Abstract
Crohn disease (CD), an inflammatory bowel disease, is a multifactorial trait with the highest frequency in Ashkenazi Jewish (AJ) individuals of Central European origin. Recently, three common predisposing CARD15 mutations (R702W, G908R, and 1007fs) and a polymorphism (P268S) were identified. To determine whether CARD15 mutations account for the higher prevalence of CD in AJ individuals, the haplotypes and allele frequencies of the common mutations and variants were assessed in 219 members of 50 AJ and 53 members of 10 Sephardi/Oriental Jewish (SOJ) multiplex families with CD, in 36 AJ patients with sporadic CD, and in 246 AJ and 82 SOJ controls. A higher frequency of CARD15 mutations was found in AJ patients from multiplex families with CD from Central (44.0%) versus Eastern (24.0%) Europe, especially for G908R and 1007fs, and in SOJ patients (34.5%) compared with AJ (10.1%) or SOJ (5.4%) controls. Contrary to expectation, the frequency of the common mutations was slightly lower in AJ patients with CD (30.1%) than in SOJ patients with CD (34.5%). The 702W allele was associated with both the P268 and 268S alleles. CARD15 mutation frequencies were greater in affected sib pairs than in sporadic CD cases but actually decreased in families with three or more affected sibs, raising the possibility of genetic heterogeneity. Similarly, our linkage evidence on chromosome 16 was diminished in the families with three or more affected sibs compared with sib pairs. Screening the CARD15 gene for rare variants revealed five novel changes (D113N, D357A, I363F, L550V, and N852S) of which N852S occurred only in AJ individuals and may be disease predisposing. Also, there was no evidence for increased risk associated with the recently described IVS+158 single-nucleotide polymorphism. Although the AJ controls appear to have a higher frequency of CARD15 mutations than the SOJ controls, it is unlikely that this difference fully explains the excess frequency of CD in the AJ population.
Introduction
Crohn disease (CD [MIM 266600]) is an inflammatory bowel disease resulting from defects in mucosal immunity and intestinal epithelial barrier function (Yang et al. 2001; Podolsky 2002; Swidsinski et al. 2002). On the basis of familial aggregation, twin, and spouse studies, it has been determined that disease susceptibility is inherited as a multifactorial trait (King et al. 1992; Binder 1998; Ahmad et al. 2001). It is of relevance that CD primarily affects white individuals, with the highest prevalence among individuals of Ashkenazi Jewish (AJ) descent, occurring two to four times more frequently than in non-Jewish white populations (Roth et al. 1989a, 1989b; Kurata et al. 1992; Yang et al. 2001). Among Jews, the prevalence of CD in the Ashkenazim is higher than in Sephardic or Oriental Jews (SOJ) (Yang et al. 1993), and, even among the Ashkenazim, those of Central European origin have a relatively higher risk for CD than those of Polish and Russian origin (Roth et al. 1989a, 1989b; Zlotogora et al. 1990; Yang et al. 1993). Although the basis for the increased frequency of CD in AJ individuals is not known, it could be hypothesized that they have a higher frequency of mutations in predisposing genes.
Recently, several groups performed genomewide scans that identified chromosomal regions containing potential predisposing genes for CD, including IBD1 (16q12) (Hugot et al. 1996), IBD2 (12q13.2) (Duerr et al. 1998), IBD3 (6q21 [major histocompatibility complex]) (Hampe et al. 1999a, 1999b), IBD4 (14q11-12) (Ma et al. 1999), and IBD5 (5q31-33) (Rioux et al. 2000, 2001), as well as regions on 1p, 3q, 4q (Cho et al. 1998), and 19p13 (Rioux et al. 2000). Subsequently, three groups independently identified the IBD1 susceptibility gene on chromosome 16 as the CARD15 (also known as “NOD2”) gene (Hampe et al. 2001; Hugot et al. 2001; Ogura et al. 2001). The LRR domain of the gene has a binding activity for bacterial peptidoglycans, and mutations in this domain abrogate nuclear factor-κB–mediated signaling (Inohara et al. 1999, 2000; Chamaillard et al. 2002).
Studies of European and North American families with CD identified three common CARD15 mutations, R702W, G908R, and 1007fs, and a polymorphism, P268S, that was always found with the mutant alleles (Hampe et al. 2001; Hugot et al. 2001; Ogura et al. 2001). The frequencies of the mutant alleles in white patients with CD were 3.5%–12.9% for R702W, 0.8%–6.0% for G908R, and 1.7%–13.7% for 1007fs, with the lower frequencies found in Norwegian patients (Hampe et al. 2001). Overall, the frequency for the three mutations in North American and Central and Western European patients with CD was 19.1%–29.0% (Ahmad et al. 2001; Hampe et al. 2001; Hugot et al. 2001; Ogura et al. 2001; Cuthbert et al. 2002; Lesage et al. 2002; Vermeire et al. 2002). In contrast, studies revealed a low frequency (2.6%) in African American patients (Bonen et al. 2002) and an absence of these mutations in Japanese (Inoue et al. 2002; Yamazaki et al. 2002) and Korean patients with CD (Croucher et al. 2003).
Further, studies of the CARD15 alleles from patients with familial CD by denaturing high-performance liquid chromatography (DHPLC) heteroduplex analysis and sequencing revealed additional CARD15 polymorphisms and rare variants that may be disease causing (Lesage et al. 2002). The Lesage et al. (2002) study indicated that 49% of the patients with familial CD had one (32%) or two (17%) mutant CARD15 alleles. Most (93%) CARD15 mutations were in the distal third of the gene, with the three common mutations (R702W, G908R, and 1007fs) accounting for 81% of identified variants.
Although the prevalence of CD is highest in the AJ population, limited information is available on the nature and frequency of the common—and, particularly, the rare—CARD15 mutations in AJ patients with familial and sporadic CD (Zhou et al. 2002; Sugimura et al. 2003), and no data have been reported for SOJ patients with CD. In this article, we report the haplotypes and allele frequencies of the common CARD15 mutations in 50 AJ and 10 SOJ multiplex families with CD, as well as the allele frequencies in AJ patients with sporadic CD and in unrelated AJ and SOJ healthy controls. In addition, we used DHPLC screening and genomic sequencing to identify five novel CARD15 lesions and several previously reported variants. It is notable that the novel conserved variant, N852S, was found in 15% of the AJ families with CD and in none of the SOJ families with CD. Importantly, 32% and 30% of the AJ and SOJ families with CD, respectively, did not have a CARD15 mutation, indicating the heterogeneity of the predisposing genes causing CD among Jewish patients.
Subjects and Methods
Patients
Fifty AJ multiplex families with CD were recruited from the New York metropolitan area and Israel, including 115 patients, 79 unaffected parents, and 25 unaffected sibs. In Israel, 10 SOJ multiplex families with CD were recruited, including 21 patients and 32 unaffected first-degree relatives. The SOJ CD group was composed of five Sephardic families of Moroccan (n=3), Turkish (n=1), and Tunisian (n=1) descent and five Oriental families of Iraqi (n=1), Kurdish (n=1), Yemeni (n=1), Iranian (n=1), and Syrian (n=1) ancestry. The multiplex family groups contained single sibships with at least two affected sibs, except for six AJ families that contained affected individuals in more than one sibship. In addition, 36 AJ patients with sporadic CD and with no family history of an inflammatory bowel disease participated in this study. All patients with CD were seen by gastroenterologists, and diagnoses were based on clinical, endoscopic, radiological, and/or histopathological findings (Lennard-Jones 1989). Families with a relative affected with ulcerative colitis or indeterminate colitis were excluded. All patients completed a questionnaire requesting demographic and medical information. Blood specimens were obtained from the patients with CD and their family members, as well as from 246 unrelated healthy AJ adults from New York and Israel and from 82 unrelated healthy Israeli SOJ adults. The healthy SOJ adults were equally of Sephardi and Oriental (Mediterranean) origin. The study was approved by the respective institutional review boards, and informed consent was obtained from each participant.
CARD15 Mutation Detection Strategy
Initially, the haplotypes for the IBD1 locus on chromosome 16q12 were established in each member of the multiplex families through use of the closely linked microsatellite markers D16S3035 and D16S3136 and the P268S CARD15 polymorphism. These haplotypes were used to determine phase and to calculate haplotype frequencies. The CARD15 P268S polymorphism and the common mutations R702W, G908R, and 1007fs were determined in all family members, patients with sporadic CD, and unrelated healthy individuals. DHPLC analysis and sequencing were performed in patients who had none or only one of the three common mutant alleles, to detect other CARD15 variants, as well as in 188 randomly chosen individuals of the 246 unrelated AJ healthy controls. The occurrence of these variants was assessed in all of the family members and in the remainder of the unrelated healthy AJ and SOJ individuals by sequencing or restriction analyses. In addition, the presence of the IVS8+158 polymorphism (Sugimura et al. 2003) was determined in all family members, patients with sporadic disease, and unrelated healthy controls.
Detection of the CARD15 Common Variants
The common CARD15 mutations (R702W, G908R, and 1007fs) and the P268S polymorphism were detected by restriction analyses. The specific PCR primers and the size of the restriction fragments before and after digestion are given in table 1. Since the R702W mutation abolished the restriction site for MspI, the samples found positive for this mutation by restriction analysis were sequenced to confirm the correct base change.
Table 1.
PCR Primers for Amplification, Restriction Analysis, and Sequencing of the CARD15 Mutations and Polymorphisms
|
Primera |
PCR or RestrictionFragment Size(bp) |
||||
| Mutation | Restriction Enzyme | Forward | Reverse | Wild Type | Mutant |
| R702W | MspI | CTTCCTGGCAGGGCTGTTGTC | CATGCACGCTCTTGGCCTCAC | 76+54+24+22 | 130+24+22 |
| G908Rb | HhaIb | AAGTCTGTAATGTAAAGCCACb | CCCAGCTCCTCCCTCTTCb | 380 | 242+138 |
| 1007fs | NlaIV | CCTGCAGTCTCTTTAACTGG | CTTACCAGACTTCCAGGATG | 168 | 128+40 |
| P268S | BamHI | TGCCTCTTCTTCTGCCTTCCb | AGTAGAGTCCGCACAGAGAGb | 422 | 247+175 |
| M863V | NcoI | CTGTTTGCATGATGGGGGGb | GGGAGATCACAGCATTAGAGb | 212+160 | 372 |
| N852S | AluI | CTGTTTGCATGATGGGGGGb | CAGCCGTCAGTCAATTTGTAG |
151 | 129+22 |
| IVS8+158 | XhoI | TGCAGTTTTCTTGGGGAGAT | TGTACCTGATCCAGCCCAAT | 125+95 | 220 |
| L248R | MspI | TGCCTCTTCTTCTGCCTTCCb | AGTAGAGTCCGCACAGAGAGb | 44+375 | 44+117+258 |
| A612T | XcmI | ATGTGCTGCTACGTGTTCTCb | CAGACACCAGCGGGCACAGb | 456 | 155+301 |
| Sequencing primers for promoter region | TGTGGTCTCAGGAAGGAAGG |
ACTGGTGAGCCTGGATCTGT |
918 | … | |
| Wild Type |
Mutant |
||||
| D113Nc | AGCCCAGGCCGACAGCCA | AGCCCAGGCCAACAGCCA |
… | … | |
| I363Fc | GTTGGTCAAGAAGACATCTTC | GTTGGTCAAGAAGACTTCTTC |
… | … | |
| L550Vc | ACCCTCCTGCACCTGGGCA | ACCCTCCTGCACGTGGGCA |
… | … | |
Modified (or, in the case of dot-blot probes, mutated) bases are underlined.
PCR primers and/or conditions according to or modified from Lesage et al. (2002).
Dot-blot probe.
DHPLC Analysis
CARD15 exons 2–12 and adjacent intronic sequences were amplified individually, with the exception of the ∼1.8-kb exon 4, which was amplified in five overlapping fragments, as described elsewhere (Lesage et al. 2002). Amplicons were analyzed by DHPLC using the WAVE System (Transgenomic). Amplicons with abnormal profiles were sequenced.
DNA Sequencing
The promoter region (−300 bp to the 10th bp of exon 1) and exons 2–12 and adjacent flanking intronic sequences of the CARD15 gene were sequenced using primers indicated in table 1 or those described elsewhere (Lesage et al. 2002).
Screening the Control Group for Common and Rare Mutations/Sequence Variants
The three common mutations (R702W, G908R, and 1007fs); the N852S variant; the P268S, M863V, and IVS8+158 polymorphisms; and the rare L248R and A612T variants were screened by restriction analysis (table 1). The D357A, E441K, R703C, and A918D variants were screened by DHPLC, and their altered profiles were confirmed by sequencing. Screening for the newly identified variants D113N, I363F, and L550S was performed by dot-blot analyses (Edelmann et al. 2002). (See the supplemental data in the appendix [online-only] for details of the laboratory methods used.)
Analysis of Haplotype Frequencies
The first stage of analysis was to calculate haplotype frequencies for the nine haplotypes depicted in figure 1 within the five groups defined above. For the nine listed haplotypes, all 45 possible pairwise combinations can be discriminated from genotype data, except for the combinations AB and CH. For the AJ control group, six individuals were either AB or CH; in this case, the haplotype frequencies were determined using gene counting (the expectation-maximization algorithm) to obtain maximum-likelihood estimates. Similarly, gene counting was used for the AJ sporadic affected group, in which two individuals were either AB or CH. For the AJ multiplex sample and the SOJ multiplex sample, chromosomes were clustered into two groups: a control group, which contained independent chromosomes not carried by any affected individual in the pedigree (n=52 for the AJ sample and n=8 for SOJ sample), and an affected group, which contained independent chromosomes carried by at least one affected individual in the pedigree (n=138 for AJ sample and n=32 for SOJ sample). In the SOJ multiplex families with CD, all parents were genotyped or could be reconstructed from unaffected sibs. In the AJ multiplex families, some parents were missing genotype information and could not be reconstructed from unaffected sibs. This and subsequent analyses focused only on chromosomes directly observed or reconstructed from unaffected sibs. Because of the genotyping of multiple individuals in the multiplex families (often including unaffected sibs), it was possible to unambiguously determine the phase of chromosomes included in both the affected and unaffected categories. In addition to the nine haplotypes listed in figure 1, we also combined haplotypes B and C into one group (corresponding to the R702W mutation) and haplotypes E and F into one group (corresponding to the 1007fs mutation).
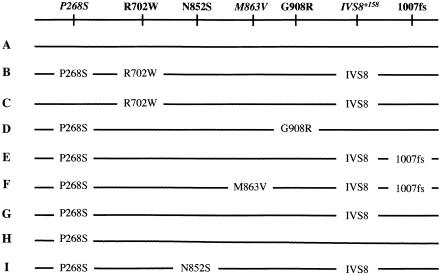
Figure 1.
Haplotypes in the AJ and SOJ patients with CD
Odds Ratios and Statistical Significance by Haplotype
Next, to determine whether each of the nine haplotypes (listed in fig. 1) was associated with the risk of CD, the odds ratios (ORs) and χ2 tests were calculated. First, the patients with sporadic AJ (n=72 chromosomes) were compared with the AJ controls (n=492 chromosomes). For individual haplotypes B, C, D, E, F, and I, ORs were calculated relative to haplotypes A+G+H (i.e., the non–mutation bearing haplotypes), combined by comparison of their frequencies in the case group versus the control group; standard Pearson χ2 tests of the 2×2 table were calculated to assess significance. ORs for haplotypes G and H were calculated relative to haplotype A only, to see if there was any difference in risk between haplotypes G and H (which do not carry mutations) and haplotype A.
For the multiplex families, analysis was based on the transmission/disequilibrium test (TDT) (Spielman et al. 1993). ORs were calculated as the ratio of the number of times a given haplotype X was transmitted versus not transmitted to affected children of heterozygous parents. For haplotypes B, C, D, E, F, and I (i.e., those carrying mutations), the heterozygous parent could have A, G, or H as the other haplotype. For haplotypes G and H, the heterozygous parent had only A as the other haplotype. Statistical significance was calculated using McNemar’s χ2 test as (T-NT)2/(T+NT), where T is the number of times X was transmitted and NT the number of times X was not transmitted (Spielman et al. 1993). The AJ multiplex families and SOJ multiplex families with CD were first analyzed separately and then combined.
Finally, a global estimate of ORs from the sporadic and multiplex groups was obtained by Mantel-Haenszel analysis, where, for each group, the natural log of the ORs was calculated along with its variance, and a combined log OR was obtained by taking the weighted sum, the weight being the inverse variance. An overall (Mantel-Haenszel) χ2 was also calculated as the square of the combined log OR divided by its variance (Kleinbaum et al. 1982).
Analysis of Mode of Inheritance
Next, the question of mode of inheritance was examined by estimating the risk of CD associated with carrying one or two mutations. For this analysis, the mutation-bearing haplotypes B, C, D, E, F, and I were grouped into a single group (called “M”) to obtain adequate numbers, and haplotypes A, G, and H were grouped into a single mutation-free group (called “W”). For the sporadic cases and controls, the ORs were estimated by counting the number of individuals by diplotype (i.e., haplotype pair) in the case versus control groups. Two ORs were calculated: OR1, based on diplotype MW versus WW, and OR2.1, based on diplotype MM versus MW. OR1 provides an estimate of the relative risk for heterozygotes (i.e., bearers of a single mutation), and OR2.1 provides an estimate of the dosage effect (i.e., relative risk for bearers of two mutations versus one mutation). The relative risk for diplotype MM versus WW is then given by OR2=OR1×OR2.1.
The multiplex families were analyzed by TDT. Here, OR1 was calculated from matings of MW×WW as the ratio of the number of MW versus WW offspring and from matings of MW×MW as half the ratio of the number of MW versus WW offspring. OR2.1 was calculated from matings of MM×MW as the ratio of the number of MM versus MW offspring and from matings of MW×MW as twice the ratio of the number of MM versus MW offspring. A combined estimate of the ORs from the sporadic (case-control) and multiplex (TDT) groups was obtained by Mantel-Haenszel analysis, similar to that described above. In addition, 95% CIs on the ORs were obtained from the log OR and its SE (Kleinbaum et al. 1982).
Determination of CARD15 Mutations and Chromosome 16q Linkage
Next, the degree to which the four mutations (R702W, G908R, 1007fs, and N852S) could account for the observed linkage evidence on chromosome 16 was determined on the basis of further analysis of the multiplex AJ and SOJ families. First, the haplotypes were categorized into two groups, as described above: a mutation-bearing group M (including haplotypes B, C, D, E, F, and I) and a mutation-free group W (haplotypes A, G, and H). The parents were then stratified into three heterozygous groups based on all SNPs tested plus the two microsatellites: (1) group W1W2, constituting parents carrying two distinguishable W haplotypes; (2) group MW, constituting parents with one M and one W haplotype; and (3) group M1M2, constituting parents with two distinguishable M haplotypes. Within each heterozygous parent class, we determined the number of affected sib pairs that shared a W haplotype, shared an M haplotype, or inherited different chromosomes from that heterozygous parent. This analysis was stratified by AJ versus SOJ, as well as by number of affected sibs in the family (two, three, or four). For the larger families, all possible affected sib pairs were formed. By this analysis, the overall degree of allele sharing in this region of chromosome 16 was examined, as well as how much of it was attributable to M chromosomes versus W chromosomes. If the linkage evidence was fully explained by the mutations included, then sharing of M chromosomes should explain the overall excess (>50%) observed.
Rare Variants
To determine whether additional rare variants could further explain linkage evidence on chromosome 16, the CARD15 gene was analyzed as described above. Rare variants found in the AJ sporadic and AJ/SOJ multiplex families were also genotyped in the AJ and SOJ controls to determine their general population frequency. In the multiplex families, identified rare variants were genotyped in the full family to determine whether the variant was shared among affected family members.
Microsatellite Distribution on Haplotypes
To study the evolutionary history and relationship of the various mutations and SNPs, the allele frequency distributions were examined for the two microsatellites (D16S3035 and D16S3136) on the nine SNP-based haplotypes included in this study. For this analysis, all independent chromosomes from the multiplex families were included, whether carried by affected individuals or not. This analysis focused on the larger, homogeneous AJ sample, which included a total of 181 chromosomes.
Results
Common and Rare CARD15 Mutations/Variants in AJ Patients with Familial and Sporadic CD and in Controls
Of the 115 patients from the AJ multiplex families with CD, 37.0% had one and 14.6% had two of the three common CARD15 mutations. These mutations were detected in 41.8% of their unaffected parents (34.1% had one mutation and 7.7% had two) and in 40% of their unaffected sibs (all with one mutation). Among the 36 AJ patients with sporadic CD, 27.7% of patients had one and two patients (5.5%) had two (R702W/R702W and R702W/G908R) of the common mutations. In contrast, 16.2% and 2.0% of the 246 unrelated AJ healthy controls had one or two of these mutations, respectively. The combined allele frequency of the three mutations was 0.101 in AJ controls, 0.208 in patients with sporadic CD, and 0.301 in patients from multiplex families with CD (table 2).
Table 2.
Haplotype Frequencies by Group[Note]
|
Frequency in AJ Group |
Frequency in SOJ Group |
||||||||
| CD Familialb |
|||||||||
| Haplotype | Control(n=492) | MultiplexUnaffecteda(n=52) | CD Sporadic(n=72) | Total(n=143) | Central Europe(n=50) | Eastern Europe(n=67) | Control(n=164) | MultiplexUnaffecteda(n=8) | CDFamilialb(n=32) |
| A | .705 | .712 | .694 | .559 | .420 | .597 | .709 | 1.0 | .563 |
| B | .012 | .000 | .056 | .028 | .040 | .030 | .018 | .000 | .094 |
| C | .016 | .019 | .014 | .028 | .000 | .045 | .000 | .000 | .000 |
| B+C (702W) | .028 | .019 | .069 | .056 | .040 | .075 | .018 | .000 | .094 |
| D (908R) | .043 | .038 | .083 | .112 | .200c | .075 | .036 | .000 | .188 |
| E | .014 | .019 | .000 | .077 | .105 | .060 | .000 | .000 | .063 |
| F | .016 | .038 | .056 | .056 | .107 | .030 | .000 | .000 | .000 |
| E+F (1007fs) | .030 | .058 | .056 | .133 | .200 | .090 | .000 | .000 | .063 |
| I (852S) | .012 | .019 | .014 | .035 | .040 | .045 | .018 | .000 | .000 |
| G | .075 | .038 | .069 | .063 | .040 | .090 | .110 | .000 | .063 |
| H | .106 | .115 | .014 | .042 | .060 | .030 | .110 | .000 | .031 |
| Common mutationsc | .101 | .115 | .208 | .301 | .440d | .240 | .054 | .000 | .345 |
Note.— n = number of chromosomes.
Independent chromosomes carried by no affected individual in family.
Independent chromosomes carried by at least one affected individual in family.
702W, 908R, and 1007fs.
Significant (P<.05) difference between Central Europe and Eastern Europe.
Common and Rare CARD15 Mutations/Variants in SOJ Patients with Familial CD and in Controls
Of the 21 familial SOJ patients with CD, 23.8% and 33.3% inherited two or one of the common mutant alleles, respectively, and 52.6% of their parents and 38.4% of their unaffected sibs had one of the common CARD15 mutations. No parent or unaffected sib carried two mutations. The combined allele frequency for the three common mutations was 0.345 among the SOJ patients from multiplex families with CD, whereas the combined mutation frequency in the SOJ healthy subjects was 0.054. It should be kept in mind that both the SOJ patients with CD and the healthy subjects were of heterogeneous ethnic Sephardi and Oriental (Mediterranean) origin. The proportion of SJ and OJ alleles among these controls was about 1:1.
Haplotype Frequencies
Haplotype analysis of the three common mutations and the common polymorphism P268S revealed six different haplotypes in the AJ and SOJ families with CD (fig. 1). These included haplotypes A (no variants), B (P268S/R702W), C (P268/R702W), D (P268S/G908R), E (P268S/1007fs), and H (P268S alone). Detection of the IVS8+158 polymorphism and rare variants increased the number of haplotypes to nine. The additional haplotypes were F (P268S/M863V/1007fs), G (P268S/IVS8+158), and I (P268S/N852S) (fig. 1). The recently reported IVS8+158 polymorphism (Sugimura et al. 2003) was present on all the haplotypes (fig. 1), with the exception of haplotypes A, D, and H.
Although prior studies have focused singly on the 702W mutation, we found it to be associated with two different haplotypes (B and C) on the basis of the presence or absence, respectively, of the P268S variant. Similarly, we found the 1007fs mutation to be associated with two different haplotypes on the basis of the absence or presence, respectively, of the M863V variant. Haplotype frequencies were calculated on the basis of independent chromosome numbers, as explained in the “Subjects and Methods” section. Haplotype and mutation frequencies in the “unaffected chromosome” group from the AJ multiplex families were slightly higher than but similar to those found in the AJ control group (table 2), ranging from 1.2% (for N852S) to 2.8% and 3.0% (for R702W and 1007fs) to 4.3% (for G908R). Although the allele frequencies are lower for R702W and G908R, the combined mutation frequency for mutations R702W, G908R, 1007fs, and N852S was 0.134 in the multiplex “unaffected chromosome” group and was 0.113 in the AJ control group. Even when the newly identified N852S sequence variant was excluded, the frequencies of the other three common mutations combined were somewhat higher than have been reported for other white populations (see the “Discussion” section). All four mutations were found at increased frequency in both the AJ sporadic group and the AJ multiplex group, but the frequencies were generally more increased in the multiplex group. For the sporadic group, the frequencies ranged from 1.4% (for N852S) to 5.6% (for 1007fs) to 6.9% (for R702W) to 8.3% (for G908R). For the multiplex group, the corresponding frequencies were 3.5%, 13.3%, 5.6%, and 11.2%. For the four mutations combined, the overall frequency was 22.2% for the sporadic group and 33.6% for the multiplex group. Most of these mutations appeared to be similarly elevated in frequency in the SOJ group (with the exception of N852S), ranging from 6.3% (for 1007fs) to 9.4% (for R702W) to 18.8% (for G908R), for a total mutation frequency of 34.5%.
The total haplotype frequency for mutation-bearing chromosomes was significantly elevated (P<.05) in AJ patients with CD in the multiplex families with Central European ancestry (0.440), compared with those from Eastern European families (0.240). The difference was due to the excess frequencies of both the 908R and 1007fs mutations in the former group, although only the frequency difference for 908R was formally significant (P<.05). This difference was consistent with the previously reported elevated frequency of CD in AJ individuals with ancestry from Central as opposed to Eastern Europe (Roth et al. 1989a, 1989b; Zlotogora et al. 1990; Yang et al. 1993) . The total mutation frequency in the patients with CD from families of SOJ origin was comparable to that of the AJ families, although the 908R mutation was found at somewhat higher frequency and the 1007fs mutation was found at a somewhat lower frequency. However, the 908R mutation was found at a comparable frequency in the AJ and SOJ healthy groups. On the other hand, the 1007fs mutation also was found at lower frequency (absent) in the SOJ controls, suggesting that this mutation is less frequent in the Mediterranean/Middle East than in Central/Eastern Europe.
Risks Associated with Individual Mutations and Haplotypes
As suggested by the results in table 2, all mutations showed significant association with CD risk (table 3). For the sporadic case-control analysis, ORs were elevated for all mutations, ranging from 1.30 to 2.78, but were significant only for R702W, G908R, and 1007fs. The OR for all mutations combined was 2.22 (P<.01). For the TDT analysis based on the multiplex families (AJ and SOJ combined), again, all ORs were elevated and significant and somewhat higher than for the sporadic case-control analysis, with values of 2.27–7.00. For all mutations combined, the OR was 3.05 (P<.001). Examination of all groups combined in the Mantel-Haenszel analysis suggested that all mutations have a similar degree of elevated risk based on the OR, which ranged from 2.87 (for R702W) to 2.26 (for G908R) to 3.03 (for 1007fs) to 3.06 (for N852S). The ORs for haplotypes B and C, both of which carry the R702W mutation, appeared to be discrepant, with a much higher OR for haplotype B than for C. Although it is possible that either the P268S variant or another variant in linkage disequilibrium with it influences—in cis with the R702W mutation—the risk of CD, it is also possible that the observed difference is simply a matter of chance, owing to the low population frequency of these haplotypes. The higher observed ORs for the multiplex group compared with the sporadic group was not unexpected, depending on the mode of inheritance, as we detail below. Table 3 also indicates a significantly decreased risk for haplotype H in the case-control analysis, but a decreased risk of only marginal significance is indicated in the combined Mantel-Haenszel analysis. This protective effect of the P268S variant alone has not been reported previously and requires further independent study to determine whether this observation is replicable.
Table 3.
ORs and χ2 Values for Sporadic Cases (by Case-Control Analysis) and Multiplex Sibships (by TDT Analysis) and Mantel-Haenszel Combined Estimates[Note]
|
AJ Sporadic |
Multiplex |
CombinedMantel-Haenszel |
||||||||||
| AJ |
SOJ |
Total |
||||||||||
| Haplotype | OR (95% CI) | χ2 | T/NT | OR | χ2 | T/NT | OR | χ2 | OR (95% CI) | χ2 | OR (95% CI) | χ2 |
| B | 5.19 (1.61–16.2) | 7.63** | 1/1 | 1.00 | .00 | 5/0 | ∞ | 5.00* | 6.00 (.94–38.5) | 3.57* | 5.40 (1.8–16.3) | 8.95** |
| C | .97 (.53–1.8) | .00 | 3/2 | 1.50 | .20 | 0/0 | … | … | 1.50 (.25–8.9) | .20 | 1.25 (.31–5.0) | .10 |
| B+C (702W) | 2.78 (1.0–7.7) | 3.88* | 4/3 | 1.33 | .14 | 5/0 | ∞ | 5.00* | 3.00 (.87–10.4) | 3.00* | 2.87 (1.3–6.5) | 6.30** |
| D (908R) | 2.22 (.88–5.6) | 2.86* | 18/5 | 3.60 | 7.35** | 7/6 | 1.17 | .08 | 2.27 (1.1–4.5) | 5.44* | 2.26 (1.3–4.0) | 7.88** |
| E | .00 | .90 | 11/3 | 3.67 | 4.57* | 5/0 | ∞ | 5.00* | 5.33 (1.8–16.0) | 8.89** | 3.71 (1.2–11.5) | 5.14* |
| F | 3.89 (1.2–12.3) | 5.38* | 10/4 | 2.50 | 2.57 | 0/0 | … | … | 2.50 (.82–7.7) | 2.57 | 3.08 (1.3–7.2) | 6.81** |
| E+F (1007fs) | 2.08 (.68–6.4) | 1.65 | 21/7 | 3.00 | 7.00* | 5/0 | ∞ | 5.00* | 3.71 (1.7–8.1) | 10.9*** | 3.03 (1.6–5.9) | 10.44** |
| I(852S) | 1.30 (.16–10.6) | .06 | 7/1 | 7.00 | 4.50* | 0/0 | … | … | 7.00 (1.2–42.3) | 4.50* | 3.06 (.69–13.7) | 2.15 |
| B+C+D+E+F+I | 2.22 (1.2–4.1) | 6.63** | 50/16 | 3.13 | 17.5*** | 17/6 | 2.83 | 5.26* | 3.05 (1.9–4.8) | 22.8*** | 2.71 (1.9–4.0) | 26.8*** |
| G | .94 (.40–2.2) | .02 | 8/13 | .62 | 1.19 | 2/3 | .67 | .20 | .63 (.29–1.4) | 1.38 | .73 (.39–1.4) | .98 |
| H | .13 (.05–.31) | 5.32* | 4/6 | .67 | .40 | 1/2 | .50 | .33 | .63 (.21–1.9) | .69 | .43 .16–1.2) | 2.83 |
Note.— For sporadic cases, OR and χ2 were derived from case-control analysis using haplotype A as reference for haplotypes G and H, and haplotypes A+G+H (grouped) as the reference for haplotypes B, C, D, E, and I. For multiplex cases, OR and χ2 were derived from TDT analysis. T/NT = number of transmitted/number of nontransmitted alleles from heterozygous parents; for transmission of haplotypes B, C, D, E, F, or I from heterozygous parents, the parent’s other haplotype could be A, G, or H; for transmission of haplotypes G or H from heterozygous parents, the parent’s other haplotype could only be A. Combined OR and χ2 based on Mantel-Haenszel analysis of sporadic (case-control) and both multiplex (TDT) values. P values for haplotypes B, C, D, E, F, and I are based on one-sided tests; P values for haplotypes G and H are based on two-sided tests. *P<.05; **P<.01; ***P<.001.
Risks for Single versus Double Dose
ORs were calculated to estimate the relative risk of MW versus WW (OR1) and MM versus MW (OR2.1), where M represents the group of mutation-bearing haplotypes (B, C, D, E, F, and I), and W represents the wild-type or mutation-free haplotypes (A, G, and H). In the AJ sporadic CD group, there were 23 WW, 10 WM, and 3 MM genotypes, whereas, in the control group, there were 195 WW, 46 WM, and 5 MM genotypes. For this analysis, the Hardy-Weinberg expected frequencies (193.18 for WW, 49.63 for WM, and 3.19 for MM) were used to obtain more-precise estimates. From the AJ sporadic case-control study, OR1 is estimated as 1.69 and OR2.1 as 4.67. The TDT analysis of the combined AJ and SOJ multiplex families had similar values. Matings of WM×WW resulted in 12 WW and 18 WM offspring; matings of WM×WM had 0 WW, 8 WM, and 15 MM offspring; and MM×WM matings had 2 WM and 6 MM offspring. OR1 was estimated as 1.83, and OR2.1 was estimated as 3.50. The Mantel-Haenszel estimate for the case-control and TDT analyses combined was 1.78 for OR1 and 3.71 for OR2.1, both of which are statistically significant (table A [online only]). These results indicate both an increased risk for heterozygotes—that is, those carrying a single CARD15 mutation—and a dosage effect, with a significantly increased risk for those carrying two mutations versus one. These results also provide the explanation for the somewhat increased ORs observed for single haplotypes in the TDT analysis of multiplex families versus the case-control analysis of the sporadic cases (table 3). Analysis of ORs for single haplotypes is based on an average of ORs for one-dose and two-dose cases. The sampling of multiplex sibships gave rise to a higher proportion of affected individuals in those families carrying two mutations compared with the sporadic cases, leading to a higher estimated OR for the former. When analysis was stratified by dose, the ORs for the sporadic cases and multiplex families were not different.
Table A.
Estimated Relative Risks (ORs) by Gene Dosage[Note]
|
No. with Genotype |
OR |
||||
| Sample | WW | WM | MM | WM vs. WW | MM vs. WM |
| AJ (Case-Control): | |||||
| Cases | 23 | 10 | 3 | 1.69 | 4.67 |
| Controlsa | 193.18 | 49.63 | 3.19 | … | … |
| AJ+SOJ (Multiplex, TDT): | |||||
| WM×WW | 12 | 18 | … | 1.5 | … |
| WM×WM | 0 | 8 | 15 | ∞ | 3.75 |
| MM×WM | … | 2 | 6 | … | 3.00 |
| Combined | 1.83 | 3.50 | |||
| Combined Mantel-Haenszel | 1.78 (95% CI 1.06–2.97) | 3.71 (95% CI 1.89–7.29) | |||
Note.— W = non-mutation-bearing haplotypes (A+G+H); M = all mutation-bearing haplotypes (B+C+D+E+F+I).
Values are Hardy-Weinberg expected frequencies; actual frequencies were 195 (WW), 46 (WM), and 5 (MM).
CARD15 Mutations versus Chromosome 16q Linkage
A major question regarding CARD15 is whether the identified mutations can explain the linkage evidence in this region of chromosome 16q in multiplex families with CD. Our collection of 50 AJ and 10 SOJ multiplex families provided the opportunity to address this question. We investigated the distribution of allele sharing by affected sib pairs stratified by the heterozygous parent genotype and the number of affected sibs (table B [online only]). The overall allele sharing in the entire sample was 57.8% (74 shared and 54 unshared), comparable to prior linkage studies in this region (Cavanaugh 2001). Sharing was greater in the AJ families (61.8%; 63 shared and 39 unshared) than in the SOJ families (42.3%; 11 shared and 15 unshared), although the number of SOJ families was small (χ2=3.1). Also noticeable was the difference in allele sharing for affected sib pairs (60.7%) versus the larger families (trios and quads), where sharing was 51.3% (20 shared versus 19 unshared) (χ2=1.0). Although the allele sharing differences between AJ and SOJ and between pairs versus trios plus quads were not significant, the latter result was consistent with the observation of greater sharing in affected pairs than in larger sibships from a pooled analysis of a large number of families with CD (Cavanaugh 2001).
Table B.
Affected Sib Pair Analysis: Distribution of Shared and Unshared Alleles by Heterozygous Parental Genotype[Note]
| AJ |
AJ+SOJ |
Total |
||||||||||
| HeterozygousParentalGenotypea | SA | SM | ST | U | SA | SM | ST | U | SA | SM | ST | U |
| W1W2: | ||||||||||||
| Pairs | 22 | 12 | 2 | 4 | 24 | 16 | ||||||
| Trios | 9 | 6 | 2 | 4 | 11 | 10 | ||||||
| Quads | 5 | 7 | 5 | 7 | ||||||||
| Total | 36 | 25 | 4 | 8 | 40 | 33 | ||||||
| WM: | ||||||||||||
| Pairs | 2 | 19 | 21 | 9 | 1 | 3 | 4 | 7 | 3 | 22 | 25 | 16 |
| Trios | 0 | 1 | 1 | 2 | 0 | 3 | 3 | 0 | 0 | 4 | 4 | 2 |
| Total | 2 | 20 | 22 | 11 | 1 | 6 | 7 | 7 | 3 | 26 | 29 | 18 |
| M1M2: | ||||||||||||
| Pairs | 5 | 3 | … | … | 5 | 3 | ||||||
| All: | ||||||||||||
| Pairs | 48 | 24 | 6 | 11 | 54 | 35 | ||||||
| Trios+quads | 15 | 15 | 5 | 4 | 20 | 19 | ||||||
| Total | 63 | 39 | 11 | 15 | 74 | 54 | ||||||
Note.— SA = number of sib pairs sharing a W haplotype; SM = number of sib pairs sharing an M haplotype; ST = number of sib pairs sharing a W or M haplotype; U = number of sib pairs not sharing a haplotype.
W = non-mutation haplotypes (A+G+H); M = mutation-bearing haplotypes (B+C+D+E+F+I); W1W2 = parent heterozygous for two different W haplotypes; M1M2 = parent heterozygous for two different M haplotypes.
When we considered allele sharing by parental genotype, overall sharing was slightly higher from MW parents (61.7%), where 26 of the 29 shared alleles were M, than from W1W2 parents, where the overall sharing was 54.8% (40 shared and 33 unshared). When we considered affected sib pairs only, however, we found similar sharing from MW and W1W2 parents, at 61.0% and 60.0%, respectively. This observation suggests that the four identified mutations in CARD15, despite being positively associated with risk of CD, do not fully explain the linkage evidence on chromosome 16q.
It is interesting that there is also a substantial difference in the distribution of parental genotypes for the pairs versus the trios plus quads. For the pairs, 40 parents are W1W2 heterozygotes, 41 are MW heterozygotes and 8 are M1M2 heterozygotes. In contrast, for the trios plus quads, 9 parents are W1W2 heterozygotes compared with 2 who are MW and 0 who are M1M2 . Thus, for the sib pairs, there are 57 M haplotypes (32.0%) out of 178 total among the parents, whereas, for the trios plus quads, there are 2 M haplotypes (9.1%) out of 22 total. This result counters what might have been expected—namely, an increase in the number of M haplotypes among the parents of the larger sibships—and suggests the possibility of genetic heterogeneity, whereby the larger sibships are segregating for another gene unrelated to CARD15.
Additional Variants
Because it appeared that the three previously identified mutations in CARD15 (R702W, G908R, and 1007fs) and the newly identified N852S did not fully explain our chromosome 16q linkage evidence, we searched for additional rare variants on the “mutation-free” haplotypes (A, G, and H), as well as for common variants. In the 43 AJ patients with familial CD and 35 AJ patients with sporadic CD who had only one or none of the common CARD15 mutations, DHPLC heteroduplex analysis and sequencing revealed that all had a novel or previously reported variant (table 4). These included the novel changes D113N, D357A, and N852S and the previously reported variants L248R, E441K, A612T, M863V, A918D, 5′-UTR-33T→G, 5′-UTR-15T→A, R459R, R587R, A611A, IVS4+10A→C, V955I (Lesage et al. 2002), and IVS8+158 (Sugimura et al. 2003). Of the six SOJ patients with familial CD who had only one or none of the common mutations, four had rare CARD15 variants, including two novel (I363F and L550V) and two previously reported (R703C and A612T) changes (Lesage et al. 2002) (table 4). These changes occurred in four unrelated SOJ subjects with CD. The rare amino acid substitutions (D113N, L248R, D357A, I363F, E441K, L550V, A612T, R703C, and A918D) all occurred with the P268 allele (i.e., on haplotype A) (table 4). We examined the segregation pattern of the seven rare amino acid substitutions identified in the multiplex families, one of which occurred in two different families and another of which occurred in three families (table 4). In four of seven informative affected sib pairs, the identified variant was shared between the affected sibs, and in the other three it was not shared. Thus, these additional rare variants cannot be shown (by statistical evidence) to be disease predisposing, and they do not provide an explanation for the residual linkage evidence observed on chromosome 16q in our study.
Table 4.
Additional CARD15 Sequence Variants Detected in AJ Controls, AJ Patients with Sporadic and Multiplex CD, SOJ Controls, and SOJ Patients from Multiplex Families with CD
|
No. with Variant/Total in Group |
|||||||
| AJ Patients with CD |
|||||||
| Variant | Haplotype | Multiplex | Sporadic | AJ Controls | SOJ Patientsfrom MultiplexFamilies with CD | SOJ Controls | Notes |
| Previously reported missense changes: | |||||||
| L248R | A | 2/138 | 0/72 | 1/376 | 0/32 | 0/164 | In one AJ family, unshared by affected sib pair; other AJ family uninformative |
| E441K | A | 1/138 | 0/72 | 0/376 | 0/32 | 0/164 | Shared by AJ affected sib pair |
| A612T | A | 2/138 | 0/72 | 4/376 | 1/32 | 0/164 | In one AJ family, shared by affected father and son; one AJ family unshared by affected sib pair; one SOJ family uninformative |
| R703C | A | 0/138 | 0/72 | 0/376 | 1/32 | 0/164 | Shared by SOJ affected sib pair |
| A918D | A | 1/138 | 0/72 | 0/376 | 0/32 | 0/164 | Shared by AJ affected sib pair |
| A725G | A | 0/86 | 0/72 | 0/376 | 0/12 | 2/164 | |
| M863V | I | 8/138 | 4/72 | 8/492 | 0/32 | 0/164 | |
| V955I | A | 3/86 | 5/72 | 1/12 | |||
| Previously reported silent or intronic changes: | |||||||
| 5′ UTR −33 T→G | All, other than A | 27/86 | 22/72 | 5/12 | |||
| 5′ UTR −15 T→A | A | 2/86 | 0/72 | 0/12 | |||
| S178S | A | 25/86 | 30/72 | 5/12 | |||
| R459R (SNP6) | All, other than A | 29/86 | 23/72 | 5/12 | |||
| R587R (SNP7) | A | 25/86 | 32/72 | 4/12 | |||
| A611A | A | 1/86 | 1/72 | 0/12 | |||
| IVS4+10 A→C | A | 2/86 | 2/72 | 7/376 | 0/12 | 2/164 | |
| IVS8+158 C→T | B, C, E, F, G, I | 50/138 | 15/72 | 72/492 | 6/32 | 24/164 | |
| Previously unreported missense changes: | |||||||
| D113N | A | 0/138 | 1/72 | 0/376 | 0/32 | 0/164 | |
| D357A | A | 0/138 | 1/72 | 1/376 | 0/32 | 0/164 | |
| I363F | A | 0/138 | 0/72 | 0/376 | 1/32 | 1/164 | Shared by SOJ affected sib pair |
| L550V | A | 0/138 | 0/72 | 0/376 | 1/32 | 0/164 | Unshared by SOJ affected sib pair |
| I939V | A | 0/138 | 0/72 | 0/376 | 0/32 | 2/164 | |
Evolutionary History of Haplotypes
The presence of information on the two microsatellite loci D16S3035 and D16S3136 on haplotypes A–I allowed consideration of some historical and evolutionary aspects of these chromosomes. This analysis focused on the much larger and homogeneous collection of AJ chromosomes, although the results were not substantially different for the SOJ group. On the basis of the presence of the various SNPs, haplotype H could have been derived from haplotype A by a single mutation (P268S). Further single changes occurred on haplotype H to give rise to haplotype D (mutation G908R) and haplotype G (variant IVS8). Subsequent single mutations then occurred on haplotype G to give rise to haplotype I (variant N852S), haplotype E (mutation 1007fs), and haplotype B (mutation R702W). Haplotype E sustained another single variant (M863V) to give rise to haplotype F. Haplotype C, without the P268S variant but carrying both R702W and IVS8, may have arisen through recombination of haplotype B with an A haplotype. This is the only haplotype requiring an ancestral recombination or gene conversion event to explain its existence. The microsatellite data in table 5 support such a model. Haplotype H has a similar allele frequency distribution to that of haplotype A, suggesting that the P268S change was a relatively early event. Haplotype D, however, shows a distinct pattern at D16S3136, carrying the 204 allele at high frequency, which suggests a relatively more recent event for the G908R mutation. Haplotype G also shows a somewhat altered pattern from haplotype A (again a high frequency of the 204 allele at D16S3136). Haplotypes I and E, representing single mutation events on haplotype G, both show a very distinctive pattern, with haplotype I carrying a 268 allele at D16S3035 (rare on haplotype G) and haplotype E carrying a 264 allele at D16S3035 (also rare on haplotype G). Haplotype F shows an identical pattern to that of haplotype E at D16S3035 and a more restricted distribution at D16S3136 than haplotype E, consistent with its derivation from haplotype E. Haplotypes B and C also show a distinctive and similar pattern, with the predominance of the 202 allele at D16S3136. All the mutation-bearing haplotypes show a much narrower range of microsatellite allele sizes than the common A haplotype, consistent with their recent origin.
Table 5.
Microsatellite Allele Distribution On Haplotypes In AJ and SOJ Individuals
|
Frequency of D16S3035 Allele |
Frequency of D16S3136 Allele |
|||||||||||
| Haplotype (SNPs)and Ethnicity | No. of Chromosomes | ⩽264 | 266 | 268 | 270 | ⩽198 | 200 | 202 | 204 | 206 | 208 | 210 |
| A: | ||||||||||||
| AJ | 107 | .11 | .81 | .06 | .02 | .07 | .17 | .33 | .32 | .09 | .03 | .00 |
| SOJ | 22 | .05 | .86 | .05 | .05 | .09 | .05 | .50 | .18 | .14 | .05 | .00 |
| H (268S): | ||||||||||||
| AJ | 11 | .00 | 1.0 | .00 | .00 | .00 | .00 | .45 | .45 | .10 | .00 | .00 |
| SOJ | 1 | .00 | 1.0 | .00 | .00 | .00 | .00 | 1.0 | .00 | .00 | .00 | .00 |
| D (268S+908R): | ||||||||||||
| AJ | 18 | .06 | .89 | .06 | .00 | .00 | .00 | .11 | .83 | .06 | .00 | .00 |
| SOJ | 6 | .00 | .83 | .17 | .00 | .00 | .00 | .17 | .83 | .04 | .00 | .00 |
| G (268S+IVS8): | ||||||||||||
| AJ | 10 | .00 | .90 | .00 | .10 | .00 | .11 | .11 | .67 | .00 | .11 | .00 |
| SOJ | 2 | .00 | 1.0 | .00 | .00 | .00 | .00 | .00 | 1.0 | .00 | .00 | .00 |
| I (268S+IVS8+852S): | ||||||||||||
| AJ | 6 | .00 | .00 | 1.0 | .00 | .00 | .00 | .00 | .00 | .00 | .00 | 1.0 |
| SOJ | 0 | … | … | … | … | … | … | … | … | … | … | … |
| E (268S+IVS8+1007fs): | ||||||||||||
| AJ | 11 | 1.0 | .00 | .00 | .00 | .09 | .00 | .09 | .73 | .09 | .00 | .00 |
| SOJ | 2 | 1.0 | .00 | .00 | .00 | .00 | .00 | .00 | 1.0 | .00 | .00 | .00 |
| F (268S+IVS8+1007fs +863V): | ||||||||||||
| AJ | 11 | 1.0 | .00 | .00 | .00 | .00 | .00 | .00 | 1.0 | .00 | .00 | .00 |
| SOJ | 0 | … | … | … | … | … | … | … | … | … | … | … |
| B (268S+IVS8+702W): | ||||||||||||
| AJ | 3 | .00 | 1.0 | .00 | .00 | .00 | .00 | 1.0 | .00 | .00 | .00 | .00 |
| SOJ | 3 | .00 | .67 | .00 | .33 | .00 | .00 | 1.0 | .00 | .00 | .00 | .00 |
| C (IVS8+702W): | ||||||||||||
| AJ | 4 | .25 | .75 | .00 | .00 | .00 | .00 | .75 | .25 | .00 | .00 | .00 |
| SOJ | 0 | … | … | … | … | … | … | … | … | … | … | … |
Discussion
Recently, a CD susceptibility gene at the IBD1 locus on chromosome 16 was identified as the CARD15/NOD2 gene. To date, three common (R702W, G908R, and 1007fs) and >25 rare CARD15 variants have been identified (Hampe et al. 2001; Hugot et al. 2001; Ogura et al. 2001; Lesage et al. 2002). The results presented here confirm the association of the three common mutations with CD in both AJ and SOJ patients. In the unaffected controls, the total allele frequency for the three mutations combined in the Ashkenazi population (10.1%) was higher than in the (mixed Mediterranean/Middle Eastern) Sephardi/Oriental group (5.4%). This was also true for all three mutations individually, but it was particularly the case for the 1007fs mutation. On the other hand, the same was not true in the CD cases, for which the overall allele frequency was slightly higher in the SOJ multiplex group (34.5%) than in the AJ multiplex group (30.1%). Similarly, other studies of AJ patients with CD have consistently found an increased frequency of the G908R mutation compared with non-Jewish populations, but this has not been true for the other two mutations (Zhou et al. 2002; Bonen et al. 2003; Sugimura et al. 2003).
The prevalence of CD in the Ashkenazim is unusually high, having a two- to fourfold greater frequency than in non-Jewish European or North American whites (Roth et al. 1989a, 1989b; Sonnenberg et al. 1991; Kurata et al. 1992; Probert et al. 1993; Yang et al. 2001). Among AJ patients with CD, a higher prevalence has been noted in those with origins from Central Europe than in those from Russia and Poland (Roth et al. 1989a, 1989b; Zlotogora et al. 1990; Yang et al. 1993). Consistent with this latter observation, in the multiplex AJ families with CD studied here, there also was a higher frequency of the three common CARD15 mutations in AJ patients with origins in Central Europe (44.0%) than in those from Eastern Europe (24.0%). The excess frequency was observed primarily for the G908R and 1007fs mutations.
One potentially interesting contributor to differences between AJ CD and non-Jewish CD may be mutation R702W. In AJ individuals, this mutation was present on two different haplotype backgrounds, one with the P268S allele (haplotype B) and the other with the P268 allele (haplotype C). The P268S allele was completely associated with the other two mutations G908R and 1007fs. In our study, an increased risk was found for the R702W allele only in the context of haplotype B (with P268S) and not with haplotype C (with P268) (table 3). Also, haplotype B occurred only in AJ patients with CD whose ancestry was from Central Europe. Other studies of non-Jewish CD have reported only haplotype B. It will be interesting to determine, in other studies of AJ patients with CD, whether this background effect of P268S on R702W expression can be confirmed.
Another important question is whether CARD15 is the only CD susceptibility gene on chromosome 16. This question is addressed by considering the degree to which identified CARD15 mutations explain the linkage evidence observed in multiplex families. The overwhelming answer to this question now appears to be that CARD15 does not fully explain the linkage evidence. At least five studies now show that CD affected sib pair families that segregate none of the three common CARD15 mutations still demonstrate linkage on chromosome 16 (Hugot et al. 2001; Hampe et al. 2002; Cavanaugh et al. 2003; Sugimura et al. 2003; present study). In our study, the mean allele sharing in families with CARD15 mutations was 34/55 (61.8%), versus 40/73 (54.8%) in families without CARD15 mutations.
It also appears that the residual allele sharing observed on chromosome 16 cannot be explained by additional CARD15 variants. In our study, we employed DHPLC heteroduplex analysis and sequencing of the CARD15 gene in 43 AJ patients with familial CD and 34 AJ patients with sporadic CD who had only one or no CARD15 mutation. Among these patients, three novel (D113N, D357A, and N852S) and several previously reported sequence changes were identified, including L248R, E441K, A612T, M863V, and A918D. The new variants D113N, D357A, and N852S were in the CARD1, NBD, and the fourth LRR domain of CARD15, respectively. Of these, N852S, which is a conserved amino acid in the mouse CARD15 gene, was found in six AJ families with CD and in one AJ patient with sporadic CD, whereas the conserved L248R and A612T were each detected in two AJ families with CD. In six SOJ subjects with CD without two common CARD15 mutations, DHPLC analysis revealed two previously reported variants, R703C and A612T, and two novel variants, I363F and L550V. Both novel variants were in the NBD domain of CARD15, L550V being conserved in mouse. However, in AJ multiplex families with CD, although moderately convincing evidence was found for variant N852S (with a frequency of ∼1% in the AJ population) to be disease predisposing, excess allele sharing was not found for the other rare variants. Also, support for another recently reported high-frequency variant (IVS+158C→T) being associated with CD (Sugimura et al. 2003) was not found. None of the additional reported variants, either in this or in previous studies, was able to fully explain the residual linkage evidence observed on chromosome 16.
Of note, both the linkage evidence and presence of CARD15 mutations were related to the number of affected individuals in the family. In this study, both the linkage evidence (mean allele sharing) and the presence of CARD15 mutations decreased in families with three or more CD cases versus those with two. The pooled linkage analysis also found decreased allele sharing in families with three or more CD affected sibs (Cavanaugh 2001). According to any simple model of inheritance, the frequency of CARD15 mutations should increase with the number of CD cases in the family. The observed pattern raises the likelihood of genetic complexity in the form of genetic heterogeneity. The evidence supports both an additional susceptibility locus on chromosome 16 as well as another locus (or loci) elsewhere.
Acknowledgments
The authors wish to thank the many patients and families with CD for their participation in these studies. We especially thank the many physicians who referred patients for these studies, including David Sachar, Arthur Kornbluth, Simon Lichtiger, Adrian Greenstein, Steven H. Itzkowitz, David Jaffe, Susan Rose, Thomas Alman, Mark Babyatsky, and Saul Agus. We acknowledge Monica Erazo, Eric Smith, and Suresh Pattumudi for their excellent technical assistance. This research was supported by a grant from the New York Crohn’s Foundation.
Appendix: Supplemental Methods
DNA Isolation and PCR Amplification
Genomic DNA was isolated from peripheral blood collected in EDTA from patients, family members, and normal controls, through use of the Puregene Isolation Kit, according to the manufacturer’s instructions (Genetra Systems). For future studies, lymphoblast cell lines were established and maintained by standard procedures (Anderson and Gusella 1984).
Genomic DNA was PCR-amplified in 96-well microtitre plates in an oil-free system using a DNA Engine PTC-200 Thermal Cycler (MJ Research). Reaction mixtures (50-μl) contained 50 ng of genomic DNA, 2 mM MgCl2, 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 200 nM of each primer (see table 1), 0.2 mM dNTPs, and 2 U of Taq DNA polymerase (AmpliTaq Gold). For the microsatellite markers, the reactions were performed in 10-μl volumes with the same concentrations of reagents. For PCR, the reaction mixtures were initially incubated at 95°C for 10 min and then were amplified for 35 cycles for mutation detection or 27 cycles for microsatellite amplification, with denaturation at 94°C for 30 s, annealing at a specific temperature (52–58°C) for each fragment for 30 s, extension at 72°C for 30 s, and a final extension step at 72°C for 7 min.
Haplotype Analysis
All members of the 60 families with CD were initially haplotyped at the IBD1 locus with two closely linked microsatellite markers, D16S3035 and D16S3136, and the CARD15 intragenic polymorphism, P268S, as described elsewhere (Hugot et al. 1996; Lesage et al. 2002). Forward primers for microsatellite analyses were fluorescently dye-labeled (Invitrogen Life Technologies). PCRs were performed as described above and were analyzed with either an ABI Prism 3100 Genetic Analyzer or on an ABI Prism 377 DNA Sequencer, using GeneScan Analysis Software (version 3.1.2) and Genotyper Software (version 2.5) (Perkin-Elmer-Cetus).
Detection of the CARD15 Common Variants
The common CARD15 mutations (R702W, G908R, and 1007fs) and the P268S polymorphism were detected by restriction analyses. The specific PCR primers and the size of the restriction fragments before and after digestion are given in table 1. For the G908R mutation, PCR amplification and digestion with HhaI was performed as described elsewhere (Lesage et al. 2002). Restriction analyses were developed for the R702W mutation and the IVS8+158 polymorphism, which abolish MspI and XhoI restriction sites, respectively, and for the 1007fs, M863V, and P268S variants, which introduce NlaIV, NcoI, and BamHI sites, respectively. The samples found positive for the R702W mutation by restriction analysis were sequenced to confirm the correct base change. For detection of the N852S mutation, a modified reverse primer was used to introduce an AluI restriction site by altering a base two nucleotides downstream from the mutation site. The PCR products were incubated with the relevant restriction enzyme for at least 3 h at 37°C, and the fragments were separated on 2%–4% agarose gels, depending on the expected fragment sizes.
DHPLC Analysis
CARD15 exons 2–12 and adjacent intronic sequences were amplified individually, with the exception of the ∼1.8-kb exon 4, which was amplified in five overlapping fragments, as described elsewhere (Lesage et al. 2002). Amplicons were analyzed by DHPLC using the Transgenomic WAVE System (Transgenomic). Prior to DHPLC, amplicons were denatured at 95°C for 5 min and than gradually cooled to room temperature over 45 min. An aliquot (7 μl) of the denatured amplicon was automatically loaded on the DNAsep column (Transgenomic) and was eluted on a linear gradient of acetonitrile in 0.1 M triethylamine acetate (TEAA) buffer, pH 7, at a constant flow rate of 0.9 ml/min. The gradient start and end points were adjusted according to amplicon size. The rehybridized DNA molecules eluted from the column were detected by a scanning UV-C detector. The partially denaturing temperature, which is required to separate heteroduplexes from homoduplexes, was predicted for each amplicon by using Navigator software, version 1.3 (Transgenomic) and was fine tuned. Heteroduplex profiles were visually identified as superimposed multiple peaks on the normal chromatograms. Amplicons with abnormal profiles were sequenced.
DNA Sequencing
The promoter region (−300 bp to the 10th bp of exon 1) and exons 2–12 and adjacent flanking intronic sequences of the CARD15 gene were individually PCR amplified from genomic DNA as noted above, through use of primers indicated in table 1 or described elsewhere (Lesage et al. 2002). Each amplicon was sequenced on an ABI Prism 3700 Capillary Array Sequencer using the ABI Prism BigDye Terminator Ready Reaction Mix (Perkin-Elmer-Cetus). Electrophorograms were inspected using ABI Prism Sequencing Analysis software (version 3.4.1) and were aligned to the reference sequence.
Screening the Control Group for Common and Rare Mutations/Sequence Variants
The three common mutations (R702W, G908R, and 1007fs), the N852S variant, and the P268S, M863V, and IVS8+158 polymorphisms were screened as described above. The rare L248R and A612T variants were screened by restriction analysis. The L248R variant creates an MspI site in the exon 4a amplicon, whereas the A612T variant creates an XcmI site in the exon 4d PCR product (table 1). The D357A, E441K, R703C, and A918D variants were screened by DHPLC, and their altered profiles were confirmed by sequencing.
Screening for the newly identified variants, D113N, I363F, and L550S, was performed by dot-blot analyses (Edelmann et al. 2002). Oligonucleotide probes designed to hybridize to the normal or mutant sequences are indicated in table 1. Prehybridization (3 h) and hybridization (16 h) were performed in PerfectHyb buffer, (Sigma) at 45°C. Membranes were washed in 5× SSC buffer for 10 min at 48°C, followed by washing in 0.5× SSC buffer at 48°C and then in 0.1% SDS for 20 min. A final wash was performed at 48°C in 0.1× SSC and 0.1% SDS for 20 min, and then the membranes were exposed to Kodak Biomax MS film for 2–16 h.
Electronic-Database Information
The URL for data presented herein is as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for CD) [PubMed]
References
- Ahmad T, Satsangi J, McGovern D, Bunce M, Jewell DP (2001) Review article: the genetics of inflammatory bowel disease. Aliment Pharmacol Ther 15:731–748 10.1046/j.1365-2036.2001.00981.x [DOI] [PubMed] [Google Scholar]
- Anderson MA, Gusella JF (1984) Use of cyclosporin A in establishing Epstein-Barr virus-transformed human lymphoblastoid cell lines. In Vitro 20:856–858 [DOI] [PubMed] [Google Scholar]
- Binder V (1998) Genetic epidemiology in inflammatory bowel disease. Dig Dis 16:351–355 10.1159/000016891 [DOI] [PubMed] [Google Scholar]
- Bonen DK, Nicolae DL, Moran T, Turkyilmaz MA, Ramos R, Karaliukas R, Brant SR, Duerr RH, Kirschner BS, Hanauer SB, Cho JH (2002) Racial differences in NOD2 variation: characterization of NOD2 in African-Americans with Crohn's disease. Gastroenterol 122 Suppl:A29 [Google Scholar]
- Bonen DK, Ogura Y, Nicolae DL, Inohara N, Saab L, Tanabe T, Chen FF, Foster SJ, Duerr RH, Brant SR, Cho JH, Nunez G (2003) Crohn’s disease-associated NOD2 variants share a signaling defect in response to lipopolysaccharide and peptidoglycan. Gastroenterology 124:140–146 10.1053/gast.2003.50019 [DOI] [PubMed] [Google Scholar]
- Cavanaugh J (2001) International collaboration provides convincing linkage replication in complex disease through analysis of a large pooled data set: Crohn disease and chromosome 16. Am J Hum Genet 68:1165–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh JA, Adams KE, Quak EJ, Bryce ME, O’Callaghan NJ, Rodgers HJ, Magarry GR, Butler WJ, Eaden JA, Roberts-Thomson IC, Pavli P, Wilson SR, Callen DF (2003) CARD15/NOD2 risk alleles in the development of Crohn’s disease in the Australian population. Ann Hum Genet 67:35–41 10.1046/j.1469-1809.2003.00006.x [DOI] [PubMed] [Google Scholar]
- Chamaillard M, Philpott D, Chareyre F, Zouali H, Lesage S, Girardin S, The Hung B, Zaehringer U, Sansonnetti P, Hugot JP, Thomas G (2002) Functional analysis of genetic variations of CARD15 in Crohn’s Disease predisposition. Am J Hum Genet Suppl 71:191 [Google Scholar]
- Cho JH, Nicolae DL, Gold LH, Fields CT, LaBuda MC, Rohal PM, Pickles MR, Qin L, Fu Y, Mann JS, Kirschner BS, Jabs EW, Weber J, Hanauer SB, Bayless TM, Brant SR (1998) Identification of novel susceptibility loci for inflammatory bowel disease on chromosomes 1p, 3q, and 4q: evidence for epistasis between 1p and IBD1. Proc Natl Acad Sci USA 95:7502–7507 10.1073/pnas.95.13.7502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croucher PJ, Mascheretti S, Hampe J, Huse K, Frenzel H, Stoll M, Lu T, Nikolaus S, Yang SK, Krawczak M, Kim WH, Schreiber S (2003) Haplotype structure and association to Crohn’s disease of CARD15 mutations in two ethnically divergent populations. Eur J Hum Genet 11:6–16 10.1038/sj.ejhg.5200897 [DOI] [PubMed] [Google Scholar]
- Cuthbert AP, Fisher SA, Mirza MM, King K, Hampe J, Croucher PJ, Mascheretti S, Sanderson J, Forbes A, Mansfield J, Schreiber S, Lewis CM, Mathew CG (2002) The contribution of NOD2 gene mutations to the risk and site of disease in inflammatory bowel disease. Gastroenterology 122:867–874 [DOI] [PubMed] [Google Scholar]
- Duerr RH, Barmada MM, Zhang L, Davis S, Preston RA, Chensny LJ, Brown JL, Ehrlich GD, Weeks DE, Aston CE (1998) Linkage and association between inflammatory bowel disease and a locus on chromosome 12. Am J Hum Genet 63:95–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann L, Dong J, Desnick RJ, Kornreich R (2002) Carrier screening for mucolipidosis type IV in the American Ashkenazi Jewish population. Am J Hum Genet 70:1023–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampe J, Cuthbert A, Croucher PJ, Mirza MM, Mascheretti S, Fisher S, Frenzel H, King K, Hasselmeyer A, MacPherson AJ, Bridger S, van Deventer S, Forbes A, Nikolaus S, Lennard-Jones JE, Foelsch UR, Krawczak M, Lewis C, Schreiber S, Mathew CG (2001) Association between insertion mutation in NOD2 gene and Crohn’s disease in German and British populations. Lancet 357:1925–1928 10.1016/S0140-6736(00)05063-7 [DOI] [PubMed] [Google Scholar]
- Hampe J, Grebe J, Nikolaus S, Solberg C, Croucher PJ, Mascheretti S, Jahnsen J, Moum B, Klump B, Krawczak M, Mirza MM, Foelsch UR, Vatn M, Schreiber S (2002) Association of NOD2 (CARD 15) genotype with clinical course of Crohn’s disease: a cohort study. Lancet 359:1661–1665 10.1016/S0140-6736(02)08590-2 [DOI] [PubMed] [Google Scholar]
- Hampe J, Schreiber S, Shaw SH, Lau KF, Bridger S, Macpherson AJ, Cardon LR, Sakul H, Harris TJ, Buckler A, Hall J, Stokkers P, van Deventer SJ, Nurnberg P, Mirza MM, Lee JC, Lennard-Jones JE, Mathew CG, Curran ME (1999a) A genomewide analysis provides evidence for novel linkages in inflammatory bowel disease in a large European cohort. Am J Hum Genet 64:808–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampe J, Shaw SH, Saiz R, Leysens N, Lantermann A, Mascheretti S, Lynch NJ, MacPherson AJ, Bridger S, van Deventer S, Stokkers P, Morin P, Mirza MM, Forbes A, Lennard-Jones JE, Mathew CG, Curran ME, Schreiber S (1999b) Linkage of inflammatory bowel disease to human chromosome 6p. Am J Hum Genet 65:1647–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O’Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G (2001) Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 411:599–603 10.1038/35079107 [DOI] [PubMed] [Google Scholar]
- Hugot JP, Laurent-Puig P, Gower-Rousseau C, Olson JM, Lee JC, Beaugerie L, Naom I, Dupas JL, Van Gossum A, Orholm M, Bonaiti-Pellie C, Weissenbach J, Mathew CG, Lennard-Jones JE, Cortot A, Colombel JF, Thomas G (1996) Mapping of a susceptibility locus for Crohn’s disease on chromosome 16. Nature 379:821–823 10.1038/379821a0 [DOI] [PubMed] [Google Scholar]
- Inohara N, Koseki T, del Peso L, Hu Y, Yee C, Chen S, Carrio R, Merino J, Liu D, Ni J, Nunez G (1999) Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-κB. J Biol Chem 274:14560–14567 10.1074/jbc.274.21.14560 [DOI] [PubMed] [Google Scholar]
- Inohara N, Koseki T, Lin J, del Peso L, Lucas PC, Chen FF, Ogura Y, Nunez G (2000) An induced proximity model for NF-κB activation in the Nod1/RICK and RIP signaling pathways. J Biol Chem 275:27823–27831 [DOI] [PubMed] [Google Scholar]
- Inoue N, Tamura K, Kinouchi Y, Fukuda Y, Takahashi S, Ogura Y, Inohara N, Nunez G, Kishi Y, Koike Y, Shimosegawa T, Shimoyama T, Hibi T (2002) Lack of common NOD2 variants in Japanese patients with Crohn’s disease. Gastroenterology 123:86–91 10.1053/gast.2002.34155 [DOI] [PubMed] [Google Scholar]
- King RA, Rotter JI, Motulsky AG (1992) The genetic basis of common diseases. Oxford University Press, New York [Google Scholar]
- Kleinbaum DG, Kupper LL, Morgenstern H (1982) Epidemiologic research principles and quantitative methods. Lifetime Learning Publications, Belmont, CA [Google Scholar]
- Kurata JH, Kantor-Fish S, Frankl H, Godby P, Vadheim CM (1992) Crohn’s disease among ethnic groups in a large health maintenance organization. Gastroenterology 102:1940–1948 [DOI] [PubMed] [Google Scholar]
- Lennard-Jones JE (1989) Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl 170:2–6 [DOI] [PubMed] [Google Scholar]
- Lesage S, Zouali H, Cezard JP, Colombel JF, Belaiche J, Almer S, Tysk C, O’Morain C, Gassull M, Binder V, Finkel Y, Modigliani R, Gower-Rousseau C, Macry J, Merlin F, Chamaillard M, Jannot AS, Thomas G, Hugot JP (2002) CARD15/NOD2 mutational analysis and genotype-phenotype correlation in 612 patients with inflammatory bowel disease. Am J Hum Genet 70:845–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Ohmen JD, Li Z, Bentley LG, McElree C, Pressman S, Targan SR, Fischel-Ghodsian N, Rotter JI, Yang H (1999) A genome-wide search identifies potential new susceptibility loci for Crohn’s disease. Inflamm Bowel Dis 5:271–278 [DOI] [PubMed] [Google Scholar]
- Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nunez G, Cho JH (2001) A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 411:603–606 10.1038/35079114 [DOI] [PubMed] [Google Scholar]
- Podolsky DK (2002) Inflammatory bowel disease. N Engl J Med 347:417–429 10.1056/NEJMra020831 [DOI] [PubMed] [Google Scholar]
- Probert CS, Jayanthi V, Hughes AO, Thompson JR, Wicks AC, Mayberry JF (1993) Prevalence and family risk of ulcerative colitis and Crohn’s disease: an epidemiological study among Europeans and south Asians in Leicestershire. Gut 34:1547–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux JD, Daly MJ, Silverberg MS, Lindblad K, Steinhart H, Cohen Z, Delmonte T, et al (2001) Genetic variation in the 5q31 cytokine gene cluster confers susceptibility to Crohn disease. Nat Genet 29:223–228 10.1038/ng1001-223 [DOI] [PubMed] [Google Scholar]
- Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, McLeod RS, Griffiths AM, Green T, Brettin TS, Stone V, Bull SB, Bitton A, Williams CN, Greenberg GR, Cohen Z, Lander ES, Hudson TJ, Siminovitch KA (2000) Genomewide search in Canadian families with inflammatory bowel disease reveals two novel susceptibility loci. Am J Hum Genet 66:1863–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth MP, Petersen GM, McElree C, Feldman E, Rotter JI (1989a) Geographic origins of Jewish patients with inflammatory bowel disease. Gastroenterology 97:900–904 [DOI] [PubMed] [Google Scholar]
- Roth MP, Petersen GM, McElree C, Vadheim CM, Panish JF, Rotter JI (1989b) Familial empiric risk estimates of inflammatory bowel disease in Ashkenazi Jews. Gastroenterology 96:1016–1020 [DOI] [PubMed] [Google Scholar]
- Sonnenberg A, McCarty DJ, Jacobsen SJ (1991) Geographic variation of inflammatory bowel disease within the United States. Gastroenterology 100:143–149 [DOI] [PubMed] [Google Scholar]
- Spielman RS, McGinnis RE, Ewens WJ (1993) Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus (IDDM). Am J Hum Genet 52:506–516 [PMC free article] [PubMed] [Google Scholar]
- Sugimura K, Taylor KD, Lin YC, Hang T, Wang D, Tang YM, Fischel-Ghodsian N, Targan SR, Rotter JI, Yang H (2003) A novel NOD2/CARD15 haplotype conferring risk for Crohn disease in Ashkenazi Jews. Am J Hum Genet 72:509–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidsinski A, Ladhoff A, Pernthaler A, Swidsinski S, Loening-Baucke V, Ortner M, Weber J, Hoffmann U, Schreiber S, Dietel M, Lochs H (2002) Mucosal flora in inflammatory bowel disease. Gastroenterology 122:44–54 [DOI] [PubMed] [Google Scholar]
- Vermeire S, Wild G, Kocher K, Cousineau J, Dufresne L, Bitton A, Langelier D, Pare P, Lapointe G, Cohen A, Daly MJ, Rioux JD (2002) CARD15 genetic variation in a Quebec population: prevalence, genotype-phenotype relationship, and haplotype structure. Am J Hum Genet 71:74–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Takazoe M, Tanaka T, Kazumori T, Nakamura Y (2002) Absence of mutation in the NOD2/CARD15 gene among 483 Japanese patients with Crohn’s disease. J Hum Genet 47:469–472 10.1007/s100380200067 [DOI] [PubMed] [Google Scholar]
- Yang H, McElree C, Roth MP, Shanahan F, Targan SR, Rotter JI (1993) Familial empirical risks for inflammatory bowel disease: differences between Jews and non-Jews. Gut 34:517–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Taylor KD, Rotter JI (2001) Inflammatory bowel disease. I. Genetic epidemiology. Mol Genet Metab 74:1–21 10.1006/mgme.2001.3199 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Lin XY, Akolkar PN, Gulwani-Akolkar B, Levine J, Katz S, Silver J (2002) Variation at NOD2/CARD15 in familial and sporadic cases of Crohn’s disease in the Ashkenazi Jewish population. Am J Gastroenterol 97:3095–3101 10.1016/S0002-9270(02)05522-3 [DOI] [PubMed] [Google Scholar]
- Zlotogora J, Zimmerman J, Rachmilewitz D (1990) Crohn’s disease in Ashkenazi Jews. Gastroenterology 99:286–287 [DOI] [PubMed] [Google Scholar]