Abstract
Purpose
To investigate the effect of race and sex on long-term survival of oral and oropharyngeal cancer.
Methods
The Surveillance, Epidemiology and End Results database was queried for adult oral and oropharyngeal cancer patients with at least 25-year follow-up. Kaplan–Meier survival curves and cox proportional hazards model were used to identify differences.
Results
Of the 22,162 patients identified, 70.3 % were males. Only 8.9 % were alive at 25 years post-diagnosis. Black males show the poorest overall and disease-specific survival rates (p < 0.001). After controlling for covariates, Blacks had a 40 % higher hazard of mortality compared with Whites (HR 1.40, 95 % CI 1.35–1.46), while females had a 9 % reduction in mortality risk (HR 0.91, 95 % CI 0.88–0.94).
Conclusions
Overall and disease-specific survival is poor for oral and oropharyngeal cancer patients, and Black men fare worst. This illustrates the need for long-term cancer survival plans incorporating disparity effects in overall cancer outcomes.
Keywords: Racial and sex disparities, Oral cavity cancer, Oropharyngeal cancer, Long-term survival, Outcomes
Introduction
Oral cavity and oropharyngeal cancer (OCOPC) is found in the lip, tongue, mouth, and the oropharynx (Warnakulasuriya 2009), and it is one of the top 8 commonly diagnosed cancers among males in the USA (American Cancer Society 2015). OCOPC accounts for 3 % of all new cases of cancer in the USA, and it is estimated that in 2015 there will be 45,780 new cases resulting in 8650 deaths (American Cancer Society 2015). The major causal factors associated with OCOPC include tobacco, alcohol and human papillomavirus (HPV) (Neville and Day 2002; American Cancer Society 2015). While tobacco- and alcohol-associated OCOPC are decreasing, HPV-associated head and neck cancer, especially oropharyngeal cancer, has increased by approximately 225 % since the 1980s (D’Souza and Dempsey 2011; Malloy et al. 2013; Marur et al. 2010).
OCOPC has poorer 5-year relative survival rates (all stage) when compared with other commonly diagnosed cancers such as prostate, breast, colorectal, and cervical cancers (American Cancer Society 2015). Blacks fare worse than Whites in 5-year relative survival (45 vs. 67 %) (American Cancer Society 2015), and Black males have higher age-adjusted incidence rates than females for cancer of the oral cavity and pharynx (Goodwin et al. 2008).
Although OCOPC survival rates are relatively poor, survival has gradually increased in the last two decades (Barker et al. 2005; Jemal et al. 2010; Lin et al. 2014; Pulte and Brenner 2010). In 2013, there were at least 280,763 (185,242 males and 95,521 females) head and neck cancer survivors in the USA (de Moor et al. 2013). Some factors that have contributed to the changing oropharyngeal and oral cavity cancer survival landscape include the increasing incidence in HPV-associated head and neck cancer, improvement in treatment modalities, and the shift in age and profile of survivors from sixth or seventh generation to much younger individuals (Chaturvedi et al. 2011; Deschler et al. 2014; Lin et al. 2014; Patel et al. 2011; Pulte and Brenner 2010; Schantz and Yu 2002). However, the increased survival of oral cavity and oropharyngeal cancer is also associated with long-term sequelae, which include several toxicities associated with treatment modalities, structural, functional, and esthetic compromise, and other quality of life issues (Barker and Barker 2001; Epstein et al. 2012; Nicolatou-Galitis et al. 2011; Vickery et al. 2003). This is excluding significant costs associated with managing complications (de Moor et al. 2013; de Souza and Seiwert 2014).
The growing number of OCOPC survivors, as well as the unintended outcomes of their cancer treatments, necessitates longer-term surveillance than the commonly reported 5- to 10-year survival rates (Tiwana et al. 2014). Increasing survival rates creates the need for surveillance, survivorship plan, and management of late treatment complications and comorbidities, and longer-term survival rates may provide a better prognostic estimate of overall survival. There is dearth of information about longer-term disparities associated with survival of OCOPC, even though a major focus of public health service in the USA has been health disparities, exemplified by the Healthy People 2020 overarching objective of eliminating health disparities (Services; Tiwana et al. 2014).
The objective of this study is to describe disparities in long-term survival after diagnosis of OCOPC, stratified by race and sex. We hypothesize that early disease-specific survival racial and sex disparities observed in other studies will persist over long-term follow-up.
Methods
Data were obtained from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program’s database with a focus on sex and racial disparities for oral and oropharyngeal cancers. We selected oral and oropharyngeal cancer cases diagnosed between 1975 and 1986 to assure that each patient had the opportunity to be followed for at least 25 years from time of cancer diagnosis, since the most complete SEER database followed patients until 2011. Sites were identified based on the SEER site recode ICD-O-3/WHO 2008 variable which corresponds to ICD-O-3 site codes: C000–C029, C030–C069, C090–C109, C140, C142, and C148. Using the SEER categorization system tongue includes both oral tongue and tongue base. Cases were not selected based on histologic subtype. The sites we included are: lip, tongue, floor of mouth, gum and other mouth, tonsil, oropharynx, and other oral cavity and pharynx. We excluded nasopharynx and hypopharynx from our analysis. There are organizations such as the American Cancer Society (http://www.cancer.gov/types/head-and-neck/patient/oral-prevention-pdq#section/_7) and The Oral Cancer Foundation (http://oralcancerfoundation.org/cdc/cdc_chapter1.php) that group oral cavity and oropharynx together and do not include nasopharynx and hypopharynx. For this study, we chose to group oral cavity and oropharynx; while these are two separate subsites within the head and neck, they share some common features including risk factors and the opportunity for transoral screening. Although some organizations group oral cavity and oropharynx with these other pharyngeal sites (including nasopharynx and hypopharynx) due to anatomic proximity, we excluded them because nasopharyngeal and hypopharyngeal cancers typically have distinct clinical presentations and epidemiology.
Records required pathologically proven cancers to be included and records limited to data from death certificates only were excluded. We also excluded patients <18 years old.
From the SEER 18 database (1973–2012), we selected states with the SEER 9 records so that trends in survival could be followed for a longer follow-up in the same cohort. Race was defined as White, Black, and Other (American Indian/AK Native, Asian/Pacific Islander, and unknown) categories. The “Other” group was used rather than more specific groupings to allow for examination of long-term survival trends.
Data abstracted included age, cancer site, marital status, treatment type, and county-level demographics. A socioeconomic status index variable was created using a composite of three county-level variables available in the SEER database based on methods employed by Du et al. (2006) and others (Danzig et al. 2014; Robert et al. 2004): specifically, (1) poverty percentage, (2) median family income, and (3) percentage of high school graduates. The variables were separated into quintiles and coded so that the highest quintile represented the highest SES. The three variables were then combined into a SES Index variable representing lowest to highest SES. We also abstracted data on observed survival and disease-specific survival.
Statistical analyses were completed using SPSS version 22.0 (IBM SPSS Statistics for Windows 2013). Kaplan–Meier curves were produced to examine differences in survival (overall and disease specific) based on race and sex. The log-rank test was used to determine whether there were significant differences between groups. In an effort to evaluate possible mechanisms of sex and racial disparities, a Cox proportional hazards survival analysis was performed using the following variables: sex, race, age, marital status, cancer site, and treatment (radiation and/or surgery), stage (local, regional, distant), and the SES Index variable. Potential variables were evaluated in univariate analysis and then significant variables were included in the multivariate analysis. Statistical significance was set at α = 0.05.
Results
Participant characteristics
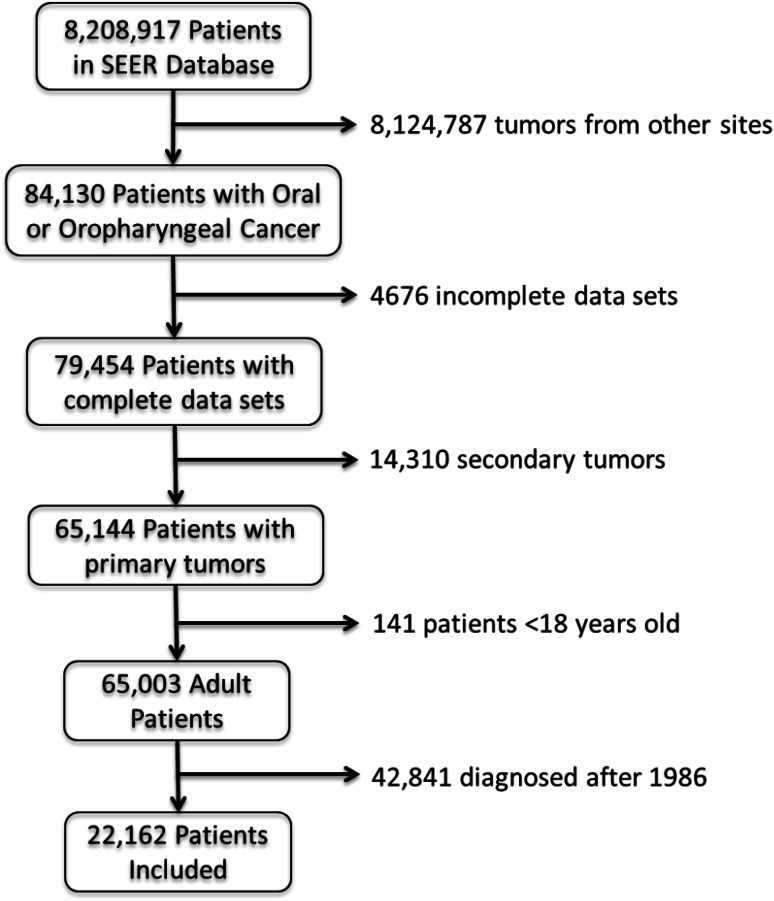
Our cohort included 22,162 cancer patients (Fig. 1); the majority were males (70.3 %) and minority were Blacks (8.9 %). Most of the cohort was married at the time of diagnosis (60.4 %). The most common cancer sites were tongue (24.0 %), lip (21.0 %), and gum/other mouth (20.7 %). The majority were treated with surgery (64.3 %), while just under half (47.1 %) had radiation as part of their treatment regimen (Table 1).
Fig. 1.
Patient selection flowchart. This figure describes how sample population was derived from the SEER cancer database, using the inclusion and exclusion criteria
Table 1.
Population characteristics
| Patient characteristic | Entire cohort, n = 22,162 | White, n = 19,628 (88.6 %) | Black, n = 1964 (8.9 %) | Other, n = 570 (2.6) |
|---|---|---|---|---|
| Age, mean (SD) (min–max 19–99) | 62.50 (12.33) | 63.15 (12.22) | 56.34 (10.82) | 61.27 (14.68) |
| Sex [n (%)] | ||||
| Male | 15,580 (70.3) | 13,798 (70.3) | 1415 (72.0) | 367 (64.4) |
| Female | 6582 (29.7) | 5830 (29.7) | 549 (28.0) | 203 (35.6) |
| Marital status | ||||
| Single, never married | 2226 (10.9) | 1741 (9.6) | 419 (23.3) | 66 (12.7) |
| Married | 12,362 (60.4) | 11,404 (62.8) | 639 (35.6) | 319 (61.6) |
| Divorced or separated | 2641 (12.9) | 2187 (12.0) | 415 (23.1) | 39 (7.5) |
| Widowed | 3236 (15.8) | 2820 (15.5) | 322 (17.9) | 94 (18.1) |
| Cancer site | ||||
| Tongue | 5322 (24.0) | 4581 (23.3) | 544 (27.7) | 197 (34.6) |
| Oropharynx | 723 (3.3) | 633 (3.2) | 76 (3.9) | 14 (2.5) |
| Floor of mouth | 3299 (14.9) | 2871 (14.6) | 338 (17.2) | 90 (15.8) |
| Gum and other mouth | 4588 (20.7) | 3967 (20.2) | 462 (23.5) | 159 (27.9) |
| Lip | 4662 (21.0) | 4592 (23.4) | 25 (1.3) | 45 (7.9) |
| Tonsil | 2699 (12.2) | 2243 (11.4) | 403 (20.5) | 53 (9.3) |
| Other | 869 (3.9) | 741 (3.8) | 116 (5.9) | 12 (2.1) |
| Surgery, yes | 14,242 (64.3) | 12,920 (65.8) | 973 (49.5) | 349 (61.2) |
| Radiation, yes | 10,210 (47.1) | 8725 (45.3) | 1200 (65.1) | 285 (50.3) |
Overall survival and racial disparities
The 10-year overall survival rate was 30.8 % and continued to decline throughout the period of follow-up, with only 8.9 % still alive at 25 years post-diagnosis and treatment. Figures 2 and 3 show the overall and disease-specific survival, respectively, and racial survival disparities were observed in patients diagnosed between 1975 and 1986. Overall observed survival curves show lower survival rates among Black males compared with all other racial groups, log rank χ 2 = 542.43, p < 0.001 (Fig. 2). There was also a significant difference in disease-specific survival, log rank χ 2 = 661.35, p < 0.001 (Fig. 3). Specific survival rates by race can be seen in Table 2, survival rates by race and sex are presented in Table 3.
Fig. 2.
Overall survival by race and sex. This figure is a survival curve showing overall survival of patients. Survival curve for Black females eventually converges with White males and females around the 20-year mark. For males, the curve fails to converge even at the 25-year mark
Fig. 3.
Disease-specific survival. This figure is a survival curve showing oral cavity and oropharyngeal cancer disease-specific survival. Curves indicate the disparities that exist between Blacks, both male and females, versus Whites. Black males fare worse
Table 2.
Survival rates (% surviving) by race and sex
| Race | 2 years (%) | 5 years (%) | 10 years (%) | 15 years (%) | 20 years (%) | 25 years (%) |
|---|---|---|---|---|---|---|
| White | 65.2 | 48.1 | 32.0 | 21.2 | 13.9 | 9.1 |
| White male | 65.6 | 48.0 | 31.7 | 21.0 | 13.6 | 8.9 |
| White female | 64.2 | 48.6 | 32.6 | 21.8 | 14.6 | 9.6 |
| Black | 45.4 | 28.2 | 16.7 | 9.4 | 6.3 | 4.0 |
| Black male | 41.7 | 23.7 | 12.9 | 6.9 | 4.0 | 2.4 |
| Black female | 55.2 | 39.9 | 26.4 | 15.7 | 12.2 | 8.3 |
| Other | 62.8 | 53.2 | 40.2 | 31.9 | 24.0 | 19.6 |
| Other male | 59.9 | 49.2 | 35.2 | 26.6 | 19.2 | 15.1 |
| Other female | 67.9 | 60.5 | 49.4 | 41.1 | 33.0 | 27.9 |
Table 3.
Multivariate cox regression analysis
| Patient characteristic | Overall mortality | Disease-specific mortality | ||
|---|---|---|---|---|
| HR (95 % CI)a | p value | HR (95 % CI)a | p value | |
| Race | ||||
| White (ref) | – | – | – | – |
| Black | 1.43 (1.39–1.48) | <0.001 | 1.40 (1.35–1.46) | <0.001 |
| Other | 0.82 (0.78–0.87) | <0.001 | 0.97 (0.90–1.03) | 0.34 |
| Sex, female | 0.80 (0.78–0.81) | <0.001 | 0.91 (0.88–0.94) | <0.001 |
| Age | 1.04 (1.04–1.04) | <0.001 | 1.02 (1.02–1.02) | <0.001 |
| Marital status | ||||
| Married (ref) | – | – | – | – |
| Single, never married | 1.34 (1.31–1.39) | <0.001 | 1.36 (1.31–1.41) | <0.001 |
| Divorced or separated | 1.41 (1.37–1.45) | <0.001 | 1.38 (1.33–1.43) | <0.001 |
| Widowed | 1.33 (1.29–1.37) | <0.001 | 1.39 (1.33–1.45) | <0.001 |
| Cancer site | ||||
| Tongue (ref) | – | – | – | – |
| Oropharynx | 1.24 (1.18–1.31) | <0.001 | 1.17 (1.10–1.24) | <0.001 |
| Floor of mouth | 1.21 (1.17–1.24) | <0.001 | 1.10 (1.05–1.15) | <0.001 |
| Gum and other mouth | 0.96 (0.93–0.99) | 0.007 | 0.95 (0.91–0.98) | 0.005 |
| Lip | 0.67 (0.65–0.70) | <0.001 | 0.20 (0.18–0.22) | <0.001 |
| Tonsil | 0.85 (0.82–0.88) | <0.001 | 0.77 (0.74–0.80) | <0.001 |
| Other | 1.48 (1.41–1.56) | <0.001 | 1.51 (1.43–1.61) | <0.001 |
| Stage | ||||
| Localized | – | – | – | – |
| Regional | 1.46 (1.43–1.50) | <0.001 | 1.98 (1.87–2.10) | <0.001 |
| Distant | 2.56 (2.47–2.66) | <0.001 | 3.82 (3.56–4.11) | <0.001 |
| Unstaged | 1.36 (1.30–1.41) | <0.001 | 1.72 (1.58–1.86) | <0.001 |
| SES index | ||||
| Quintile 1 | 1.15 (1.12–1.19) | <0.001 | 0.94 (0.88–1.00) | 0.07 |
| Quintile 2 | 1.05 (1.02–1.08) | 0.002 | 0.96 (0.91–1.02) | 0.24 |
| Quintile 3 | 1.07 (1.04–1.11) | <0.001 | 0.77 (0.71–0.85) | <0.001 |
| Quintile 4 | 0.98 (0.95–1.02) | 0.38 | 0.95 (0.89–1.02) | 0.13 |
| Quintile 5 (ref) | – | – | – | – |
| Surgery, yes | 0.61 (0.60–0.63) | <0.001 | 0.56 (0.54–0.58) | <0.001 |
| Radiation, yes | 1.01 (0.98–1.04) | 0.27 | 1.04 (1.01–1.08) | 0.02 |
a HR hazard ratio, 95 % CI 95 % confidence interval
The largest absolute racial disparity in disease-specific survival was 24.9 % and occured 11–13 years after diagnosis. After this period the disease-specific survival curves for all groups began to plateau; however, survival for Black males was consistently lower than that for all other groups. Meanwhile, females of other racial groups showed the best survival throughout long-term follow-up. Black females and White overall survival eventually converged beyond 20 years, whereas disease-specific survival showed persistent disparities (see Fig. 2). With regard to disease-specific survival, opposite trends were also observed by sex between racial groups with Black males showing poorer survival than Black females while White males had better survival than White females (Fig. 3).
Multivariate cox proportional hazards analysis
Results from the final adjusted Cox proportional hazards model are presented in Table 3. After adjusting for age, marital status, cancer site, stage, treatment type, and socioeconomic status, there was still a significant effect of race and sex on overall survival. Blacks had a 43 % higher hazard of mortality compared with Whites (HR 1.43, 95 % CI 1.39–1.48), while those of other racial backgrounds saw a 28 % lower hazard of mortality (HR 0.82, 95 % CI 0.78–0.87) after an oral or oropharyngeal cancer diagnosis. Females also had a 20 % lower hazard of mortality after the cancer diagnosis compared with males (HR 0.80, 95 % CI 0.78–0.81). This relationship holds for disease-specific survival, with a 40 % higher hazard of mortality due to the cancer diagnosis for Blacks compared with Whites (HR 1.40, 95 % CI 1.35–1.46). Females showed a 9 % lower hazard of cancer-related mortality compared with males (HR 0.91, 95 % CI 0.88–0.94). Socioeconomic status had a modest, but statistically significant association with survival; lower socioeconomic status was an indicator of higher hazard of mortality when compared with those with the highest socioeconomic status (HR 1.15, 95 % CI 1.12–1.19). Marital status was also a significant predictor of overall and specific survival. For OCOPC-specific survival, patients who were single, divorced, or widowed had 36–39 % higher hazard of mortality, compared with those that were married.
Discussion
The objective of this study was to assess whether racial and sex disparities existed in long-term survival of oral and oropharyngeal cancer in the USA. We found significant disparities between racial groups, both in overall and in disease-specific survival. These results are consistent with our hypothesis that racial disparities will persist over long-term follow-up. Our findings are novel as the numerous studies that have examined racial disparities in survival of several head and neck cancer subsites have only reported 5- to 10-year survival rates (Amit et al. 2013; McMahon et al. 2011; Ruggeri et al. 2005; Tomita et al. 2010) and none have reported racial disparities after 25 years of follow-up. The longest term follow-up reported to date is 25 years, from a cancer database in British Columbia, Canada (Tiwana et al. 2014); however, this study did not include race in its analysis. Several explanations have been given why racial disparities in cancer outcomes occur, both biologic, genetic and environmental factors (Arbes et al. 1999; Freeman 1989; Henschke et al. 1973; Tomar et al. 2004; Whitworth 2006; Yoo et al. 2004). Among these factors include stage at diagnosis, lack of access to quality care, lower socioeconomic status, lack of health insurance, and possibly comorbidities (Arbes et al. 1999; Goodwin et al. 2008; Morse and Kerr 2006; Piccirillo 2000; Tomar et al. 2004). A recent paper from our institution (Osazuwa-Peters 2015) indicates that head and neck cancer patients without health insurance are more than 10 times more likely to present with advanced stage disease than those with health insurance. The role of HPV positivity of head and neck tumors in survival continues to be explored; some studies have shown that Whites have a higher proportion of HPV-positive tumors, which is associated with a more favorable prognosis (D’Souza et al. 2007; Lin et al. 2013; Panwar et al. 2014; Settle et al. 2009). However, we were not able to include HPV status in our study as the SEER database did not have this information available. Most previously published studies that analyzed race show a disparity in survival for Blacks, although this disparity is attenuated after controlling for other covariables, such as age, marital status, tumor stage, socioeconomic status, treatment type, and anatomic site (Arbes et al. 1999; Franco et al. 1993; Moore et al. 2001). The same trend was shown in our results; hazard ratio was lower though still significant after controlling for covariables such as age, sex, marital status, cancer site, stage, SES index, and treatment type. The knowledge that racial disparities in survival persists after two decades of survival of OCOPC illustrates the need for interventions aimed at mitigating the underlying mechanisms associated with disparities and health inequality.
We also found there was a sex disparity in long-term survival of OCOPC patients, with males in general having a higher hazard of death compared with females and Black males faring worse than all other races and sex, both in overall and in disease-specific survival. This trend was observed from 2-year survival right up to 25-year survival. This survival advantage in females has been observed in a previously published study (Franco et al. 1993; Funk et al. 2002; Moore et al. 2001; Tiwana et al. 2014). An explanation offered for this sex disparity included compliance to treatment; females are more likely than males to seek care and comply to treatment (Thies and Travers 2006). It could also be that males are more likely to engage in behaviors linked with tobacco-associated OCOPC, while females are more likely than males to present with HPV-positive oropharyngeal cancer, which has better survival rates than the tobacco-associated OCOPC.
This study is significant as it describes two independent predictors of long-term survival of OCOPC in the United States. Understanding factors associated with survivorship in cancer generally and OCOPC specifically has become increasing important as overall survivorship continues to improve. The National Institute of Medicine had previously stated that there is a need to improve the quality of follow-up care for each cancer survivor in the US healthcare system (Hewitt et al 2006), as toxicities and chronic side effects of treatment persist long after cancer therapy and cancer patients are often “lost in transition” (Hewitt et al 2006; Institute of Medicine 2013). This has become even more important in head and neck cancer care as patients present at a younger age and survive longer.
There are some limitations in the present study. The study is limited to data available through the SEER database. While this is a high-quality database, it suffers from limitations inherent in retrospective cohort studies. Many covariates were included, but HPV status, which has become very important in outcomes of head and neck cancer, was not examined due to reasons previously stated. We also did not examine the role of comorbidities in survival. The authors hope to explore the potential effect of comorbidities in long-term survivorship of OCOPC in the future. Another limitation was that we could only present county-level measures and compute a valid proxy measure for SES (Geronimus and Bound 1998; Krieger 1992; Kwok and Yankaskas 2001). Despite these limitations, the study benefits from a large sample size, multiple sites, quality controls, and population-based long-term follow-up.
We conclude that the overall survival for patients with OCOPC is poor, and Black males experience the worst outcomes. We also conclude from our results that racial and sex disparities exist in survival of those with OCOPC, and these disparities have not shown in appreciable improvement since 1975. Access to care is pivotal to reducing health disparities, which is the overarching goal of the Healthy People 2020 initiative. Realizing this goal will require a multidimensional approach to the myriad of causal and risk factors associated with survival disparities elucidated in this study.
Acknowledgments
We would like to acknowledge Mario Schootman, PhD, Lauren D. Arnold, PhD, MPH, Shannon E. Nicks, MPH, Kendra L. Ratnapradipa, MSW, and Bram Cleaver, MA, for reviewing and providing feedback on the earlier draft of this manuscript.
Funding
Authors received no funding for this study.
Compliance with ethical standards
Conflict of interest
None of the authors have any conflict of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. No ethics approval was sought for this study by authors as data used were the National Cancer Institute’s Surveillance, Epidemiology, and End Result (SEER) database, which is a publicly available de-identified population-based database.
References
- American Cancer Society (2015) Cancer facts and figures 2015. American Cancer Society, Atlanta [Google Scholar]
- Amit M et al (2013) Improvement in survival of patients with oral cavity squamous cell carcinoma: an international collaborative study. Cancer 119:4242–4248 [DOI] [PubMed] [Google Scholar]
- Arbes SJ, Olshan AF, Caplan DJ, Schoenbach VJ, Slade GD, Symons MJ (1999) Factors contributing to the poorer survival of black Americans diagnosed with oral cancer (United States). Cancer Causes Control 10:513–523 [DOI] [PubMed] [Google Scholar]
- Barker BF, Barker GJ (2001) Oral management of the patient with cancer in the head and neck region. J Calif Dent Assoc 29:619–623 [PubMed] [Google Scholar]
- Barker GJ, Epstein JB, Williams KB, Gorsky M, Raber-Durlacher JE (2005) Current practice and knowledge of oral care for cancer patients: a survey of supportive health care providers. Support Care Cancer 13:32–41 [DOI] [PubMed] [Google Scholar]
- Chaturvedi AK et al (2011) Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 29:4294–4301. doi:10.1200/jco.2011.36.4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzig MR, Weinberg AC, Ghandour RA, Kotamarti S, McKiernan JM, Badani KK (2014) The association between socioeconomic status, renal cancer presentation, and survival in the United States: a survival, epidemiology, and end results analysis. Urology 84:583–589 [DOI] [PubMed] [Google Scholar]
- de Moor JS et al (2013) Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev 22:561–570. doi:10.1158/1055-9965.epi-12-1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza JA, Seiwert TY (2014) A value framework in head and neck cancer care. In: American Society of Clinical Oncology educational book/ASCO American Society of Clinical Oncology Meeting, pp e304–309. doi:10.14694/EdBook_AM.2014.34.e304 [DOI] [PubMed]
- Deschler DG, Richmon JD, Khariwala SS, Ferris RL, Wang MB (2014) The “new” head and neck cancer patient—young, nonsmoker, nondrinker, and HPV positive: evaluation. Otolaryngol Head Neck Surg 151:375–380. doi:10.1177/0194599814538605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza G, Dempsey A (2011) The role of HPV in head and neck cancer and review of the HPV vaccine. Prev Med 53:S5–S11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza G et al (2007) Case–control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 356:1944–1956 [DOI] [PubMed] [Google Scholar]
- Du XL et al (2006) Racial disparity and socioeconomic status in association with survival in older men with local/regional stage prostate carcinoma. Cancer 106:1276–1285 [DOI] [PubMed] [Google Scholar]
- Epstein JB et al (2012) Oral complications of cancer and cancer therapy: from cancer treatment to survivorship. CA Cancer J Clin 62:400–422. doi:10.3322/caac.21157 [DOI] [PubMed] [Google Scholar]
- Franco EL, Dib LL, Pinto DS, Lombardo V, Contesini H (1993) Race and gender influences on the survival of patients with mouth cancer. J Clin Epidemiol 46:37–46 [DOI] [PubMed] [Google Scholar]
- Freeman HP (1989) Cancer in the socioeconomically disadvantaged. CA Cancer J Clin 39:266–288 [DOI] [PubMed] [Google Scholar]
- Funk GF, Karnell LH, Robinson RA, Zhen WK, Trask DK, Hoffman HT (2002) Presentation, treatment, and outcome of oral cavity cancer: a national cancer data base report. Head Neck 24:165–180. doi:10.1002/hed.10004 [DOI] [PubMed] [Google Scholar]
- Geronimus AT, Bound J (1998) Use of census-based aggregate variables to proxy for socioeconomic group: evidence from national samples. Am J Epidemiol 148:475–486 [DOI] [PubMed] [Google Scholar]
- Goodwin WJ et al (2008) Unequal burden of head and neck cancer in the United States. Head Neck 30:358–371. doi:10.1002/hed.20710 [DOI] [PubMed] [Google Scholar]
- Henschke UK, Leffall LD, Mason CH, Reinhold AW, Schneider RL, White JE (1973) Alarming increase of the cancer mortality in the US black population (1950–1967). Cancer 31:763–768 [DOI] [PubMed] [Google Scholar]
- IBM (2013) SPSS Statistics for Windows, Version 22.0. IBM Corp, Armonk, NY
- Institute of Medicine (2013) Delivering high-quality cancer care charting a new course for a system in crisis. http://iom.edu/~/media/Files/Report%20Files/2013/Quality-Cancer-Care/qualitycancercare_rb.pdf. Accessed 22 Aug 2015 [PubMed]
- Hewitt M, Greenfield S, Stovall E (eds) (2006) From Cancer Patient to Cancer Survivor: Lost in Transition. National Academies Press, Washington, DC
- Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60:277–300. doi:10.3322/caac.20073 [DOI] [PubMed] [Google Scholar]
- Krieger N (1992) Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health 82:703–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok RK, Yankaskas BC (2001) The use of census data for determining race and education as SES indicators: a validation study. Ann Epidemiol 11:171–177 [DOI] [PubMed] [Google Scholar]
- Lin BM et al (2013) Long-term prognosis and risk factors among patients with HPV-associated oropharyngeal squamous cell carcinoma. Cancer 119:3462–3471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SS, Massa ST, Varvares MA (2014) Improved overall survival and mortality in head and neck cancer with adjuvant concurrent chemoradiotherapy in national databases. Head Neck. doi:10.1002/hed.23869 [DOI] [PubMed] [Google Scholar]
- Malloy KM, Ellender SM, Goldenberg D, Dolan RW (2013) A survey of current practices, attitudes, and knowledge regarding human papillomavirus-related cancers and vaccines among head and neck surgeons. JAMA Otolaryngol Head Neck Surg 139:1037–1042. doi:10.1001/jamaoto.2013.4452 [DOI] [PubMed] [Google Scholar]
- Marur S, D’Souza G, Westra WH, Forastiere AA (2010) HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol 11:781–789. doi:10.1016/s1470-2045(10)70017-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon J et al (2011) Oral and oropharyngeal cancer in the West of Scotland—long-term outcome data of a prospective audit 1999–2001. Br J Oral Maxillofac Surg 49:92–98 [DOI] [PubMed] [Google Scholar]
- Moore RJ, Doherty DA, Do K-A, Chamberlain RM, Khuri FR (2001) Racial disparity in survival of patients with squamous cell carcinoma of the oral cavity and pharynx. Ethn Health 6:165–177 [DOI] [PubMed] [Google Scholar]
- Morse DE, Kerr AR (2006) Disparities in oral and pharyngeal cancer incidence, mortality and survival among black and white Americans. J Am Dent Assoc 137:203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville BW, Day TA (2002) Oral cancer and precancerous lesions. CA Cancer J Clin 52:195–215 [DOI] [PubMed] [Google Scholar]
- Nicolatou-Galitis O et al (2011) Oral mucositis, pain and xerostomia in 135 head and neck cancer patients receiving radiotherapy with or without chemotherapy. Open Cancer J 4:7–17 [Google Scholar]
- Osazuwa-Peters N, Christopher KM, Hussaini A, Behera A, Walker RJ, Varvares MA (2015) Predictors of stage at presentation and outcomes of head and neck cancers in a university hospital setting. Head Neck (in press) [DOI] [PubMed]
- Panwar A, Batra R, Lydiatt WM, Ganti AK (2014) Human papilloma virus positive oropharyngeal squamous cell carcinoma: a growing epidemic. Cancer Treat Rev 40:215–219 [DOI] [PubMed] [Google Scholar]
- Patel SC et al (2011) Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J Clin Oncol 29:1488–1494. doi:10.1200/jco.2010.31.7883 [DOI] [PubMed] [Google Scholar]
- Piccirillo JF (2000) Importance of comorbidity in head and neck cancer. Laryngoscope 110:593–602 [DOI] [PubMed] [Google Scholar]
- Pulte D, Brenner H (2010) Changes in survival in head and neck cancers in the late 20th and early 21st century: a period analysis. Oncologist 15:994–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert SA, Trentham-Dietz A, Hampton JM, McElroy JA, Newcomb PA, Remington PL (2004) Socioeconomic risk factors for breast cancer: distinguishing individual-and community-level effects. Epidemiology 15:442–450 [DOI] [PubMed] [Google Scholar]
- Ruggeri EM et al (2005) Long-term survival in locally advanced oral cavity cancer: an analysis of patients treated with neoadjuvant cisplatin-based chemotherapy followed by surgery. Head Neck 27:452–458 [DOI] [PubMed] [Google Scholar]
- Schantz SP, Yu GP (2002) Head and neck cancer incidence trends in young Americans, 1973–1997, with a special analysis for tongue cancer. Arch Otolaryngol Head Neck Surg 128:268–274 [DOI] [PubMed] [Google Scholar]
- Settle K et al (2009) Racial survival disparity in head and neck cancer results from low prevalence of human papillomavirus infection in black oropharyngeal cancer patients. Cancer Prev Res (Phila) 2:776–781. doi:10.1158/1940-6207.capr-09-0149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies KM, Travers JF (2006) Handbook of human development for health care professionals. Jones & Bartlett Learning, Sudbury [Google Scholar]
- Tiwana MS, Wu J, Hay J, Wong F, Cheung W, Olson RA (2014) 25 year survival outcomes for squamous cell carcinomas of the head and neck: population-based outcomes from a Canadian province. Oral Oncol 50:651–656. doi:10.1016/j.oraloncology.2014.03.009 [DOI] [PubMed] [Google Scholar]
- Tomar SL, Loree M, Logan H (2004) Racial differences in oral and pharyngeal cancer treatment and survival in Florida. Cancer Causes Control 15:601–609 [DOI] [PubMed] [Google Scholar]
- Tomita N et al (2010) Long-term follow-up and a detailed prognostic analysis of patients with oropharyngeal cancer treated with radiotherapy. J Cancer Res Clin Oncol 136:617–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services Healthy People 2020. https://www.healthypeople.gov/2020/topics-objectives/2020-Topics-and-Objectives-Objectives-A-Z. Accessed 22 Aug 2015
- Vickery LE, Latchford G, Hewison J, Bellew M, Feber T (2003) The impact of head and neck cancer and facial disfigurement on the quality of life of patients and their partners. Head Neck 25:289–296. doi:10.1002/hed.10206 [DOI] [PubMed] [Google Scholar]
- Warnakulasuriya S (2009) Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 45:309–316. doi:10.1016/j.oraloncology.2008.06.002 [DOI] [PubMed] [Google Scholar]
- Whitworth A (2006) New research suggests access, genetic differences play role in high minority cancer death rate. J Natl Cancer Inst 98:669 [DOI] [PubMed] [Google Scholar]
- Yoo GH et al (2004) Microsatellite alterations in African Americans with head and neck cancer. Laryngoscope 114:1619–1624 [DOI] [PubMed] [Google Scholar]