gastric cancer cells increases the migration and invasion capacity of gastric cancer cells. a Western Blot analysis of HOXB8 levels in the AGS established cell lines. b qRT-PCR analysis of HOXB8 levels in the AGS established cell lines. c Gastric cancer cells with high expression of HOXB8 possessed stronger invaded abilities in transwell and Matrigel assay. P < 0.01 is based on the Student’s t test. Error bars SD
Fig. 6.
Overexpression of HOXB8 in AGS cell increases the rate of tumor metastasis in vivo. a More number of mice with distant metastasis was found in mouse injected with AGS-HOXB8 cells. b, c More metastasis foci in gastric cancer cells were counted in each mouse injected with AGS-HOXB8 cells. P < 0.01 is based on the Student’s t test. Error bars SD
HOXB8 regulates the transition between epithelial and mesenchymal phenotypes in gastric cancer cells
Expression level of protein markers of EMT was assessed to evaluate the relationship between HOXB8 and EMT. In the HOXB8 silencing cell lines, upregulation of epithelial cell marker (E-cadherin and α-catenin) and downregulation of mesenchymal cell markers (N-cadherin, fibronectin, and vimentin) were detected by Western blot (Fig. 7a) and qRT-PCR (Fig. 7b) assays. Also, overexpression of HOXB8 increased the mesenchymal cell markers and decreased the epithelial cell markers significantly (Fig. 8a, b). These results indicated that HOXB8 play important roles in the transition of epithelial and mesenchymal.
Fig. 7.
Silencing HOXB8 in NCI-N87 inhibits epithelial–mesenchymal transition. a Western blot analysis showed that silencing HOXB8 causes the epithelial cell markers (E-cadherin and α-catenin) upregulated and mesenchymal cell markers (N-cadherin, fibronectin, and vimentin) downregulated. b qRT-PCR analysis showed that silencing HOXB8 causes the epithelial cell markers upregulated and mesenchymal cell markers downregulated. P < 0.01 is based on the Student’s t test. Error bars SD
Fig. 8.
Overexpression of HOXB8 in AGS promotes epithelial–mesenchymal transition. a Western blot analysis showed that silencing HOXB8 causes the epithelial cell markers (E-cadherin and α-catenin) downregulated and mesenchymal cell markers (N-cadherin, fibronectin, and vimentin) upregulated. b qRT-PCR analysis showed that silencing HOXB8 causes the epithelial cell markers downregulated and mesenchymal cell markers upregulated. P < 0.01 is based on the Student’s t test. Error bars SD
HOXB8 can regulate the expression of ZEB2
To better understand the mechanisms by which HOXB8 engaged in the development and progression of gastric cancer cell, microarray assay was performed using cell line NCI-N87-shHOXB8 #2 and its control cells with empty vector. Microarray results indicated that a list of genes significantly differentially expressed after HOXB8 silencing (Fig. 9a). Furthermore, gene set enrichment analysis indicated that ZEB2-related gene signatures were significantly enriched in HOXB8 knockdown cells (Fig. 9b), supporting the idea that HOXB8 regulates EMT and cancer invasion and metastasis and which may be mediated by ZEB2.
Fig. 9.
HOXB8 regulates the expression level of ZEB2. a Clustering of the genes differentially expressed after silencing HOXB8. b The enrichment scores of differential expressing genes in HOXB8 silencing cell line
To further verify the relationship between HOXB8 and ZEB2, we detect the expression level of ZEB2 in gastric cancer cell lines constructed above using Western blot and qRT-PCR. In cell lines with HOXB8 silencing, the expression level of ZEB2 decreased significantly compared with control vector cells (Fig. 10a, b), while overexpression HOXB8 can increase the ZEB expression in protein and mRNA level (Fig. 10c, d). To recognize any clinical correlation of HOXB8 and ZEB2, we analyzed ZEB2 mRNA expression in the same human AGS tissues. Highly positive correlation between HOXB8 and ZEB2 expression was drawn and similar to HOXB8 expression (Fig. 10e).
Fig. 10.
ZEB2 expression was affected by HOXB8. The ZEB2 expression levels in HOXB8 silencing cell line were assayed by Western blot (a) and qRT-PCR (b). Detecting ZEB2 expression levels in HOXB8 overexpression cell line by using Western blot (c) and qRT-PCR (d). e ZEB2 expression was positively correlated with HOXB8 expression in gastric cancer tissue arrays. P < 0.01 is based on the Student’s t test. Error bars SD
To detect whether HOXB8 regulates ZEB2 on the transcriptional level, regulation of ZEB2 gene promoter activity by changing HOXB8 expression in NCI-N87 and AGS cells was analyzed by luciferase assay. The results show that silencing HOXB8 expression in NCI-N87 decreases the ZEB2 gene promoter activity (Fig. 11a), while ectopic expression of HOXB8 in AGS cells significantly increases the ZEB2 gene promoter activity (Fig. 11b). Quantitative chromatin immunoprecipitation (qChIP) assays were performed in MAGS97H-shHOXB8, Huh7-HOXB8, and theirs control cells. Antibodies against HOXB8 and IgG were used to pull down the chromatin complex, and three pairs of primers against the ZEB2 gene promoter region (#1, #2, and #2) were used to assess the occupancy of the ZEB2 gene promoter (Fig. 11c). Silencing HOXB8 expression was associated with decreased levels at #1 and #2 of the ZEB2 gene promoter region in NCI-N87-shHOXB8s cells (Fig. 11d, e). More occupancy of those ZEB2 gene promoter regions by HOXB8 was detected in AGS-HOXB8 cells (Fig. 11f, g). These results clearly indicate that HOXB8 induces transcriptional activation of ZEB2 expression through increasing HOXB8 to the ZEB2 gene promoter in AGS cells.
Fig. 11.
HOXB8 binding to the promoter of ZEB2 were assayed by ChIP-qPCR. a Regulation of ZEB2 gene promoter activity by knocking down of HOXB8 expression in NCI-N87 cells was analyzed by luciferase assay in NCI-N87 cells. b Regulation of ZEB2 gene promoter activity overexpression of HOXB8 in AGS cells was analyzed by luciferase assay. c Schematic diagram of ZEB2 promoter region. d qChIP was performed in HOXB8 silencing cell lines. f qChIP was performed in HOXB8 overexpression cell lines. e, g IgG was used as negative control. P < 0.01 is based on the Student’s t test. Error bars SD
ZEB2 is a mediator for HOXB8-induced migration, invasion, and EMT in AGS cells
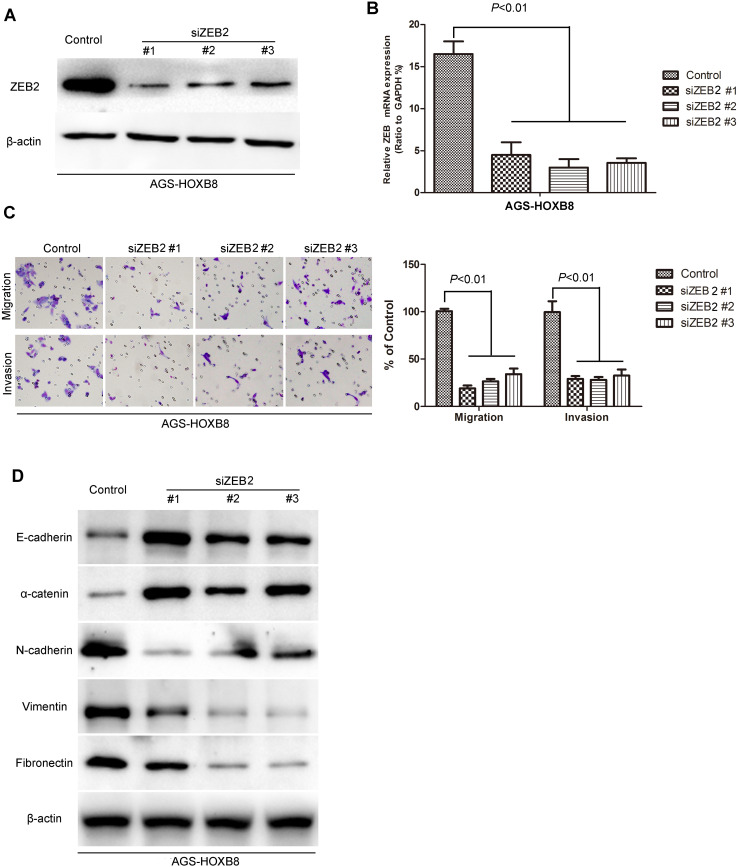
To test whether HOXB8-induced metastatic capacity was mediated by ZEB2, siRNAs were used to silence ZEB2 gene expression by virally transfecting AGS-HOXB8 cells with 3 distinct ZEB2 siRNAs and 3 cell lines with ZEB2 silencing were verified by Western blot and qRT-PCR (Fig. 12a, b). Knockdown of ZEB2 decreased the migration and invasion capacity of AGS-HOXB8 cells (Fig. 12c). Epithelial markers, E-cadherin and α-catenin, were increased while mesenchymal markers, N-cadherin, vimentin, and fibronectin, were decreased (Fig. 12d), indicating the transformation from mesenchymal to epithelial. Taken together, these results show that ZEB2 mediates HOXB8-induced EMT, migration, and invasion in AGS cells.
Fig. 12.
Silencing ZEB2 in AGS-HOXB8 cells decreases the migration and invasion capacity of gastric cancer cells. a Western Blot analysis of ZEB2 levels in the established AGS-HOXB8 cells. b qRT-PCR analysis of ZEB2 levels in the established AGS-HOXB8 cells. c Silencing ZEB2 reverses the HOXB8-induced migration and invasion in AGS-HOXB8 cells. d Silencing ZEB2 reverses the HOXB8-induced EMT markers change in AGS-HOXB8 cells. P < 0.01 is based on the Student’s t test. Error bars SD
Discussion
To our knowledge, this is the first study to show that HOXB8 plays a functional role in gastric cancer EMT and distant metastasis. Overexpression of HOXB8 in gastric cancer cells promotes the EMT, migration, invasion and EMT in vitro, and increases metastatic capacities in vivo. On the contrary, silencing of HOXB8 reversed these events in otherwise aggressive and invasive AGS cells. Microarray data showed that HOXB8 affects the expression of ZEB2 in gastric cancer cells. Silencing ZEB2 in HOXB8 overexpression cell lines resulted in the similar phenomenon caused by HOXB8 knockdown.
Our results suggested the expression of HOXB8 in gastric cancer cells was higher than that in normal cells. Previous study has shown that abnormal expression of HOX genes in tumor tissues, for example, some studies found that overexpression of HOX genes can enhance tumor cell proliferation (Hsu et al. 2013; Liu et al. 2015b). For example, HoxA9, HoxB8, and HoxB9 were overexpressed in some tumors, and HoxB2, HoxB13, and HoxD8 were low expressed in some tumors (Haria and Naora 2013). Abnormal expression of HOX family members such as HoxA6, HoxA13, HoxB2, HoxB4, HoxB5, HoxB6 is appeared in breast cancer (Haria and Naora 2013). In conclusion, HOXB8 may associate closely with the occurrence and development of cancer. In this study, the other evidence that HOXB8 functions as an oncogene in cancer cells was the effects on cell migration, invasion, and metastasis. Silencing HOXB8 can significantly reduce the migration and invasion ability of cancer cells in vitro. Also, it weakened the metastasis ability of cancers in vivo. Overexpression of HOXB8 confers the cell opposite performance. Interestingly, our study points to a novel function of HOXB8 in gastric cancer cells metastasis through regulating EMT.
The epithelial–mesenchymal transition (EMT) is a process by which epithelial cells lose their cell polarity and cell–cell adhesion, and gain migratory and invasive properties to become mesenchymal stem cells; these are multipotent stromal cells that can differentiate into a variety of cell types (Liang et al. 2016; Wang et al. 2014). EMT is essential for numerous developmental processes including mesoderm formation and neural tube formation (Liang et al. 2016; Liu et al. 2015a). The happening of EMT accompanied by the altered expression of molecular markers; thus, EMT can be determined by detecting the expression of these molecular markers (Grigore et al. 2016; Nantajit et al. 2015). Different expression of HOXB8 caused the different cell status. Overexpression of HOXB8 in gastric cancer cells promotes the transformation from epithelial to mesenchymal. Our results also indicate that HOXB8 not only promotes EMT, but silencing HOXB8 also leads to MET. This observation suggests that EMT-MET is a fluid process. High expression of HOXB8 also caused increased numbers of distant metastases in vivo. This phenomenon is consistent with the previous theory that EMT is essential for tumor cells to disseminate from adjacent tissues and seed new tumors in distant sites. These results indicate that HOXB8 affects the cells’ migration and invasion via regulating EMT progress.
To investigate the mechanism of HOXB8 regulating the development of cancer, microarray was carried out. We identified zinc finger E-box-binding homeobox 2, short for ZEB2, as an effective mediator of these HOXB8-induced phenomena. Silencing ZEB2 in HOXB8 overexpression cell lines resulted in the similar phenomenon caused by HOXB8 knockdown. It has been proved that the ZEB2 protein is a transcription factor that plays a role in the transforming growth factor β (TGF-β) signaling pathways that are essential during early fetal development (Techasen et al. 2014; Zhang et al. 2013). It is reasonable that HOXB8 involves in the development of AGS cells through the ZEB2 transcription factor.
In conclusion, we report that upregulation of HOXB8 might be correlated with gastric cancer development. HOXB8-mediated induction of EMT is ZEB2-dependent, and inhibition of HOXB8 results in induction of a mesenchymal phenotype. These findings suggest that HOXB8 activation may play an important role in promoting ZEB2 in gastric cancer, which may shed light to potential new targets in gastric cancer prevention and therapy.
Acknowledgments
Authors’ contributions
WJD extracted mRNA, performed qRT-PCR and WB analyses, and drafted the manuscript. CYQ assisted with data interpretation, designed the project, secured the funding, and drafted the manuscript. MZ and MMC provided patient samples and clinicopathological data. WJD assisted with data interpretation and revised the manuscript. CYQ provided patient samples and clinicopathological data, assisted with data interpretation, and revised the manuscript. All authors have read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
All the authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional and/or National Research Committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Funding
This study was funded by National Natural Science Foundation of China (No. 81400610); The Project-sponsored by SRF for ROCS, SEM (No. 20144802); Program of Shanghai Municipal Commission of Health and Family Planning for Youth (No. 20134Y043).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Wen-Jin Ding and Min Zhou have contributed equally to this work.
References
- Ang YS et al (2011) Wdr5 mediates self-renewal and reprogramming via the embryonic stem cell core transcriptional network. Cell 145:183–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A, Ghangal R, Garg R, Jain M (2015) Genome-wide analysis of homeobox gene family in legumes: identification, gene duplication and expression profiling. PLoS ONE 10:e0119198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SK, Kurdyukov S, Kereszt A, Wang XD, Gresshoff PM, Rose RJ (2009) The association of homeobox gene expression with stem cell formation and morphogenesis in cultured Medicago truncatula. Planta 230:827–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WM et al. (2016a) Expression of Helios in gastric tumor cells predicts better survival in gastric cancer patients. J Cancer Res Clin Oncol 142(11):2375–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y et al (2016) SOX2 inhibits metastasis in gastric cancer. J Cancer Res Clin Oncol 142:1221–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J et al (2010) Ubiquitin ligase cullin 7 induces epithelial–mesenchymal transition in human choriocarcinoma cells. J Biol Chem 285:10870–10879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino T, Yamazaki Y, Largaespada DA, Jenkins NA, Copeland NG, Hirokawa K, Nakamura T (2001) Inhibition of myeloid differentiation by Hoxa9, Hoxb8, and Meis homeobox genes. Exp Hematol 29:856–863 [DOI] [PubMed] [Google Scholar]
- Grigore AD, Jolly MK, Jia D, Farach-Carson MC, Levine H (2016) Tumor Budding: The Name is EMT. Partial EMT. J Clin Med. doi:10.3390/jcm5050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haria D, Naora H (2013) Homeobox gene deregulation: impact on the hallmarks of cancer. Cancer Hallm 1:67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SH et al (2013) Proinflammatory homeobox gene, ISX, regulates tumor growth and survival in hepatocellular carcinoma. Cancer Res 73:508–518 [DOI] [PubMed] [Google Scholar]
- Liang L, Sun H, Zhang W, Zhang M, Yang X, Kuang R, Zheng H (2016) Meta-analysis of EMT datasets reveals different types of EMT. PLoS ONE 11:e0156839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhang X, Li J, Sun B, Qian H, Yin Z (2015a) The biological and clinical importance of epithelial–mesenchymal transition in circulating tumor cells. J Cancer Res Clin Oncol 141:189–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Zhu Y, Yuan HX, Zhang JP, Guo JM, Lin ZM (2015b) Overexpression of HOXC11 homeobox gene in clear cell renal cell carcinoma induces cellular proliferation and is associated with poor prognosis. Tumour Biol 36:2821–2829 [DOI] [PubMed] [Google Scholar]
- Nantajit D, Lin D, Li JJ (2015) The network of epithelial–mesenchymal transition: potential new targets for tumor resistance. J Cancer Res Clin Oncol 141:1697–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmanidis M et al (2013) Hoxb8 regulates expression of microRNAs to control cell death and differentiation. Cell Death Differ 20:1370–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63:11–30 [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65:5–29 [DOI] [PubMed] [Google Scholar]
- Stavnes HT et al (2013) HOXB8 expression in ovarian serous carcinoma effusions is associated with shorter survival. Gynecol Oncol 129:358–363 [DOI] [PubMed] [Google Scholar]
- Techasen A, Namwat N, Loilome W, Duangkumpha K, Puapairoj A, Saya H, Yongvanit P (2014) Tumor necrosis factor-alpha modulates epithelial mesenchymal transition mediators ZEB2 and S100A4 to promote cholangiocarcinoma progression. J Hepato-biliary Pancreat Sci 21:703–711 [DOI] [PubMed] [Google Scholar]
- Wang Y et al (2014) CUL4A induces epithelial–mesenchymal transition and promotes cancer metastasis by regulating ZEB1 expression. Cancer Res 74:520–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YC et al (2009) A tumorigenic homeobox (HOX) gene expressing human gastric cell line derived from putative gastric stem cell. Eur J Gastroenterol Hepatol 21:1016–1023 [DOI] [PubMed] [Google Scholar]
- Zhang H et al (2013) KLF8 involves in TGF-beta-induced EMT and promotes invasion and migration in gastric cancer cells. J Cancer Res Clin Oncol 139:1033–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]