Abstract
The present article describes the first patient with a deficiency of ribose-5-phosphate isomerase (RPI) (Enzyme Commission number 5.3.1.6) who presented with leukoencephalopathy and peripheral neuropathy. Proton magnetic resonance spectroscopy of the brain revealed highly elevated levels of the polyols ribitol and D-arabitol, which were subsequently also found in high concentrations in body fluids. Deficient activity of RPI, one of the pentose-phosphate-pathway (PPP) enzymes, was demonstrated in fibroblasts. RPI gene–sequence analysis revealed a frameshift and a missense mutation. Recently, we described a patient with liver cirrhosis and abnormal polyol levels in body fluids, related to a deficiency of transaldolase, another enzyme in the PPP. RPI is the second known inborn error in the reversible phase of the PPP, confirming that defects in pentose and polyol metabolism constitute a new area of inborn metabolic disorders.
White-matter disorders of unknown etiology constitute a vexing problem in child neurology. Children with neurological deficits of central origin often demonstrate white-matter abnormalities on magnetic resonance (MR) imaging (MRI). Despite an extensive laboratory work-up, ⩾50% of these children remain without a specific diagnosis (Kristjansdottir et al. 1996; van der Knaap et al. 1999a). Both MRI and proton MR spectroscopy (MRS) have been used with success to identify and define “new” disease entities among the children with a leukoencephalopathy of unknown origin (Hanefeld et al. 1993; Schiffmann et al. 1994; van der Knaap et al. 1995, 1997, 2002, 2003; Leegwater et al. 2001a, 2001b). For this reason, we apply these techniques systematically in the work-up of patients with an unclassified leukoencephalopathy.
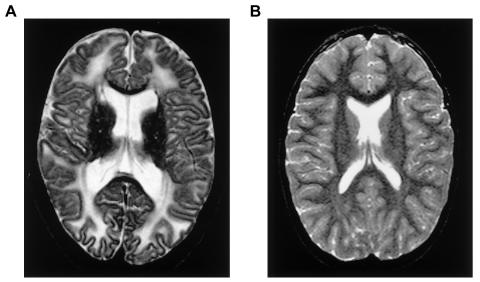
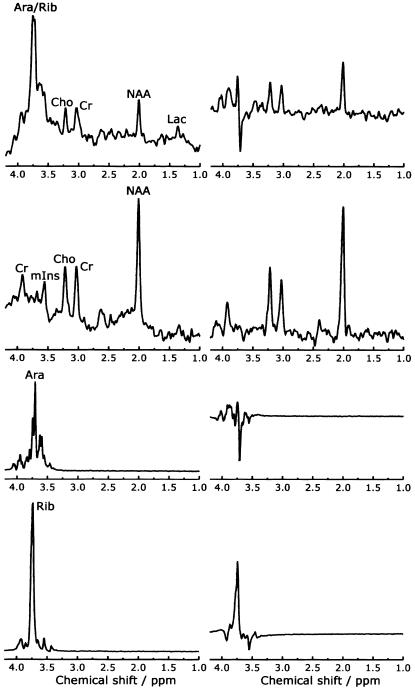
In 1999, we found highly elevated peaks in the sugar and polyol region of the MR spectrum of a boy with leukoencephalopathy of unknown etiology (van der Knaap et al. 1999b). The boy was born in 1984, the only child of healthy, unrelated parents. He had psychomotor retardation from early in life and developed epilepsy at age 4 years. From age 7 years, a slow neurological regression occurred with prominent cerebellar ataxia, some spasticity, optic atrophy, and a mild sensorimotor neuropathy. Neither organomegaly nor dysfunction of the internal organs was present. Extensive metabolic investigations failed to reveal an underlying metabolic defect. MRI of the brain at ages 11 years and 14 years showed extensive abnormalities of the cerebral white matter (fig. 1). Proton MRS of the brain revealed highly elevated abnormal peaks, which could be identified as representing the pentitols ribitol and D-arabitol (fig. 2). Quantitation of the patient’s spectrum revealed a D-arabitol concentration of 8.9 and a ribitol concentration of 2.9 mmol/liter of white matter.
Figure 1.
The transverse T2-weighted image of the brain, showing extensive abnormalities of the cerebral hemispheric white matter in the patient (A), as compared with that of a normal individual (B).
Figure 2.
The first and second rows show proton MR spectra of cerebral white matter of the patient and a control individual, respectively. The third and fourth rows show spectra of pure solutions of 50 mM D-arabitol and ribitol, respectively. The spectra on the left were obtained with a short echo time (STEAM, repetition time 6,000 milliseconds, echo time 20 milliseconds); the spectra on the right were obtained with a longer echo time (PRESS, repetition time 3,000 milliseconds, echo time 135 milliseconds). The spectra of the patient and the control individual were plotted on the same vertical scale to allow a qualitative comparison. The spectra of the D-arabitol and ribitol solutions were plotted on the same vertical scale. Note the high peaks between 3.6 and 3.8 ppm in the short-echo-time spectrum of the patient, not present in the control individual. With a longer echo time, a part of these peaks is below the baseline, related to so-called J-coupling effects. D-arabitol and ribitol are represented by resonances between 3.6 and 3.8 ppm. Comparing the short- and long-echo-time spectra, the same effects of J-coupling are seen again, in particular in D-arabitol. These data indicate that the resonances between 3.6 and 3.8 ppm in the patient can be ascribed to D-arabitol and, to a lesser extent, ribitol. For further comments on the spectral changes in the patient, see van der Knaap et al. (1999b). Ara = D-arabitol; Rib = ribitol; Cho = choline-containing compounds; Cr = total creatine; NAA = N-acetylaspartate; Lac = lactate; mIns = myoinositol.
Polyols are sugar alcohols, subdivided on the basis of C-atom number. For example, pentitols are polyols with five C atoms. Numerous studies have shown that polyols are present in human body fluids (Jansen et al. 1986; Kusmierz et al. 1989), but assessment of their levels is not part of the general metabolic screening. We subsequently analyzed the levels of sugars and polyols in body fluids of the patient, using the method described by Jansen et al. (1986) and their reference ranges for urine. Reference values for sugars and polyols in plasma and cerebrospinal fluid (CSF) were determined in our laboratory. The results of repeated analyses of urine, plasma, and CSF of the patient are shown in table 1. Over the years, ribitol and D-arabitol were consistently elevated in all body fluids. There was a high brain:CSF:plasma ratio of 70:40:1 for D-arabitol and 125:55:1 for ribitol (van der Knaap et al. 1999b). Decreased concentrations of erythritol and myoinositol were found in urine and CSF, whereas the concentrations of xylitol, xylulose, treitol, arabinose, ribulose, and ribose were elevated (table 1).
Table 1.
Sugar and Polyol Levels in Body Fluids of the Patient and Control Individuals
|
Level (μmol/liter) in CSF of |
Level (μmol/liter)in Plasma of |
Level (mmol/mol creatinine)in Urine of |
||||
| Sugaror Polyol | Patient(n=3) | Control Individuals(aged 0–18 years) | Patient (n=5) | Control Individuals(aged 0–18 years) | Patient (n=3) | Control Individuals(aged 6–18 years) |
| Ribose | 47–146 | <5 | <5 | <5 | <5–102 | <5 |
| Ribitol | 891–1,249 | <6 | 6–35 | <5 | 123–186 | <5–11 |
| Arabinose | 66–135 | 6–23 | <5–7 | <5 | 37–41 | 8–46 |
| Arabitol | 3,342–5,535 | 9–39 | 32–198 | <5 | 1,021–1,612 | 16–89 |
| Xylulose | 88–166 | <5 | <5–10 | <5 | 39–52 | <5 |
| Xylitol | 33–96 | <5 | <5 | <5 | 15–34 | <5–7 |
| Treitol | 29–67 | <7 | <5–8 | <5 | 38–81 | 7–83 |
| Erythritol | <5 | 12–33 | <5 | <5 | 20–49 | 35–179 |
| Galactose | <5 | <5 | <5 | <5 | <5–62 | <5–18 |
| Mannitol | <5 | <5 | <5 | <5 | 9–25 | <5–74 |
| Myoinositol | 44–54 | 112–337 | 23–24 | 21–49 | <5–8 | <5–22 |
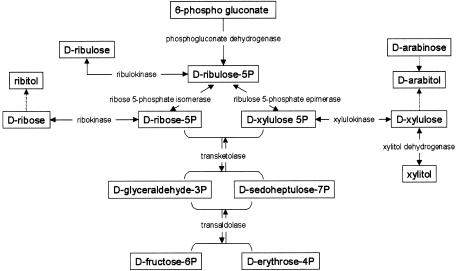
The consistent abnormalities in the pentitol levels in the present patient suggested a defect in their metabolism. It is unfortunate that little is known about the metabolic routes of polyols in humans. We hypothesized that pentitols derive from their corresponding pentoses and pentose phosphates, the latter being intermediates of the pentose-phosphate pathway (PPP) (fig. 3). We obtained an additional piece of information when we identified a patient with liver cirrhosis who also had elevated concentrations of ribitol, D-arabitol, and erythritol in urine and plasma and in whom we could demonstrate a deficiency of transaldolase, a major enzyme of the PPP, and mutations in the related gene (Verhoeven et al. 2001). We hypothesized that the present patient could also have a defect in the PPP. We developed assays to study the PPP enzymes xylulokinase, transaldolase, and transketolase in erythrocytes and lymphoblasts, but we failed to demonstrate a deficient activity of any of those enzymes in the patient.
Figure 3.
Schematic representation of the presumed reactions leading to the formation of the pentitols ribitol, D-arabitol, and xylitol
We then developed a new sensitive method for measuring intermediates of the PPP in blood spots (Huck et al. 2003). This method can be used to study two additional enzymes of the PPP in cultured cell lines, ribose-5-phosphate isomerase (RPI) (Enzyme Commission [EC] number 5.3.1.6) and ribulose 5-phosphate epimerase (RPE) (EC: 5.1.3.1).
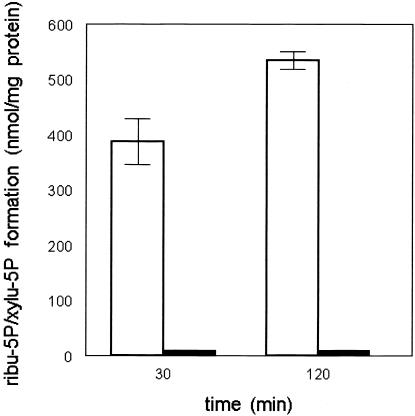
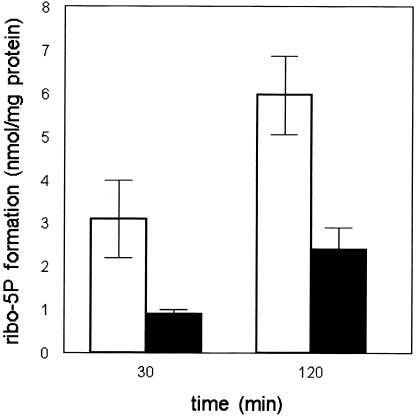
In the PPP, ribulose 5-phosphate is converted both to ribose-5-phosphate by RPI and to xylulose 5-phosphate by RPE, providing the substrates for transketolase and further conversion into glycolytic intermediates (fig. 3). To assay both RPI and RPE, we incubated fibroblast homogenates with ribose-5-phosphate. Formation of the reaction products ribulose 5-phosphate and xylulose 5-phosphate was monitored using the new method based on LC-MS/MS (API-3000 [Applied Biosystems]) (Huck et al. 2003). For the enzyme assay, we used fibroblast homogenates of the patient and three control individuals. The reaction was performed at 37°C in a volume of 300 μl, with concentrations of 45 mM Tris HCl buffer (pH 8.5), 21 mM Mg2+, 0.1 mM thiamine pyrophosphate, and 4 mM ribose-5-phosphate (all from Sigma). After taking 50-μl samples at 0 min, 30 min, and 120 min, sample clean-up was performed by adding 50 μl 5% perchloric acid, containing 5 μM stable isotope-labeled glucose 6-phosphate (internal standard), to the 50-μl incubation samples and keeping the samples at −20°C for ⩾30 min. To neutralize the solution, 30 μl of 1 M phosphate buffer (pH 11.5) was added, and the samples were subsequently centrifuged for 5 min at 21,000g at 4°C. After the supernatants were transferred to glass vials, the samples were subjected to LC-MS/MS with an ion-pair-loaded C18 high-performance liquid chromatography column. Figure 4 shows the combined activities of RPI and RPE in the patient and control individuals. LC-MS/MS does not allow separate quantitation of the reaction products ribulose 5-phosphate and xylulose 5-phosphate. In contrast to the findings for control individuals, no increase in the combined ribulose 5-phosphate and xylulose 5-phosphate peak could be observed in the patient, suggesting a deficiency of RPI.
Figure 4.
Combined activities of RPI and RPE in cultured fibroblast homogenates, measured by monitoring the combined formation of ribulose 5-phosphate and xylulose 5-phosphate from ribose-5-phosphate in three different control cell lines (unblackened bars) and in three independent experiments in the patient cell line (blackened bars). Results are expressed as the amount of the combined ribulose 5-phosphate + xylulose 5-phosphate formed during 2 h of incubation. Mean values are given; error bars indicate the SD in the results of the control cell lines.
An additional enzyme assay was developed to study RPI separately. We incubated the same fibroblast lines with 6-phosphogluconate, which is a substrate for phosphogluconate dehydrogenase (EC: 1.1.1.44) and is converted into ribulose 5-phosphate. Subsequent ribulose 5-phosphate conversion to ribose-5-phosphate by RPI was monitored by LC-MS/MS. The assay was performed at 37°C in a volume of 3 ml of 100 mM Tris/HCl buffer (pH 8.6), with concentrations of 33 μM 6-phosphogluconate, 0.2 mM NAD+, and 5 mM MgCl2. After taking 100 μl samples at 0 min, 30 min, and 120 min, clean-up of the samples and sugar phosphate determination was performed as described above. Formation of ribose-5-phosphate by fibroblasts, from the patient and the three control individuals, during incubation with 6-phosphogluconate is shown in figure 5. A decreased formation of ribose-5-phosphate could be observed in the patient as compared with control individuals, again suggesting a defect in RPI. Fifty percent of the expected ribose-5-phosphate was still produced when incubating the fibroblasts with 6-phosphogluconate, which could suggest residual RPI activity. However, interconversions of pentoses also may result in ribose-5-phosphate formation.
Figure 5.
RPI activity in cultured fibroblast homogenates, measured by LC-MS/MS by monitoring the formation of ribose-5-phosphate from 6-phosphogluconate in three different control cell lines (unblackened bars) and in three independent experiments in the patient cell line (blackened bars). Results are expressed as the amount of ribose-5-phosphate formed during 2 h of incubation. Mean values are given; error bars indicate the SD in the results of the cell lines.
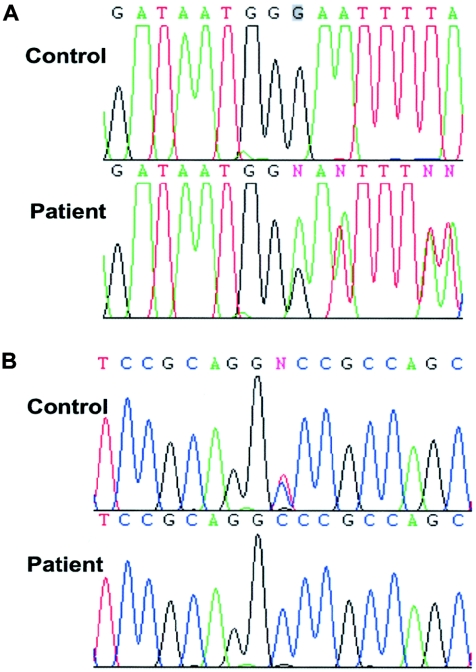
Because of the biochemical findings, we decided to sequence the gene RPI. cDNA was synthesized from total RNA derived from lymphoblasts of the patient by Omniscript Transcriptase (RT) (Qiagen). The cDNA sequence of RPI has been published (Apel et al. 1995; GenBank accession number NM_144563). RT-PCRs to amplify RPI cDNA were performed using a set of RPI-specific primers and Hot Startaq (Qiagen). Primer sequences will be provided on request. An automated DNA sequencer (ABI 3100 [PE Biosystems]) was used to analyze RPI cDNA, by use of BigDye terminator and cycle sequencing (PE Biosystems). Sequence analysis of full-length RPI cDNA demonstrated a single base-pair deletion (c.540delG) in the patient, causing a frameshift change with asparagine 181 as the first affected amino acid and a shifted reading frame ending in a stop at codon 17 (p.N181fsX17) (fig. 6A). This predicts a truncated protein of 196 amino acids. In addition, a single transition, c.182C→T, was found, which leads to a substitution of alanine for a valine at codon 61 (p.A61V) (fig. 6B). The alanine at this position is conserved in the RPI of the two known species (mouse and rat). Direct sequencing of PCR-amplified gDNA exon 4 and exon 7 of the patient confirmed both the frameshift and the missense mutations. The mother of the patient was found to be heterozygous for the frameshift mutation. DNA of the father was not available for investigation. These mutations were not found in 220 control chromosomes of individuals of Northern European descent, making it unlikely that the mutations represent polymorphisms.
Figure 6.
RPI cDNA sequence from a control lymphoblast sample and from the patient lymphoblast sample, showing a single base-pair deletion (c.540delG) in exon 7 (A), resulting in a frameshift and premature termination of translation of the RPI protein, and a single transition, c.182C→T, in exon 4 (B), resulting in the substitution of an alanine for a valine at codon 61 (p.A61V) in the RPI protein.
RPI deficiency is a novel inborn error in the PPP. The most likely explanation for the biochemical abnormalities in our patient is that deficient conversion of ribulose 5-phosphate into ribose-5-phosphate leads to accumulation of pentoses and pentose phosphates, which in turn lead to accumulation of ribitol and D-arabitol as metabolic end products. The elevated levels of pentoses found in CSF and urine are in agreement with this. In normal individuals, polyols are particularly abundant in the CNS (Kusmierz et al. 1989; Shetty et al. 1995), suggesting active production of polyols in nervous tissue or selective transport toward the brain. These data may explain the extreme accumulation of polyols in the nervous system in our patient.
The disease is clinically characterized by a leukoencephalopathy and mild peripheral polyneuropathy. The extremely high levels of D-arabitol and ribitol in brain tissue suggest that the leukoencephalopathy and neuropathy of the patient may be related to polyol toxicity. This hypothesis is supported by the findings in two other disorders. In diabetes mellitus and galactosemia, elevated levels of the polyols sorbitol and galactitol are present, and evidence has been provided that they may be important in the pathophysiology of the neurological damage (Greene and Stevens 1996; Berry et al. 2001). Peripheral neuropathy is a common complication of diabetes mellitus, whereas cerebral white-matter abnormalities occur in galactosemia (Berry 1995; Sundkvist et al. 2000). In nervous tissue of diabetic and galactosemic animals, accumulations of sorbitol and galactitol, respectively, have been found (Sredy et al. 1991; Greene and Stevens 1996), accompanied by a decrease in myoinositol. Evidence has been provided for a role of both osmotic stress as a result of polyol accumulation (Kinoshita 1974) and defective Na+/K+-ATPase regulation as a result of myoinositol depletion (Greene et al. 1988) in the pathogenesis of nervous-tissue complications in diabetes mellitus and galactosemia.
The patient in the present study has leukoencephalopathy, highly elevated ribitol and D-arabitol levels in his brain and body fluids, and a defect in the PPP (i.e., RPI deficiency). Recently, we reported on a patient with liver cirrhosis, abnormal polyol levels in her body fluids, and another defect in the PPP (i.e., transaldolase deficiency) (Verhoeven et al. 2001). Our findings confirm that defects involving pentose and polyol metabolism constitute a new area of inborn metabolic disorders, yet to be explored.
Acknowledgments
We thank Petra J. W. Pouwels, Ph.D., Department of Clinical Physics and Informatics, Vrije Universiteit Medical Center, Amsterdam, for her assistance in the MRS studies. James M. Powers, M.D., Department of Pathology, University of Rochester Medical Center, Rochester, NY, is acknowledged for critical reading of the manuscript.
Electronic-Database Information
The accession number and URL for data presented herein are as follows:
- GenBank, http://www.ncbi.nih.gov/Genbank/ (for NM_144563)
References
- Apel TW, Scherer A, Adachi T, Auch D, Ayane M, Reth M (1995) The ribose 5-phosphate isomerase-encoding gene is located immediately downstream from that encoding murine immunoglobulin kappa. Gene 156:191–197 10.1016/0378-1119(94)00901-4 [DOI] [PubMed] [Google Scholar]
- Berry GT (1995) The role of polyols in the pathophysiology of hypergalactosemia. Eur J Pediatr Suppl 154:S53–S64 7671966 [DOI] [PubMed] [Google Scholar]
- Berry GT, Hunter JV, Wang Z, Dreha S, Mazur A, Brooks DG, Ning C, Zimmerman RA, Segal S (2001) In vivo evidence of brain galactitol accumulation in an infant with galactosemia and encephalopathy. J Pediatr 138:260–262 10.1067/mpd.2001.110423 [DOI] [PubMed] [Google Scholar]
- Greene DA, Lattimer SA, Sima AA (1988) Are disturbances of sorbitol, phosphoinositide, and Na+-K+-ATPase regulation involved in pathogenesis of diabetic neuropathy? Diabetes 37:688–693 [DOI] [PubMed] [Google Scholar]
- Greene DA, Stevens MJ (1996) The sorbitol-osmotic and sorbitol-redox hypotheses. In: LeRoith D, Taylor SI, Olefsky JM (eds) Diabetes mellitus. Lippincott Raven, Philadelphia, pp 801–809 [Google Scholar]
- Hanefeld F, Holzbach U, Kruse B, Wilichowski E, Christen HJ, Frahm J (1993) Diffuse white matter disease in three children: an encephalopathy with unique features on magnetic resonance imaging and proton magnetic resonance spectroscopy. Neuropediatrics 24:244–248 [DOI] [PubMed] [Google Scholar]
- Huck JHJ, Struys EA, Verhoeven NM, Jakobs C, van der Knaap MS (2003) Profiling of pentose phosphate pathway intermediates in blood spots by tandem mass spectrometry: application to transaldolase deficiency. Clin Chem 49:1375–1380 10.1373/49.8.1375 [DOI] [PubMed] [Google Scholar]
- Jansen G, Muskiet FA, Schierbeek H, Berger R, van der Slik SW (1986) Capillary gas chromatographic profiling of urinary, plasma and erythrocyte sugars and polyols as their trimethylsilyl derivatives, preceded by a simple and rapid prepurification method. Clin Chim Acta 157:277–293 10.1016/0009-8981(86)90303-7 [DOI] [PubMed] [Google Scholar]
- Kinoshita JH (1974) Mechanisms initiating cataract formation. Invest Ophthalmol 13:713–724 [PubMed] [Google Scholar]
- Kristjansdottir R, Uvebrant P, Hagberg B, Kyllerman M, Wiklund LM, Blennow G, Flodmark O, Gustavsson L, Ekholm S, Mansson JE (1996) Disorders of the cerebral white matter in children: the spectrum of lesions. Neuropediatrics 27:295–298 [DOI] [PubMed] [Google Scholar]
- Kusmierz J, DeGeorge JJ, Sweeney D, May C, Rapoport SI (1989) Quantitative analysis of polyols in human plasma and cerebrospinal fluid. J Chromatogr 497:39–48 [DOI] [PubMed] [Google Scholar]
- Leegwater PA, Vermeulen G, Könst AA, Naidu S, Mulders J, Visser A, Kersbergen P, Mobach D, Fonds D, van Berkel CG, Lemmers RJ, Frants RR, Oudejans CB, Schutgens RB, Pronk JC, van der Knaap MS (2001a) Subunits of the translation initiation factor eIF2B are mutant in leukoencephalopathy with vanishing white matter. Nat Genet 29:383–388 10.1038/ng764 [DOI] [PubMed] [Google Scholar]
- Leegwater PA, Yuan BQ, van der SJ, Mulders J, Könst AA, Boor PK, Mejaski-Bosnjak V, van der Maarel SM, Frants RR, Oudejans CB, Schutgens RB, Pronk JC, van der Knaap MS (2001b) Mutations of MLC1 (KIAA0027), encoding a putative membrane protein, cause megalencephalic leukoencephalopathy with subcortical cysts. Am J Hum Genet 68:831–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann R, Moller JR, Trapp BD, Shih HH, Farrer RG, Katz DA, Alger JR, Parker CC, Hauer PE, Kaneski CR, Heiss JD, Kaye EM, Quarles RH, Brady RO, Barton NW (1994) Childhood ataxia with diffuse central nervous system hypomyelination. Ann Neurol 35:331–340 [DOI] [PubMed] [Google Scholar]
- Shetty HU, Holloway HW, Rapoport SI (1995) Capillary gas chromatography combined with ion trap detection for quantitative profiling of polyols in cerebrospinal fluid and plasma. Anal Biochem 224:279–285 10.1006/abio.1995.1041 [DOI] [PubMed] [Google Scholar]
- Sredy J, Sawicki DR, Notvest RR (1991) Polyol pathway activity in nervous tissues of diabetic and galactose-fed rats: effect of dietary galactose withdrawal or tolrestat intervention therapy. J Diabet Complications 5:42–47 1830318 [DOI] [PubMed] [Google Scholar]
- Sundkvist G, Dahlin LB, Nilsson H, Eriksson KF, Lindgarde F, Rosen I, Lattimer SA, Sima AA, Sullivan K, Greene DA (2000) Sorbitol and myo-inositol levels and morphology of sural nerve in relation to peripheral nerve function and clinical neuropathy in men with diabetic, impaired, and normal glucose tolerance. Diabet Med 17:259–268 10.1046/j.1464-5491.2000.00261.x [DOI] [PubMed] [Google Scholar]
- van der Knaap MS, Barth PG, Gabreëls FJ, Franzoni E, Begeer JH, Stroink H, Rotteveel JJ, Valk J (1997) A new leukoencephalopathy with vanishing white matter. Neurology 48:845–855 [DOI] [PubMed] [Google Scholar]
- van der Knaap MS, Barth PG, Stroink H, Van Nieuwenhuizen O, Arts WFM, Hoogenraad F, Valk J (1995) Leukoencephalopathy with swelling and a discrepantly mild clinical course in eight children. Ann Neurol 37:324–334 [DOI] [PubMed] [Google Scholar]
- van der Knaap MS, Breiter SN, Naidu S, Hart AA, Valk J (1999a) Defining and categorizing leukoencephalopathies of unknown origin: MR imaging approach. Radiology 213:121–133 [DOI] [PubMed] [Google Scholar]
- van der Knaap MS, Leegwater PA, Könst AA, Visser A, Naidu S, Oudejans CB, Schutgens RB, Pronk JC (2002) Mutations in each of the five subunits of translation initiation factor eIF2B can cause leukoencephalopathy with vanishing white matter. Ann Neurol 51:264–270 10.1002/ana.10112 [DOI] [PubMed] [Google Scholar]
- van der Knaap MS, van der Voorn P, Barkhof F, van Coster R, Krägeloh-Mann I, Feigenbaum A, Blaser S, Vles JSH, Rieckmann P, Pouwels PJW (2003) A new leukoencephalopathy with brainstem and spinal cord involvement and high lactate. Ann Neurol 53:252–258 10.1002/ana.10456 [DOI] [PubMed] [Google Scholar]
- van der Knaap MS, Wevers RA, Struys EA, Verhoeven NM, Pouwels PJ, Engelke UF, Feikema W, Valk J, Jakobs C (1999b) Leukoencephalopathy associated with a disturbance in the metabolism of polyols. Ann Neurol 46:925–928 [DOI] [PubMed] [Google Scholar]
- Verhoeven NM, Huck JHJ, Roos B, Struys EA, Salomons GS, Douwes AC, van der Knaap MS, Jakobs C (2001) Transaldolase deficiency: liver cirrhosis associated with a new inborn error in the pentose phosphate pathway. Am J Hum Genet 68:1086–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]