Abstract
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is an inherited vascular dementia characterized by the degeneration of smooth-muscle cells in small cerebral arteries. CADASIL is caused by mutations in NOTCH3, one of the four mammalian homologs to the Drosophila melanogaster NOTCH gene. Disease-associated mutations are distributed throughout the 34 epidermal growth factor–like repeats (EGFRs) that compose the extracellular domain of the Notch3 receptor and result in a loss or a gain of a cysteine residue in one of these EGFRs. In human adults, Notch3 expression is highly restricted to vascular smooth-muscle cells. In patients with CADASIL, there is an abnormal accumulation of Notch3 in the vessel. Molecular pathways linking NOTCH3 mutations to degeneration of vascular smooth-muscle cells are as yet poorly understood. In this study, we investigated the effect of CADASIL mutations on Notch3 activity. We studied five naturally occurring mutations: R90C and C212S, located in the previously identified mutational hotspot EGFR2–5; C428S, shown in this study to be located in the ligand-binding domain EGFR10–11; and C542Y and R1006C, located in EGFR13 and EGFR26, respectively. All five mutant proteins were correctly processed. The C428S and C542Y mutant receptors exhibited a significant reduction in Jagged1-induced transcriptional activity of a RBP/JK responsive luciferase reporter, relative to wild-type Notch3. Impaired signaling activity of these two mutants arose through different mechanisms; the C428S mutant lost its Jagged1-binding ability, whereas C542Y retained it but exhibited an impaired presentation to the cell surface. In contrast, the R90C, C212S, and R1006C mutants retained the ability to bind Jagged1 and were associated with apparently normal levels of signaling activity. We conclude that mutations in Notch3 differently affect Jagged1 binding and Notch3 signaling via the RBP/JK pathway.
CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy) (MIM 125310) is an adult-onset disorder characterized by recurrent ischemic strokes, dementia, and premature death (Tournier-Lasserve et al. 1993; Chabriat et al. 1995; Dichgans et al. 1998). The pathological hallmark is a systemic nonamyloid and nonatherosclerotic vasculopathy, which affects predominantly the small cerebral arteries. Vessel changes are characterized by progressive degeneration of smooth-muscle cells, as well as by the accumulation, in the vessel wall, of an unknown material that appears by electron microscopy as granular osmiophilic material (GOM) (Baudrimont et al. 1993; Ruchoux et al. 1995).
CADASIL is caused by mutations in Notch3, a member of the highly conserved Notch transmembrane receptors family (Joutel et al. 1996). NOTCH3 (MIM 600276) encodes a single-pass transmembrane receptor with a large extracellular domain containing 34 tandem epidermal growth factor–like repeats (EGFRs). Notch3, initially synthesized as a ∼280-kDa precursor, undergoes proteolytic processing like the other Notch receptors. This results in the formation of a mature heterodimeric transmembrane receptor, consisting of a 210-kDa extracellular domain (Notch3ECD) that is noncovalently attached to a 97-kDa transmembrane/cytosolic fragment (Notch3TMIC) (Joutel et al. 2000a). Notch receptors are activated by members of the Delta/Jagged transmembrane ligands family (Artavanis-Tsakonas et al. 1999). In response to ligand binding, Notch receptors undergo sequential proteolytic cleavages, producing the active Notch intracellular domain that forms a complex with the transcription factor RBP/JK (De Strooper et al. 1999; Brou et al. 2000; Mumm et al. 2000). This complex regulates the transcription of downstream genes and modulates numerous cell fate decisions during development (Artavanis-Tsakonas et al. 1999). So far, the precise function of Notch3 in human adult tissues has not been elucidated.
NOTCH3 mutations in patients are distributed throughout the 34 EGFRs and include essentially single amino acid substitutions. Mutations are highly stereotyped, leading to the addition or the loss of a cysteine residue and, therefore, to an EGFR with an odd number of cysteine residues (Joutel et al. 1997; Oberstein et al. 1999; Dichgans et al. 2000; Joutel et al. 2000b; Markus et al. 2002). EGFR2–5, which have no predicted functional role, have been identified as forming a mutational hotspot (Joutel et al. 1997). We and others have recently identified pathogenic mutations in EGFR10 and EGFR11 that are predicted to be required for ligand binding, by homology to the Drosophila melanogaster Notch receptor (Rebay et al. 1991; Lawrence et al. 2000; Arboleda-Velasquez et al. 2002; Markus et al. 2002; A.J., F.R., and E.T.-L., unpublished data).
We recently demonstrated that vascular smooth-muscle cells are the primary targets of the pathogenic process. In normal human adults, expression of Notch3 is highly restricted to these cells. In patients with CADASIL, there is an abnormal accumulation of the Notch3 extracellular domain (ECD) that takes place at the plasma membrane of vascular smooth-muscle cells (Joutel et al. 2000a). However, the molecular pathways linking Notch3 mutations to degeneration of vascular smooth-muscle cells are poorly understood. Different hypotheses have been proposed. First, the pathogenic effect of mutations may result from a primary signaling defect of mutated receptors. Two studies have shown that four different Notch3 mutants retained the ability to interact with soluble Delta1, and one mutant appeared not to affect signaling mediated by Jagged1 or Delta1 (Haritunians et al. 2002; Karlstrom et al. 2002). Another hypothesis is that accumulation of the Notch3 ECD may dominantly inhibit the normal Notch3 pathway through competitive inhibition of ligand binding or ligand sequestering. Finally, it has been proposed that excess Notch3 or GOM deposits may be toxic for vascular smooth-muscle cells, by analogy with several other progressive adult-onset neurologic diseases in which abnormal protein accumulation has been recurrently demonstrated and is suspected to play a major role in pathogenesis (Spinner 2000). We recently observed that vascular smooth-muscle cell defects precede Notch3 accumulation and GOM deposits in transgenic mice mimicking CADASIL arteriopathy (Ruchoux et al. 2003). These findings did not support the two latest hypotheses, prompting us to further investigate the possibility that mutant Notch3 receptors may exhibit an impaired signaling activity.
In the present study, we investigated five naturally occurring NOTCH3 mutations for their ability to transduce ligand-mediated signaling: two mutations located in the mutational hotspot region, one mutation lying in a region that we showed in this study to be required for ligand binding and signaling activity, and two mutations located in other EGFRs. Below, we demonstrate that two of these five mutations result in impaired ligand-induced Notch3 activity, mediated by the RBP/JK transcription factor through different molecular mechanisms, whereas three other mutations appear to retain normal activity.
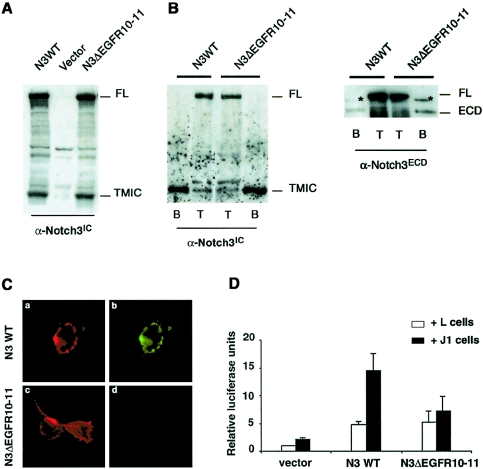
We first assessed the requirement of EGFR10 and EGFR11 of Notch3 for ligand binding and signaling activity. Using a PCR-based approach with wild-type Notch3 as a template, we made a Notch3 construct lacking the 78-aa coding sequence of EGFR10–11 (Notch3ΔEGF10-11). Immunoblot analysis of lysates from 293T cells and NIH3T3 cells transiently transfected with the wild-type or Notch3ΔEGF10-11 construct revealed that both proteins were correctly processed, generating the 210-kDa ECD and the 97-kDa transmembrane intracellular (TMIC) fragments that use, respectively, the 5E1 monoclonal antibody to the ECD (fig. 1A) and the bc4 polyclonal antibody to the intracellular domain (data not shown). We further examined whether wild-type and Notch3ΔEGF10-11 receptors correctly reached the cell surface in 293T cells and NIH3T3 cells. Transiently transfected cells were treated with NHS-SS-biotin and were subsequently lysed and immunoprecipitated with immobilized streptavidin. Immunoblot analysis showed that the 97-kDa TMIC and the 210-kDa ECD fragments, but not the 280-kDa full-length receptor, were detected at the cell surface. Moreover, the relative amounts of cell-surface–expressed receptors did not differ between wild-type Notch3 (fig. 1) and Notch3ΔEGF10-11–expressing cells. As a potential relevant ligand of Notch3, we focused on Jagged1 for the following reasons: (1) of the five known Delta/Jagged ligands, Delta4, Jagged1, and Jagged2 have been shown to be expressed in the vessel during development; (2) Delta4 and Jagged2 appear to be expressed in endothelial cells and Jagged1 in both endothelial and smooth-muscle cells (Villa et al. 2001); and (3) we found that, in human adults, Jagged1 was coexpressed with Notch3 in vascular smooth-muscle cells (data not shown). To assess for Jagged1 binding, we used a soluble form of Jagged1 that contained only the ECD (aa 1–990) fused in frame with a human IgG Fc sequence (sJ1-Fc) in a ligand-binding assay, as described elsewhere (Hicks et al. 2000). (The Jagged1 cDNA sequence is available on the National Center for Biotechnology Information Web site.) A conditioned medium containing sJ1-Fc was prepared by transient transfection of the sJ1-Fc construct into 293T cells, and the sJ1-Fc amount was quantified by western blot analysis with a horseradish peroxidase–conjugated anti-Fc antibody and a known quantity of purified human IgG1. The 293T cells, transiently transfected with either wild-type Notch3, Notch3ΔEGFR10-11 constructs, or the empty vector, were incubated with a sJ1-Fc–conditioned medium that was preclustered with a fluorescein isothiocyanate (FITC)–conjugated anti-Fc antibody. Notch3-expressing cells were detected using the 1E4 monoclonal antibody to the ECD, and immunostaining without prior cell permeabilization further showed that both wild-type Notch3 and Notch3ΔEGFR10-11 were correctly targeted to the cell surface (fig. 1C, left column, red fluorescence). The binding of sJ1-Fc was detected using the FITC fluorescence intrinsic to the Fc-clustering antibody (fig. 1C, right column, green fluorescence). sJ1-Fc binding was detected in wild-type Notch3-expressing cells but not in Notch3ΔEGF10-11-expressing cells. Moreover, sJI-Fc was not detected in Notch3-expressing cells in the absence of sJ1-Fc preclustering, nor in Notch3-expressing cells incubated with a preclustered control-conditioned medium supplemented with purified IgG1 immunoglobulin (data not shown).
Figure 1.
EGFR10 and 11 of Notch3 are required for Jagged1 binding and Jagged1-stimulated transcriptional activity of RBP/JK. A, Protein extracts of 293T cells were transiently transfected with either wild-type Notch3 (N3WT), empty vector (vector), or Notch3 construct lacking EGFR10–11 (N3ΔEGFR10–11). These extracts were analyzed by western blot by use of the bc4 α-Notch3IC polyclonal antibody, revealing both the 280-kDa full-length unprocessed (FL) and the processed 97-kDa TMIC proteins in N3WT- and N3ΔEGFR10–11-expressing cells. B, Cell surface proteins of 293T cells (left panel) or NIH3T3 cells (right panel) transiently transfected with either wild-type Notch3 (N3WT) or Notch3 construct lacking EGFR10–11 (N3ΔEGFR10–11). The proteins were biotinylated using NHS-SS-biotin. Cells were lysed in RIPA buffer, and extracts were loaded on a 6% SDS-PAGE gel, either directly (T fraction, 2.5% and 1.7% of the extract from 293T cells and NIH3T3 cells, respectively) or after incubation on streptavidin-agarose beads (B fraction, 81% and 63% of the extract from 293T cells and NIH3T3 cells, respectively). Extracts were then immunoblotted with the bc4 α-Notch3IC antibody (left panel) or the 5E1 α-Notch3ECD antibody (right panel). Positions of the 280-kDa full-length unprocessed (FL) and the processed 97-kDa (TMIC) and 210-kDa (ECD) proteins are indicated. Note that only the processed proteins, but not the full-length precursor, were detected at the cell surface. The ratio between the biotinylated receptor (B) and the total amount of expressed receptor (T) did not differ between N3WT and N3ΔEGFR10–11. The asterisk in the right panel marks a band that likely corresponds to crossreacting material because it was detected in the B fraction of cells transfected with an unrelated construct (data not shown). C, Transfected cells, incubated with conditioned medium containing sJ1-Fc preclustered using the FITC-conjugated goat anti-human Fc antibody (1:100 dilution) (Jackson ImmunoResearch). After incubation with preclustered conditioned medium, cells were washed with PBS, fixed with paraformaldehyde 3%, and immunostained using the 1E4 α-Notch3ECD monoclonal antibody (1:5 dilution) without prior permeabilization and a Texas Red–conjugated goat anti-mouse antibody (1:100 dilution) (Jackson ImmunoResearch). Red fluorescence detected Notch3, and green fluorescence identified binding of sJ1-Fc to the Notch3-expressing cells. Both wild-type and ΔEGFR10–11 Notch3 proteins were detected at the cell surface (C.a and C.c). Binding of sJ1-Fc was detected to wild-type Notch3-expressing cells (C.b) but not to ΔEGFR10–11-Notch3–expressing cells (C.d). The immunofluorescence is one representative experiment (original magnification × 40). D, NIH3T3 cells transiently cotransfected with either wild-type Notch3 (N3WT), psG5 empty vector (vector), or Notch3 construct lacking EGFR10–11 (N3ΔEGFR10–11), as well as the RBP/JK reporter and the SV40-lacZ control plasmid, were cocultured with either Jagged1-expressing cells (J1) or the parental cells (L) and assayed for luciferase activity. The activity measured was expressed as relative luciferase units (RLU), which reflect the fold increase relative to the activity detected with NIH3T3 cells transfected with the psg5 empty vector cocultured with L cells; the results are the mean ± SEM from six independent experiments performed in triplicate. Jagged1 induced significant RBP/JK activation in the N3WT cells (compare 14.5 ± 3.1 RLU [N3WT + J1] and 2.2 ± 0.3 RLU [vector + J1], P=.006) and was significantly reduced in the N3ΔEGFR10–11 cells (7.2 ± 2.7 RLU; P=.004 versus N3WT + J1).
Previous studies have shown that, using a coculture assay of Notch- and ligand-expressing cells, Notch signaling resulted in stimulation of transcriptional activity of RBP/JK (Hicks et al. 2000). To assess for Jagged1-induced signaling activity, NIH3T3 cells growing in 100-mm dishes were transfected with 3.5 μg of either the wild-type Notch3 construct, the Notch3ΔEGFR10-11, or the psg5 empty vector, together with 1 μg of the SV40-lacZ control plasmid and 2 μg of the pGa981-6 luciferase construct, containing the hexamerized RBP/JK-responsive element. The final quantity of total plasmid DNA, which was transfected, was adjusted to 10 μg with the psg5 plasmid. NIH3T3 transfected cells were then spread over murine Jagged1-expressing cells or the LTK parental cells and were grown in 35-mm dishes. Luciferase activities were measured ∼30 h after coculture and were normalized by the LacZ activities in each sample. As shown in figure 1D, Jagged1 significantly activated RBP/JK in cells expressing wild-type Notch3 but failed to activate RBP/JK in cells expressing Notch3ΔEGFR10-11. Together, these data indicate that EGFR10–11 of Notch3 are required for Jagged1 binding and Jagged1-induced signaling.
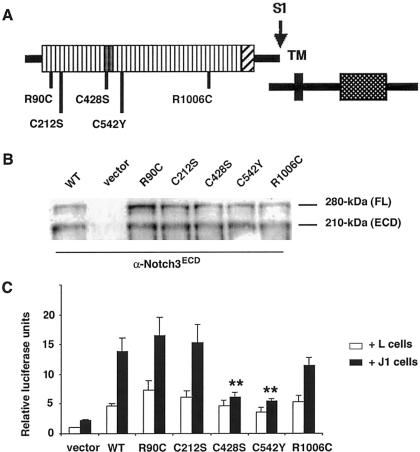
Next, we examined the ability of naturally occurring mutations to transduce Jagged1-induced signaling. We generated five Notch3 mutant constructs, representative of the mutations detected in an extended series of patients characterized in our department (A.J., F.R., and E.T.-L., unpublished data). This included two mutations located in the mutational hotspot region (i.e., R90C in EGFR2 and C212S in EGFR5), one mutation located in the ligand-binding domain (C428S in EGFR10), and two mutations located in other EGFRs (C542Y in EGFR13 and R1006C in EGFR26) (fig. 2A). The R90C and C212S mutants were generated by PCR, by use of mutated oligonucleotides and wild-type Notch3 as a template. The C428S, C542Y, and R1006C mutants were made by site-directed mutagenesis, by use of appropriate oligonucleotides containing a single-nucleotide substitution. Mutant constructs were expressed in 293T and NIH3T3 cells by transient transfection. Immunoblot analysis demonstrated that all mutant proteins were correctly processed, generating the 210-kDa Notch3ECD (fig. 2B) and the 97-kDa Notch3TMIC (data not shown).
Figure 2.
Effect of mutations on RBP/JK transcriptional activity induced by Jagged1. A, Schematic diagram of the Notch3 proteins. The missense mutants used in this study are denoted by bars beneath the diagram; numbers indicate amino acid positions. The S1 cleavage site is depicted; TM, transmembrane domain. B, NIH3T3 cells, transiently transfected with either wild-type Notch3 (WT), empty vector (vector), or mutated Notch3 constructs (R90C, C212S, C428S, C542Y, and R1006C). Extracts were immunoblotted with the α-Notch3 ECD antibody 5E1, revealing both the 280-kDa full-length unprocessed (FL) and the processed 210-kDa Notch3ECD (ECD) proteins. The ratio between the 210-kDa processed form and the 280-kDa full-length unprocessed protein are almost identical between the wild-type and the mutant Notch3 receptors. C, NIH3T3 cells, transiently cotransfected with the RBP/JK luciferase reporter and plasmids encoding either wild-type Notch3 (WT), empty vector (vector), or Notch3 constructs carrying the indicated point mutations, cocultured with either Jagged1-expressing cells (J1) or parental cells (L), and assayed for luciferase activity, as described above. The activity measured was expressed as relative luciferase units (RLU), which reflect the fold increase relative to the activity detected with NIH3T3 cells transfected with the psG5 empty vector cocultured with L cells; results are the mean ± SEM from five independent experiments performed in triplicate. Jagged1 activation of RBP/JK was not significantly affected by the R90C (16.5 ± 3.0 RLU), C212S (15.3 ± 3.1 RLU), and R1006C mutations (11.4 ± 1.4 RLU) when compared with WT-Notch3 (13.8 ± 2.3 RLU) but was significantly reduced by the C428S (6.1 ± 0.9 RLU) and C542Y mutations (5.4 ± 0.4 RLU) (P=.02 for both mutants versus N3WT + Jagged1).
We then assessed Jagged1-induced RBP/JK transcriptional activities mediated by the mutant receptors. NIH3T3 cells were transfected with 3.5 μg of either the wild-type Notch3 construct, the mutant constructs, or the psg5 empty vector, together with 2 μg of the RBP/JK-luciferase reporter, 1 μg of the SV40-lacZ control plasmid, and the final quantity of plasmid DNA, which was adjusted to 10 μg with the psg5 plasmid. NIH3T3 cells were cocultured with Jagged1-expressing cells or parental fibroblast cells and assayed for luciferase activity, as described above. As shown in figure 2C, Jagged1-mediated activation of RBP/JK was not significantly different between wild-type Notch3-expressing cells and either R90C, C212S, or R1006C mutant Notch3-expressing cells. In contrast, transcriptional activity was significantly reduced in cells expressing C428S or C542Y mutant Notch3. Because all NOTCH3 mutations in CADASIL, except one, are heterozygous mutations, we examined the effects of Notch3 mutants on the activity of the wild-type Notch3 (Tuominen et al. 2001). Simulated heterozygous states were obtained by cotransfecting equal amounts of wild-type (1.75 μg) and mutant (1.75 μg) constructs, and the amount of wild-type Notch3 construct in the wild-type Notch3 control was increased (3.5 μg) to make it comparable to the doubly transfected cells. Results showed no difference for the R90C mutant, which, at the homozygous state, did not affect ligand-induced signaling and showed intermediate behavior for both the C428S and C542Y mutants (table 1). These data indicate that the C428S and C542Y mutants demonstrate a significant reduction in Jagged1-induced RBP/JK transcriptional activity, whereas the other three mutants appear to maintain normal activity.
Table 1.
Comparison of Jagged1-Induced RBP/JK Activity in Cells Expressing either Wild-Type Notch3, Mutant Notch3, or Simulated Heterozygous Notch3 Mutants
| Type of Cell | % of Relative Activity Induced by Jagged1a | P Values versus WT-Notch3 |
| WT | 100 | … |
| R90C | 79.7 ± 13.8 | NSb |
| R90C + WT | 99.0 ± 23.8 | NSb |
| C428S | 18.0 ± 11.2 | <.001 |
| C428S + WT | 59.7 ± 10.6 | <.01 |
| C542Y | 30.0 ± 6.8 | <.001 |
| C542Y + WT | 62.3 ± 11.7 | <.01 |
Jagged1-induced activation was expressed as the ratio of normalized luciferase value induced by Jagged1-expressing cells compared with that obtained with parental L cells; relative activity reflects activation induced by Jagged1, relative to that obtained with wild-type Notch3. The mean ± SEM data from three independent experiments performed in triplicate are presented.
NS = not significant.
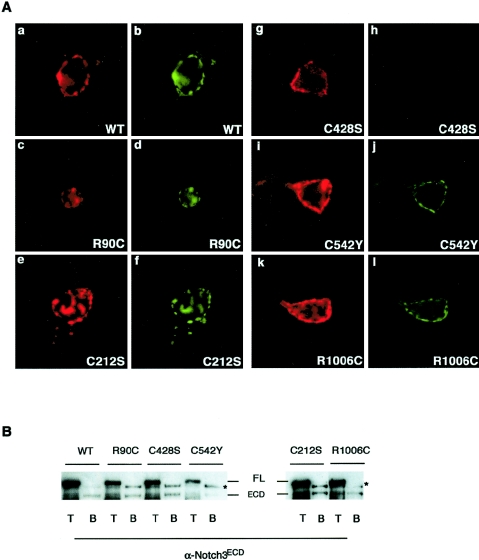
We further examined the mechanisms for impaired signaling of the C428S and C542Y mutants. We first analyzed mutant receptors for cell surface expression by using the 1E4 monoclonal antibody raised against the Notch3 ECD to immunostain unpermeabilized cells. The C428S and C542Y mutant proteins were detected at the cell surface, as were the wild-type receptor and the three other mutant proteins, R90C, C212S, and R1006C (fig. 3A, red fluorescence). We showed above that mutant receptors were correctly processed. However, examination of total Notch3 levels in the cells is not necessarily representative of what is making it to the cell surface. Moreover, the immunofluorescence assay described above did not provide a precise estimate of the number of receptors expressed at the cell surface. Therefore, we further analyzed whether mutant receptors were correctly presented at the cell surface by biotinylation experiments, as described above. Experiments were performed in NIH3T3 cells that were used for signaling. Immunoblot analysis with the 5E1 antibody to the ECD showed that only the 210-kDa ECD fragment, but not the 280-kDa full-length precursor, was detected at the cell surface for all five mutants (fig. 3B). The C542Y mutant exhibited a 5.4-fold decrease (mean value) in the relative amount of ECD detected at the cell surface, relative to wild-type Notch3. In contrast, the relative amounts of cell surface expression of the four other mutants, including C428S, were almost comparable to those of wild-type Notch3 (three independent experiments) (fig. 3B). These data indicate that all five mutant receptors reach the cell surface in the cleaved form, and the C542Y mutant, but not the other mutants, exhibits significantly fewer receptors at the cell surface. We then assayed mutant receptors for their ability to interact with Jagged1, through the use of the ligand-binding assay described above. Cells expressing the C428S mutant did not bind preclustered sJ1-Fc. In contrast, all other mutant-expressing cells, including those expressing the C542Y mutant, bound preclustered sJ1-Fc at a level comparable to that detected for the wild-type receptor (fig. 3A, green fluorescence). Together, these data suggest that the signaling defect of the C428S mutant likely arises from its loss of ligand-binding ability, but that of the C542Y mutant arises from a different mechanism that might involve an impaired presentation of the receptor to the cell surface.
Figure 3.
Effect of mutations on cell surface expression and Jagged1-binding ability. A, 293T cells transiently transfected with wild-type Notch3 or Notch3 constructs carrying the indicated point mutations. They were incubated with a preclustered conditioned medium containing sJ1-Fc and then fixed and immunostained using the 1E4 α-Notch3 ECD antibody, with no prior permeabilization, followed by a Texas Red–conjugated secondary antibody. All five mutant proteins were detected at the cell membrane (A.c, A.e, A.g, A.i, A.k; red fluorescence). The binding of sJ1-Fc to the Notch3-expressing cells was detected by the FITC-conjugated clustering α-IgG antibody. sJ1-Fc did not bind to C428S Notch3 mutant cells (A.h) but bound to all four other mutant-expressing cells (A.d, A.f, A.j, A.l; green fluorescence). The immunofluorescence is one representative experiment (original magnification ×40). B, Cell surface proteins of NIH3T3 cells transiently transfected with either wild-type Notch3 (N3WT) or Notch3 constructs carrying the indicated point mutations. They were biotinylated using NHS-SS-biotin. Cells were lysed in RIPA buffer, and extracts were loaded on a 6% SDS-PAGE gel either directly (T fraction, 1.7% of the extract) or after incubation on streptavidin-agarose beads (B fraction, 63% of the extract). Extracts were then immunoblotted with the 5E1 α-Notch3ECD antibody. Positions of the 280-kDa full-length unprocessed (FL) and the processed 210-kDa (ECD) proteins are indicated. Only the ECD protein, but not the full-length precursor, was detected at the cell surface. The ratio between the biotinylated receptor (B) and the total amount of expressed receptor (T) was lower for the C542Y mutant, compared with N3WT and the four other mutants. The western blot is from one representative experiment. The asterisks mark a band that likely corresponds to crossreacting material because it was detected in the B fraction of cells transfected with an unrelated construct (data not shown).
In this study, we investigated the biological activities of five naturally occurring NOTCH3 mutations representative of those detected in a large series of patients with CADASIL. Herein, we demonstrate for the first time, to our knowledge, (1) that EGFR10 and EGFR11 of Notch3 are required for ligand binding and signaling activity, (2) that CADASIL-associated mutations may impair or not Notch3 receptor signaling via the RBP/JK pathway, and (3) that CADASIL can be caused by a Notch3 mutant that has lost its ability to interact with the ligand.
Of the five mutants analyzed, C428S and C542Y exhibit a significant reduction in Jagged1-induced transcriptional activity of a RBP/JK-responsive luciferase reporter. Our data suggest that the impaired activity of these two mutants arises through different mechanisms. The mutations C428S and C542Y are located in and around, respectively, the ligand-binding domain. The C428S mutation appears to impair neither receptor maturation nor its trafficking but results in a loss of Jagged1-binding ability, whereas the C542Y mutation appears to retain the ability to bind Jagged1 but results in an improper presentation of the receptor to the cell surface, with fewer cleaved receptors reaching the cell surface. Whether decreased signaling activity of the C542Y mutant arises from this trafficking defect is uncertain. Indeed, the mutant murine Notch3 carrying the R142C mutation has been recently reported to exhibit an impaired S1 proteolytic cleavage, resulting in a strong reduction of cell surface expression but normal ligand-induced signaling (Karlstrom et al. 2002). Several lines of evidence indicate the important role of the EGF repeats in modulating activity of the receptor (Okajima and Irvine 2002; Li et al. 2003). Therefore, an alternative cause for the signaling defect of the C542Y mutant might be that EGFR13 of Notch3 is involved in such a role, and the C542Y mutation impairs this function.
Our data indicate that distinct CADASIL mutations have apparently different effects on Notch3 signaling via the RBP/JK pathway. We show that two mutations result in impaired activity of the receptor, whereas three others appear to have no effect. How can these data be reconciled with the highly stereotyped nature of CADASIL mutations? Different hypotheses may be raised. First, all mutations may lead to abnormal receptor activity that is mediated by the canonical RBP/JK signaling pathway, but this abnormality cannot be detected for some mutations by use of the in vitro assay employed in this study. There is evidence from several studies that the activity of Notch receptors is submitted to a tight regulation that is highly dependent on the cell context (Okajima and Irvine 2002; Li et al. 2003). Superimposed on the core components, including the ligand, the receptor, and the nuclear proteins that form a complex with the cleaved intracellular domain of Notch, is a wide array of modulators (Justice and Jan 2002). This raises the possibility that the effect of some mutations on the activity of the receptor may become apparent only in the presence of given modulators; such modulators might be not expressed in the cells employed in this study. Moreover, modulators of the Notch3 receptor remain to be identified. Second, the pathogenicity of mutations may arise through an impaired activity of the receptor that is not mediated by the canonical RBP/JK signaling pathway. In support of this second hypothesis are the recent reports that Notch receptors can signal in RBP/JK-independent modes (Shawber et al. 1996). Moreover, it has been shown that the phenotype of some Drosophila mutants, including the Abruptex mutant 59d carrying a cysteine mutation in the EGFR24 of the Notch receptor, is not dependent on Su(H) (the Drosophila RBP/JK homolog) function (Brennan et al. 1999). Finally, the pathogenicity of mutations may arise through a mechanism unrelated to the activity of the receptor.
A previous study reported that the CADASIL mutation R142C of murine Notch3, which corresponds to mutation R141C in humans, impaired the S1 proteolytic cleavage (Karlstrom et al. 2002). None of the mutant receptors investigated in our study appeared to exhibit such a defect. Indeed, we found that the ratio between the processed forms and the full-length unprocessed protein was almost identical between the wild-type and the mutant Notch3 receptors in whole-cell extracts from both 293T cells and NIH3T3 cells. However, these two studies were performed with differently expressed Notch3 receptors (stably transfected cells in the previous study versus transiently transfected cells in our study). So far, we do not know whether the discrepancy in results may have arisen from the differences in the two studies. We also observed that, for a given Notch3 construct, including wild-type Notch3, the ratio between the processed forms and the full-length, unprocessed Notch3 protein could vary from one experiment to another, depending on the total Notch3 expression level. Moreover, it is important to bear in mind that the full-length form is overrepresented in transfected cells as compared with normal human adult tissues, in which the unprocessed protein is almost undetectable and the processed forms are expressed at very low levels (Joutel et al. 2000a). These observations suggest to us that both the cell type and the expression level of Notch3 might affect the S1 processing efficiency and that the S1 cleavage efficiency should be analyzed with caution in cells overexpressing Notch3.
The mechanism of the selective Notch3ECD accumulation in CADASIL, in the absence of an associated Notch3TMIC accumulation, is not yet understood (Joutel et al. 2000a). It has been proposed that Notch3ECD accumulation may occur during or after receptor activation, on the basis of the fact that, upon receptor activation, ECD and TMIC fragments of Notch dissociate from each other through sequential proteolytical cleavages (Fortini 2001). One prediction from this model is that no or less Notch3ECD should accumulate in patients carrying a Notch3 mutation abrogating ligand-binding ability and, thus, receptor activation. We recently had the opportunity to perform Notch3 immunostaining of vessels from patients carrying either a mutation shown in this study to abolish ligand-binding ability, such as the C428S mutation, or a mutation that seems to affect neither ligand binding nor signaling activity, such as the R90C or the C21S mutations. Notch3ECD accumulation was detected in all these patients (Joutel et al. 2001). It is important to note that the amount of Notch3ECD was found to be almost identical between the C428S mutation carriers and the R90C or C212S mutation carriers (data not shown). Therefore, our results indicate that Jagged1 binding is not a prerequisite for Notch3ECD accumulation in patients. It remains to be determined whether a mutation abrogating binding to Jagged1 could abrogate binding to any other Delta/Jagged1 ligands; the ligands that actually interact with Notch3 in the vessel in vivo also remain to be identified.
In summary, we demonstrate for the first time, to our knowledge, that some CADASIL mutations impair the activity of the Notch3 receptor through distinct mechanisms. Additional studies are needed to determine whether smooth-muscle cell degeneration is actually caused by a reduction of Notch3 activity and to further elucidate the pathogenic effect of the mutations that appear, in this study, to have no effect on the activity of the receptor.
Acknowledgments
We thank G. Weinmaster for providing Jagged1-expressing cells and parental L cells, S. Artavanis-Tsakonas and R. Mann for providing a human full-length Jagged1 cDNA, G. Bornkamm and L. Strobl for providing the pGa981-6 luciferase construct, A. Traunecker for providing human IgG1 immunoglobulin Fc cDNA, and P. Aucouturier for providing purified human IgG1. We also thank J. M. Gase for his expert assistance with in situ hybridization. This work was funded by grants from the Jean Valade Foundation, the CADASIL Foundation of America, and INSERM (APEX 4X003F [to A.J.]).
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- National Center for Biotechnology Information (NCBI), http://www.ncbi.nlm.nih.gov/PubMed/ (for Jagged1 cDNA sequence [accession number U73936])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for CADASIL and NOTCH3)
References
- Arboleda-Velasquez JF, Lopera F, Lopez E, Frosch MP, Sepulveda-Falla D, Gutierrez JE, Vargas S, Medina M, Martinez De Arrieta C, Lebo RV, Slaugenhaupt SA, Betensky RA, Villegas A, Arcos-Burgos M, Rivera D, Restrepo JC, Kosik KS (2002) C455R notch3 mutation in a Colombian CADASIL kindred with early onset of stroke. Neurology 59:277–279 [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ (1999) Notch signaling: cell fate control and signal integration in development. Science 284:770–776 10.1126/science.284.5415.770 [DOI] [PubMed] [Google Scholar]
- Baudrimont M, Dubas F, Joutel A, Tournier-Lasserve E, Bousser MG (1993) Autosomal dominant leukoencephalopathy and subcortical ischemic stroke: a clinicopathological study. Stroke 24:122–125 [DOI] [PubMed] [Google Scholar]
- Brennan K, Tateson R, Lieber T, Couso JP, Zecchini V, Arias AM (1999) The abruptex mutations of notch disrupt the establishment of proneural clusters in Drosophila. Dev Biol 216:230–242 10.1006/dbio.1999.9501 [DOI] [PubMed] [Google Scholar]
- Brou C, Logeat F, Gupta N, Bessia C, LeBail O, Doedens JR, Cumano A, Roux P, Black RA, Israel A (2000) A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol Cell 5:207–216 [DOI] [PubMed] [Google Scholar]
- Chabriat H, Vahedi K, Iba-Zizen MT, Joutel A, Nibbio A, Nagy TG, Krebs MO, Julien J, Dubois B, Ducrocq X, Levasseur M, Homeyer P, Mas JL, Lyon-Caen O, Tournier-Lasserve E, Bousser MG (1995) Clinical spectrum of CADASIL: a study of 7 families. Lancet 346:934–939 10.1016/S0140-6736(95)91557-5 [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R (1999) A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature 398:518–522 10.1038/19083 [DOI] [PubMed] [Google Scholar]
- Dichgans M, Ludwig H, Muller-Hocker J, Messerschmidt A, Gasser T (2000) Small in-frame deletions and missense mutations in CADASIL: 3D models predict misfolding of Notch3 EGF-like repeat domains. Eur J Hum Genet 8:280–285 10.1038/sj.ejhg.5200460 [DOI] [PubMed] [Google Scholar]
- Dichgans M, Mayer M, Uttner I, Bruning R, Muller-Hocker J, Rungger G, Ebke M, Klockgether T, Gasser T (1998) The phenotypic spectrum of CADASIL: clinical findings in 102 cases. Ann Neurol 44:731–739 [DOI] [PubMed] [Google Scholar]
- Fortini ME (2001) Notch and presenilin: a proteolytic mechanism emerges. Curr Opin Cell Biol 13:627–634 10.1016/S0955-0674(00)00261-1 [DOI] [PubMed] [Google Scholar]
- Haritunians T, Boulter J, Hicks C, Buhrman J, DiSibio G, Shawber C, Weinmaster G, Nofziger D, Schanen C (2002) CADASIL Notch3 mutant proteins localize to the cell surface and bind ligand. Circ Res 90:506–508 10.1161/01.RES.0000013796.73742.C8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks C, Johnston SH, diSibio G, Collazo A, Vogt TF, Weinmaster G (2000) Fringe differentially modulates Jagged1 and Delta1 signalling through Notch1 and Notch2. Nat Cell Biol 2:515–520 10.1038/35019553 [DOI] [PubMed] [Google Scholar]
- Joutel A, Andreux F, Gaulis S, Domenga V, Cecillon M, Battail N, Piga N, Chapon F, Godfrain C, Tournier-Lasserve E (2000a) The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients. J Clin Invest 105:597–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joutel A, Chabriat H, Vahedi K, Domenga V, Vayssiere C, Ruchoux MM, Lucas C, Leys D, Bousser MG, Tournier-Lasserve E (2000b) Splice site mutation causing a seven amino acid Notch3 in-frame deletion in CADASIL. Neurology 54:1874–1875 [DOI] [PubMed] [Google Scholar]
- Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, Alamowitch S, Domenga V, Cecillion M, Marechal E, Maciazek J, Vayssiere C, Cruaud C, Cabanis EA, Ruchoux MM, Weissenbach J, Bach JF, Bousser MG, Tournier-Lasserve E (1996) Notch3 mutations in CADASIL, an hereditary adult-onset condition causing stroke and dementia. Nature 383:707–710 10.1038/383707a0 [DOI] [PubMed] [Google Scholar]
- Joutel A, Favrole P, Labauge P, Chabriat H, Lescoat C, Andreux F, Domenga V, Cecillon M, Vahedi K, Ducros A, Cave-Riant F, Bousser MG, Tournier-Lasserve E (2001) Skin biopsy immunostaining with a Notch3 monoclonal antibody for CADASIL diagnosis. Lancet 358:2049–2051 10.1016/S0140-6736(01)07142-2 [DOI] [PubMed] [Google Scholar]
- Joutel A, Vahedi K, Corpechot C, Troesch A, Chabriat H, Vayssiere C, Cruaud C, Maciazek J, Weissenbach J, Bousser MG, Bach JF, Tournier-Lasserve E (1997) Strong clustering and stereotyped nature of Notch3 mutations in CADASIL patients. Lancet 350:1511–1515 10.1016/S0140-6736(97)08083-5 [DOI] [PubMed] [Google Scholar]
- Justice NJ, Jan YN (2002) Variations on the Notch pathway in neural development. Curr Opin Neurobiol 12:64–70 10.1016/S0959-4388(02)00291-X [DOI] [PubMed] [Google Scholar]
- Karlstrom H, Beatus P, Dannaeus K, Chapman G, Lendahl U, Lundkvist J (2002) A CADASIL-mutated Notch3 receptor exhibits impaired intracellular trafficking and maturation but normal ligand-induced signaling. Proc Natl Acad Sci USA 99:17119–17124 10.1073/pnas.252624099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence N, Klein T, Brennan K, Martinez Arias A (2000) Structural requirements for Notch signalling with Delta and Serrate during the development and patterning of the wing disc of Drosophila. Development 127:3185–3195 [DOI] [PubMed] [Google Scholar]
- Li Y, Li L, Irvine KD, Baker NE (2003) Notch activity in neural cells triggered by a mutant allele with altered glycosylation. Development 130:2829–2840 10.1242/dev.00498 [DOI] [PubMed] [Google Scholar]
- Markus HS, Martin RJ, Simpson MA, Dong YB, Ali N, Crosby AH, Powell JF (2002) Diagnostic strategies in CADASIL. Neurology 59:1134–1138 [DOI] [PubMed] [Google Scholar]
- Mumm JS, Schroeter EH, Saxena MT, Griesemer A, Tian X, Pan DJ, Ray WJ, Kopan R (2000) A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Mol Cell 5:197–206 [DOI] [PubMed] [Google Scholar]
- Oberstein SA, Ferrari MD, Bakker E, van Gestel J, Kneppers AL, Frants RR, Breuning MH, Haan J (1999) Diagnostic Notch3 sequence analysis in CADASIL: three new mutations in Dutch patients. Dutch CADASIL Research Group. Neurology 52:1913–1915 [DOI] [PubMed] [Google Scholar]
- Okajima T, Irvine KD (2002) Regulation of notch signaling by o-linked fucose. Cell 111:893–904 [DOI] [PubMed] [Google Scholar]
- Rebay I, Fleming RJ, Fehon RG, Cherbas L, Cherbas P, Artavanis-Tsakonas S (1991) Specific EGF repeats of Notch mediate interactions with Delta and Serrate: implications for Notch as a multifunctional receptor. Cell 67:687–699 [DOI] [PubMed] [Google Scholar]
- Ruchoux MM, Domenga V, Brulin P, Maciazek J, Limol S, Tournier-Lasserve E, Joutel A (2003) Transgenic mice expressing mutant Notch3 develop vascular alterations characteristic of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Am J Pathol 162:329–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchoux MM, Guerouaou D, Vandenhaute B, Pruvo JP, Vermersch P, Leys D (1995) Systemic vascular smooth muscle cell impairment in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Acta Neuropathol 89:500–512 10.1007/s004010050281 [DOI] [PubMed] [Google Scholar]
- Shawber C, Nofziger D, Hsieh JJ, Lindsell C, Bogler O, Hayward D, Weinmaster G (1996) Notch signaling inhibits muscle cell differentiation through a CBF1-independent pathway. Development 122:3765–3773 [DOI] [PubMed] [Google Scholar]
- Spinner NB (2000) CADASIL: Notch signaling defect or protein accumulation problem? J Clin Invest 105:561–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier-Lasserve E, Joutel A, Melki J, Weissenbach J, Lathrop GM, Chabriat H, Mas JL, Cabanis EA, Baudrimont M, Maciazek J, Bach MA, Bousser MG (1993) Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy maps to chromosome 19q12. Nat Genet 3:256–259 [DOI] [PubMed] [Google Scholar]
- Tuominen S, Juvonen V, Amberla K, Jolma T, Rinne JO, Tuisku S, Kurki T, Marttila R, Poyhonen M, Savontaus ML, Viitanen M, Kalimo H (2001) Phenotype of a homozygous CADASIL patient in comparison to 9 age-matched heterozygous patients with the same R133C Notch3 mutation. Stroke 32:1767–1774 [DOI] [PubMed] [Google Scholar]
- Villa N, Walker L, Lindsell CE, Gasson J, Iruela-Arispe ML, Weinmaster G (2001) Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech Dev 108:161–164 10.1016/S0925-4773(01)00469-5 [DOI] [PubMed] [Google Scholar]