Abstract
Sotos syndrome is an overgrowth syndrome characterized by pre- and postnatal overgrowth, macrocephaly, advanced bone age, variable degrees of mental retardation, and typical facial features. Defects of the NSD1 gene account for ⩾60% of cases of Sotos syndrome, whereas the disease-causing mechanism of other cases remains unknown. Beckwith-Wiedemann syndrome (BWS) is a distinct overgrowth condition characterized by macroglossia, abdominal-wall defects, visceromegaly, embryonic tumors, hemihyperplasia, ear anomalies, renal anomalies, and neonatal hypoglycemia. Deregulation of imprinted growth-regulatory genes within the 11p15 region is the major cause of BWS, whereas the molecular defect underlying a significant proportion of sporadic BWS cases remains unknown. Owing to clinical overlaps between the two syndromes, we investigated whether unexplained cases of Sotos syndrome could be related to 11p15 anomalies and, conversely, whether unexplained BWS cases could be related to NSD1 deletions or mutations. Two 11p15 anomalies were identified in a series of 20 patients with Sotos syndrome, and two NSD1 mutations were identified in a series of 52 patients with BWS. These results suggest that the two disorders may have more similarities than previously thought and that NSD1 could be involved in imprinting of the chromosome 11p15 region.
Overgrowth syndromes are a heterogeneous group of disorders resulting from the dysfunction of various processes involving cell proliferation, cell growth, or apoptosis. Within this group, Sotos syndrome (MIM 117550) is characterized by the combination of overgrowth, specific facial features (prominent forehead with receding hairline, downslanting palpebral fissures, and pointed chin), large head circumference, and advanced bone age (Sotos 1964; Cole and Hughes 1990, 1994). Variable degrees of mental retardation are usually observed. Additional features include neonatal hypotonia, seizures, scoliosis, strabismus, congenital renal and heart defects, and tumor predisposition. Deletions and point mutations of the NSD1 gene (MIM 606681) account for ⩾60% of cases of Sotos syndrome, but the disease-causing mechanism in other cases remains unknown (Kurotaki et al. 2002; Douglas et al. 2003; Rio et al. 2003).
On the other hand, Beckwith-Wiedemann syndrome (BWS [MIM 130850]) is a distinct overgrowth condition characterized by macroglossia, anterior abdominal wall defects, visceromegaly, and tumor predisposition (Elliot 1994). Additional features include earlobe creases, posterior helical ear pits, facial naevus flammeus, neonatal hypoglycemia, renal abnormalities, and hemihypertrophy. A cluster of genes on chromosome 11p15 is involved in the pathogenesis of BWS (Li et al. 1998; Maher and Reik 2000; Reik and Murrell 2000). Although the majority of cases are sporadic, a small number of familial forms are linked to chromosome 11p15. A minority of cases result from 11p15 chromosome duplications and translocations. Genetic causes (11p15 paternal uniparental disomy or a mutation in CDKN1C) account for 30% of cases, and most patients exhibit epigenetic defects, mostly demethylation of the KvDMR1 region of the KCNQ1OT gene (MIM 604115) (60%) but also hypermethylation of the H19 gene (MIM 103280) (10%). Altogether, these genetic and epigenetic abnormalities of the 11p15 region account for ⩾80% of all BWS cases (Li et al. 1998).
Although Sotos syndrome and BWS are believed to be clinically distinct conditions, they share common clinical features—namely, macrosomia, neonatal hypoglycemia, and cardiac anomalies. On the basis of this phenotypic overlap, we hypothesized that some forms of unexplained Sotos syndrome could be related to 11p15 anomalies, whereas unexplained BWS cases could be related to NSD1 deletions or mutations.
We first analyzed genomic DNA from 52 patients with BWS for deletion of the NSD1 gene. Inclusion criteria were the presence of at least two of the four major signs—namely, (i) macrosomia, (ii) macroglossia, (iii) abdominal-wall defect, and (iv) organomegaly—and two minor signs. In all cases, routine G-banding and R-banding chromosome analyses showed a normal karyotype with no evidence of deletions or duplications. Molecular analyses of the 11p15 region ruled out chromosomal rearrangements as well as abnormal methylation patterns and mutations in the CDKN1C gene. Patients were then genotyped (according to the method of Rio et al. 2003) using four polymorphic microsatellite markers (two intragenic and two adjacent to the NSD1 gene) (UCSC). In all cases, a microdeletion encompassing NSD1 was excluded by the detection of two distinct alleles for at least one of the intragenic markers. The 12/52 patients with some degree of mental retardation were then selected for NSD1 sequencing. It is interesting that two patients with BWS were found to have NSD1 mutations—namely, a 1-bp insertion in exon 14 (patient PB) and a 4-bp deletion in exon 23 (patient BA). Both mutations were found to have occurred de novo.
Conversely, 20 patients with Sotos syndrome were investigated for chromosomal and methylation anomalies of the 11p15.5 region. They all presented with typical facial gestalt, macrocephaly (head circumference >+2 SD), overgrowth, and developmental delay. In all cases, routine G-banding and R-banding chromosome analyses showed a normal karyotype, and molecular analyses ruled out fragile X syndrome and NSD1 deletion or point mutation.
To screen for deletions or uniparental disomies of the 11p15.5 region, three polymorphic microsatellite markers (D11S4046, D11S1338, and D11S1346) (UCSC) were tested. Analysis of one patient with Sotos syndrome (patient FD) revealed the unbalanced segregation of two microsatellite DNA markers, with no maternal contribution, at loci D11S4046 and D11S1338 (table 1). Genotyping eight additional polymorphic markers confirmed the lack of maternal contribution at loci D11S1331, D11S4188, D11S902, and D11S904 and a balanced contribution at loci D11S4177 and D11S1751. Therefore, the disease region encompassed 25 Mb between D11S4046 and D11S904. FISH analyses performed using YAC 896B12 and PAC RPCI-5 908H22, located on distal chromosome 11p, detected two spots for each probe, ruling out a maternal deletion and supporting paternal isodisomy.
Table 1.
Genotypes of Patient FD and His Parents at Polymorphic Loci on Chromosome 11p15[Note]
|
Genotype of |
||||
| Locusa | Distance from Telomereb(Mb) | Father | Patient | Mother |
| D11S4177 | 1.45 | 205 | 181/205 | 181/203 |
| D11S4046 | 1.92 | 121/123 | 121 | 113/123 |
| D11S1760 | 5.34 | 87/95 | 87 | 87 |
| D11S1338 | 5.94 | 261/263 | 261 | 255/263 |
| D11S1331 | 7.25 | 192/196 | 192 | 194/196 |
| D11S909 | 8.81 | 116/118 | 118 | 114/118 |
| D11S4188 | 9.12 | 108/116 | 108 | 112 |
| D11S1346 | 11 | 268 | 268 | 268/280 |
| D11S902 | 17.52 | 157 | 157 | 159/161 |
| D11S904 | 26.71 | 194/200 | 194 | 186/188 |
| D11S1751 | 33.79 | 204/214 | 204/212 | 212 |
Note.— Bold italic characters indicate genotypes that are consistent with a maternal deletion or paternal isodisomy.
Loci are listed according to their relative chromosomal location from pter to centromere.
Allele sizes are given in base pairs.
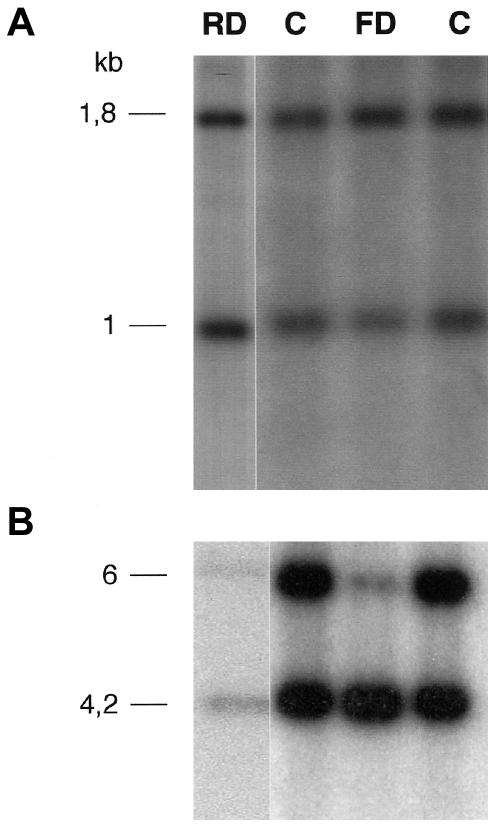
We subsequently investigated the methylation status of the 11p15 region in our 20 patients with Sotos syndrome. That region contains two distinct domains that are regulated by two independent imprinting centers separated by a nonimprinted region. The telomeric domain 1 contains the maternally expressed H19 gene and the paternally expressed IGF2 gene. The centromeric domain 2 contains six known imprinted genes, including the paternally expressed KCNQ1OT gene (LIT1) and the maternally expressed CDKN1C gene (p57KIP2). The methylation status of the KCNQ1OT and H19 genes was assessed as described elsewhere (Brannan et al. 1990; Gaston et al. 2001). As expected, patient FD, who had a paternal isodisomy, exhibited a strong demethylation pattern of the KCNQ1OT gene (methylation index [MI] 9.2%; normal mean±1 SD=51.6±2.5%) (fig. 1B). It is surprising, however, that we found an almost-normal methylation pattern of the H19 gene (MI 61%) (fig. 1A). It is interesting that a second patient with Sotos syndrome (patient RD) also displayed an abnormal methylation status, as shown by the demethylation pattern of the KCNQ1OT gene (MI 33.8%) (fig. 1B). The methylation status of the H19 gene was normal (MI 49.5%) (fig. 1A). The other patients with Sotos syndrome had a normal methylation pattern at the two loci.
Figure 1.
Methylation analysis of H19 and KCNQ1OT genes in patients (RD and FD) and normal subjects (C). A, H19 methylation was assessed by digestion with Pst1 and SmaI. The 1.8-kb Pst1 fragment is cut with SmaI, resulting in a 1-kb fragment. The H19 methylation index was determined by scanning autoradiographs and calculating the ratio (1.8-kb fragment)/(1.8-kb fragment + 1-kb fragment) × 100. B, KCNQ1OT methylation was assessed by digestion with BamHI and NotI. The 6-kb BamHI fragment is cut with NotI, resulting in a 4.2-kb fragment. The KCNQ1OT methylation index was determined by scanning autoradiographs and calculating the ratio (4.2-kb fragment)/(4.2-kb fragment + 6-kb fragment) × 100.
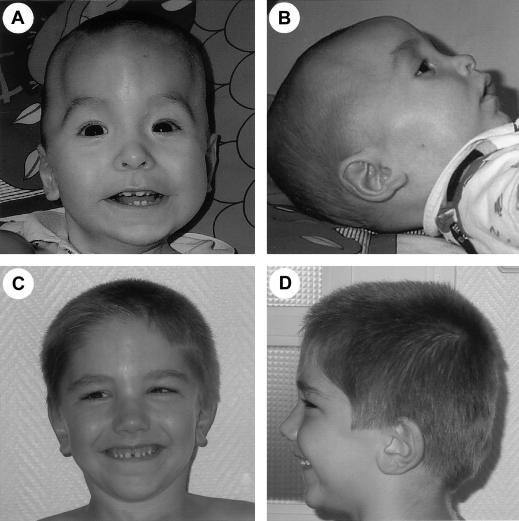
Table 2 shows the clinical findings of the two patients with BWS who had NSD1 mutations and the two children with Sotos syndrome with anomalies of the 11p15 region. In all cases, careful analysis supported the initial clinical diagnosis. Indeed, patient PB, who had a 1-bp NSD1 deletion, first received a diagnosis of BWS based on pre- and postnatal overgrowth, umbilical hernia, and hemihyperplasia. He also had mild developmental delay, craniostenosis, and multiple renal cysts. Moreover, the patient did not have the typical facial gestalt observed in patients with NSD1 anomalies (fig. 2A and 2B). Similarly, patient BA, who had a 4-bp NSD1 deletion, first received a diagnosis of BWS based on postnatal overgrowth, hypoglycemia with hyperinsulinism, a significant umbilical hernia, and persistent vesicoureteral reflux. This patient did not have the typical Sotos facies either (fig. 2C and 2D).
Table 2.
Clinical Manifestations in Children with NSD1 Mutations and 11p15 Anomalies
|
Manifestations in Patient |
||||
| Clinical Features or Diagnosis | PB | BA | FD | RD |
| Clinical Features Common for Sotos and BWS: | ||||
| Birth history: | ||||
| Duration of pregnancy | 40 wk | 38 wk | 34 wk | 40 wk |
| Birth weight (g) | 3,680 | 2,700 | 2,140 | 3,380 |
| Birth length (cm) | 54.5 | 47.5 | 44 | 49 |
| Birth head circumference (cm) | 39 | 36 | 32 | 35 |
| Complications of prematurity | No | No | No | No |
| Neonatal hypoglycemia | No | Yes, with hyperinsulinism, persistent to date | No | No |
| Postnatal overgrowth: | ||||
| Age | 5 years 6 mo | 5 years | 11 years | 10 years |
| Height (cm) | 122.5 (97th percentile) | 130 (>97th percentile) | 156 (97th percentile) | 146.5 (97th percentile) |
| Weight (kg) | 26.5 (97th percentile) | 29 (97th percentile) | 50 (>97th percentile) | 35 (50th percentile) |
| Head circumference (cm) | 57 (>97th percentile) | 57 (>97th percentile) | 59 (>97th percentile) | 58.3 (>97th percentile) |
| Heart defect | No | No | Patent ductus arteriosus and ventricular sepral defect | Septal hypertrophy |
| Genito-urinary abnormalities | 2 Renal cysts | Persistent vesicoureteral reflux | No | No |
| Advanced bone age | No | No | Yes | Yes |
| Malignant or benign tumors | No | No | No | No |
| Sotos clinical features: | ||||
| Sotos facial features: | No | No | High hairline, frontal bossing, antimongoloid slant of palpebral fissures, pointed chin | Dolichocephaly frontal bossing, antimongoloid slant of palpebral fissures |
| Development delay: | Yes (moderate) | Yes (mild) | Yes (moderate) | Yes (severe) |
| Age able to walk unaided | 2 years | 2 years | 3 years | 4 years |
| Special education | Yes | Normal education, orthophonist | Yes | Yes |
| Hands | Deep creases | Deep and brittle nails | Deep nails | Arachnodactyly |
| Cerebral malformation | No | No | Ventriculomegaly | No |
| Seizures | No | No | Yes | No |
| Skeletal abnormalities | Craniostenosis | Scoliosis | no | Severe scoliosis at age 1 year |
| BWS clinical features: | ||||
| Craniofacial features: | ||||
| Macroglossia | No | No | No | No |
| Earlobe creases | No | No | No | No |
| Posterior helical ear pits | No | No | No | No |
| Frontal angioma | No | No | No | No |
| Abdominal-wall defect | Large umbilical hernia | Abdominal-wall hypotonia | No | No |
| Visceromegaly | No | No | No | No |
| Hemihypertrophy | Yes, left side | No | No | No |
| Unclassified features: | ||||
| Craniofacial morphology (cf. fig. 3A–3D) | Scaphocephaly ptosis, epicanthus, large ears | Plagiocephaly, low hairline (diazoxyde), horizontal palpebral fissures | ||
| Initial diagnosis | BWS | BWS | Sotos syndrome | Sotos syndrome |
| Molecular diagnosis | NSD1: exon 14: 4976 ins 5(G) | NSD1: exon 23: 7968 del (GACA) | 11p15: paternal isodisomy | 11p15: demethylation of KCNQ1OT |
Figure 2.
Facial features of patients with BWS who have NSD1 mutations. A and B, Patient PB at age 1 year. Note that the craniostenosis may hide the classic facial gestalt of Sotos syndrome. Frontal bossing and antimongoloid slant of the palpebral fissure are not typical. C and D, Patient BA at age 4 years. Note the absence of typical facial characteristics of Sotos syndrome: normo-versed nares and absence of prominent forehead, dolichocephaly, and prominent chin.
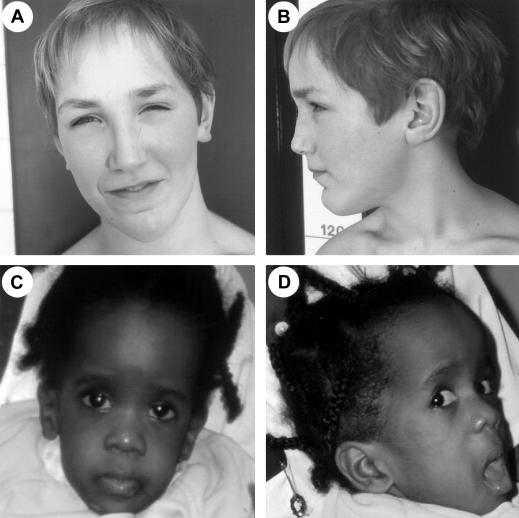
Conversely, patient RD was believed to have Sotos syndrome on the basis of the association of postnatal overgrowth, characteristic facial appearance, and developmental delay (fig. 3A and 3B). The patient had a partial isolated demethylation of KCNQ1OT, a feature that is already reported in patients with BWS and is probably due to mosaicism (Gaston et al. 2001). Similarly, facial gestalt, advanced age bone, macrosomia and overgrowth, ventriculomegaly, and ventricular septal defect were consistent with the diagnosis of Sotos syndrome in patient FD (fig. 3C and 3D). The patient had a paternal isodisomy, including both KCNQ1OT and H19 loci, and should therefore have an altered H19 methylation. His unusual methylation profile may therefore be the cause of the atypical overgrowth phenotype observed in him.
Figure 3.
Facial features in patients with Sotos who have 11p15 anomalies. A and B, Patient FD at age 11 years. Note the downslanting palpebral fissures and pointed chin. C and D, Patient RD at age 15 mo. Note the dolichocephaly, ocular hypertelorism, frontal bossing, sparseness of hair in frontoparietal region, and antimongoloid slant of the palpebral fissures.
In conclusion, this study suggests that 11p15 anomalies could account for ⩾10% of unexplained cases of Sotos syndrome and, conversely, that NSD1 mutations could account for ⩾5% of the unexplained cases of BWS. These findings illustrate the difficulty in clinically recognizing the various overgrowth syndromes, owing to their phenotypic overlap. These results suggest giving consideration to testing the NSD1 gene and 11p15 region, respectively, in individuals with atypical features of BWS (i.e., unexplained developmental delay) and Sotos syndrome (i.e., hypoglycemia secondary to hyperinsulinism). Finally, these results are also important for genetic counseling and definition of recurrence risk in affected families.
The mechanism by which mutations in the NSD1 gene and deregulation of 11p15 genes result in similar phenotypes remains unknown. NSD1 was isolated in a screen to isolate C-regulators for retinoic acid receptors (Huang et al. 1998). It encodes a nuclear protein containing an SET (su(var)3-9, enhancer-of-zeste, trithorax) domain and multiple PHD (plant homeodomain protein finger) domains. Recent analyses of the mouse protein have shown that the nsd1 SET domain has an intrinsic histone methyltransferase with a unique substrate specificity for both Lys36 of histone H3 and Lys20 of histone H4 (Rayasam et al. 2003), suggesting an essential function in the transcriptional gene silencing by histone methylation. On the basis of these findings, it is tempting to speculate that the NSD1 protein may also be involved in the establishment and/or maintenance of the imprinting of the chromosome 11p15 region. We hope that further molecular and genetic studies will elucidate the exact link between NSD1 and imprinting.
Acknowledgments
We are grateful to the patients and their families for their cooperation. This study was supported by INSERM. G.B. was supported by Fondation pour la Recherche Médicale.
Electronic-Database Information
The URLs for data presented herein are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for Sotos syndrome, BWS, NSD1, KCNQ1OT, and H19)
- University of California–Santa Cruz (UCSC) Genome Bioinformatics, http://genome.cse.ucsc.edu/ (for chromosome 11 physical map [updated July 2003])
References
- Brannan C, Dees E, Ingram R, Tilghman S (1990) The product of the H19 gene may function as a RNA. Mol Cell Biol 10:28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TRP, Hughes HE (1990) Sotos syndrome. J Med Genet 27:571–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ——— (1994) Sotos syndrome: a study of the diagnostic criteria and natural history. J Med Genet 31:20–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas J, Hanks S, Temple KI, Davies S, Murray A, Upadhayaya M, Tomkins S, Hughes HE, Cole TRP, Rahman N (2003) NSD1 mutations are the major cause of Sotos syndrome and occur in some cases of Weaver syndrome but are rare in other overgrowth phenotypes. Am J Hum Genet 72:132–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott M, Bayly R, Cole T (1994) Clinical features and natural history of Beckwith-Wiedemann syndrome: presentation of 74 new cases. Clin Genet 46:168–174 [DOI] [PubMed] [Google Scholar]
- Gaston V, Le Bouc Y, Soupre V, Burglen L, Donadieu J, Oro H, Audry G, Vazquez MP, Gicquel C (2001) Analysis of the methylation status of the KCNQ1OT and H19 genes in leukocyte DNA for the diagnosis and prognosis of Beckwith-Wiedemann syndrome. Eur J Hum Genet 9:409–418 10.1038/sj.ejhg.5200649 [DOI] [PubMed] [Google Scholar]
- Huang N, Baur E, Garnier JM, Lerouge T, Vonesh JL, Lutz Y, Chambon P, Losson R (1998) Two distinct nuclear receptor interaction domains in NSD1, a novel SET protein that exhibits characteristics of both co-repressors and co-activators. EMBO J 17:3398–3412 10.1093/emboj/17.12.3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurotaki N, Imaizumi K, Harada N, Masuno M, Kondoh T, Nagai T, Ohashi H, Naritomi K, Tsukahara M, Makita Y, Sugimoto T, Sonoda T, Hasegawa T, Chinene Y, Tomita Ha Ha, Kinoshita A, Mizuguchi T, Yoshiura Ki K, Ohta T, Kishino T, Fukushima Y, Niikawa N, Matsumoyo N (2002) Haploinsufficiency of NSD1 causes Sotos syndrome. Nat Genet 30:365–366 10.1038/ng863 [DOI] [PubMed] [Google Scholar]
- Li M, Squire JA, Weksberg R (1998) Molecular genetics of Wiedemann-Beckwith syndrome. Am J Med Genet 79:253–259 9781904 [DOI] [PubMed] [Google Scholar]
- Maher ER, Reik W (2000) Beckwith-Wiedemann syndrome: imprinting in clusters revisited. J Clin Invest 105:247–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayasam GV, Wendling O, Angrand PO, Mark M, Niederreither K, Song L, Lerouge T, Hager GL, Chambon P, Losson R (2003) NSD1 is essential for early post-implantation development and has a catalytically active SET domain. EMBO J 22:3153–3163 10.1093/emboj/cdg288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik W, Murrell A (2000) Genomic imprinting: silence across the border. Nature 405:408–409 10.1038/35013178 [DOI] [PubMed] [Google Scholar]
- Rio M, Clech L, Amiel J, Faivre L , Lyonnet S , Le Merrer M, Odent S, Lacombe D, Edery P, Brauner R, Raoul O, Gosset P, Prieur M, Vekemans M, Munnich A, Colleaux L, Cormier-Daire V (2003) Spectrum of NSD1 mutations in Sotos and Weaver syndromes. J Med Genet 40:436–440 10.1136/jmg.40.6.436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotos JF, Dodge PR, Muirhead D, Crawford JD, Talbot NB (1964) Cerebral gigantism in childhood: a syndrome of excessively rapid growth and acromegalic features and a nonprogressive neurologic disorder. N Engl J Med 271:109–116 14148233 [DOI] [PubMed] [Google Scholar]