Abstract
Individuals with mosaicism for the autosomal dominant bone dysplasia osteogenesis imperfecta (OI) are generally identified by having more than one affected child. The mosaic carriers have both normal and mutant cell populations in somatic and germline tissues but are unaffected or minimally affected by the type I collagen mutation that manifests clinically in their heterozygous offspring. We determined the proportion of mutant osteoblasts in skeletal tissue of two mosaic carriers who each have a COL1A1 mutation in a high proportion of dermal fibroblasts. Both carriers had normal height and bone histology; the first carrier had normal lumbar spine measurements (L1–L4), as determined by dual-energy x-ray absorptiometry (Z = +1.17). In cultured cells from the first carrier, studied by labeled PCR and single-cell PCR over successive passages, the collagen mutation was present in 85% of fibroblasts and 50% and 75% of osteoblasts from her right iliac crest and left patella, respectively, with minimal selection. The second carrier was studied by PCR amplification of DNA from autopsy paraffin blocks. The proportion of heterozygous cells was 40% in calvarium, 65% in tracheal ring, and 70% in aorta. Thus, in OI, substantially normal skeletal growth, density, and histology are compatible with a 40%–75% burden of osteoblasts heterozygous for a COL1A1 mutation. These data are encouraging for mesenchymal stem-cell transplantation, since mosaic carriers are a naturally occurring model for cell therapy.
Most cases of osteogenesis imperfecta (OI), or “brittle bone disease,” are caused by mutations in one of the two genes encoding type I collagen, the major structural component of the matrix of bone and skin (Byers and Cole 2002). Affected individuals have fragile bones and increased susceptibility to fractures (Marini and Chernoff 2001). Collagen mutations causing this bone dysplasia occur in two broad categories (Byers et al. 1991), structural defects and quantitative defects. Structural defects of the alpha chains are associated with the lethal, severe, and moderate forms of the disease (type II [MIM 166210], type III [MIM 259420], and type IV [MIM 166220], respectively). The quantitative defects are usually caused by a null COL1A1 allele (Willing et al. 1994), resulting in a reduced amount of structurally normal collagen and mild symptoms (OI type I [MIM 166200]).
Dominant negative mutations, such as collagen structural defects, will not be amenable to the gene-replacement approach that is being developed as therapy for recessive disorders (Marini and Gerber 1997). The gene-replacement approach aims to transfer a construct containing a normal gene into the tissue of a patient who has no functional product from the endogenous copies of the gene. Even modest expression of the construct might provide sufficient gene product for clinical correction. In OI types II, III, and IV, the defective collagen is secreted from the cell and incorporated into the matrix, where it actively contributes to a weakened structure. Furthermore, depending on whether the structural defect is in the α1 or α2 chain of type I collagen, the heterotrimeric procollagen secreted from the cell can include 75% or 50% abnormal helices, respectively. The addition of a modest amount of normal collagen from an expression construct would not be sufficient to counteract the dominant negative function of the endogenous mutant collagen. One approach to therapy for OI and other dominant skeletal dysplasias involves the transplantation of normal mesenchymal precursors (Niyibizi et al. 2002). The naturally occurring model for this approach is the mosaic carrier. This individual has two genetically distinct cell lines, one of which carries the disease-causing mutation because of a postzygotic event (Hall 1988). Almost two dozen mosaic carriers for OI have been molecularly delineated (Dalgleish 1997, 1998). Most carriers described in the literature have had children with lethal OI; a recurrence risk of 5%–7% has been estimated for lethal OI (Byers et al. 1988). Lund and colleagues have reported five parental mosaic carriers for moderate-to-severe nonlethal OI (Lund et al. 1996, 1997, 1999), suggesting that mosaicism occurs in at least a similar proportion of nonlethal mutations. Clinically, the mosaic carriers are normal or minimally affected. They are most often identified by having more than one child with the fully manifesting heterozygous condition.
In each mosaic carrier, the proportion of mutant cells varies from tissue to tissue. The proportions will depend on the postzygotic timing of the collagen mutation and its relationship to the development of the embryo, including the tissues to which the daughters of the original mutant cell contribute and the distribution of the mutant cell line within those tissues. Selective pressures may also play a variable role in different tissues. For example, the mutation might affect cell replication and senescence differently in tissues in which the mutant gene was not expressed than in those with high or low expression levels. In 10 mosaic carriers of OI, the proportion of mutant cells was determined in more than one tissue by examining fibroblasts, leukocytes, hair bulbs, and sperm, as available. In each carrier, there was wide variation in the proportion of mutant cells between tissues. For example, a mosaic carrier of a nonlethal mutation (COL1A2 G802D), which caused OI type III/IV in the individual's affected offspring, had 25% mutant alleles in leukocytes and 40% mutant alleles in sperm (Lund et al. 1996). Three carriers had a collagen mutation in at least half of their leukocytes and essentially all of their fibroblasts (Constantinou et al. 1989; Wallis et al. 1990; Edwards et al. 1992).
The proportion of mutant osteoblasts that is compatible with normal skeletal functioning is clearly crucial but has not been examined in mosaic carriers. This data cannot be estimated by extrapolation because of tissue-to-tissue variation. The relative survival, over successive passages, of bone and skin cells carrying a collagen mutation, as compared with normal cells from the same individual, also has not been reported.
We have determined the proportion of mutant osteoblasts and fibroblasts in two mosaic carriers of OI. Each mosaic carrier has a structural mutation in the α1 chain of type I collagen, which causes OI type III or IV in the children of the carrier. This information about naturally occurring mosaic carriers provides target levels for cell therapy.
The first mosaic carrier is a 51-year-old woman whose two sons have moderately severe OI type IV. She has had mild functional effects from the collagen mutation she carries, compatible with an OI type I phenotype. Her height is 160 cm, which is in the 25th percentile for adult women but which is 8 cm less than the height predicted by parental heights. Her 168-cm span is moderately longer than her height and her upper segment to lower segment ratio of 0.82 is >−2 SDs below average for adult women and confirms that her slightly short stature is associated with a short trunk. Head circumference is relatively large (57 cm; 95th percentile for adult women). She has light blue sclerae. Dentinogenesis imperfecta was detected on her Panorex, although it is not apparent on oral inspection. Wormian bones were detected by radiography on a lateral skull film. Lumbar spine (L1–L4), as measured by dual-energy x-ray absorptiometry (DEXA), is in the normal range (1.083 gm/cm2; Z=+1.17), without prior administration of bisphosphonates. She sustained two fractures from minor accidents, a fractured wrist at age 13 years in a bicycle fall and a shattered left patella at age 46 years from a fall in the bathroom at home. Moderately severe low-frequency conductive hearing loss (R 30 dB; L 50 dB) developed in her 40s, without external risk factors such as noise exposure, but she does not require amplification. Her sons developed hearing loss in their 20s. Her skin thickness and elasticity are normal, with well-healed surgical scars. Both large and small joints have normal mobility.
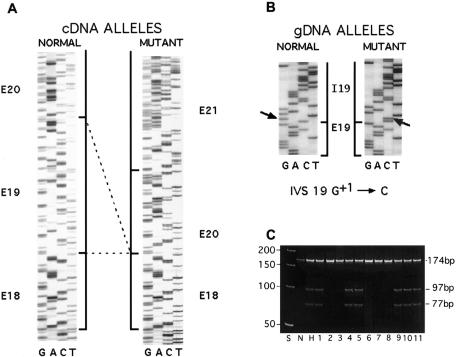
The type I collagen mutation causing OI type IV in her sons was determined to be a COL1A1 intron 19 G+1→C point mutation by conformation-sensitive gel electrophoresis (Korkko et al. 1998) of PCR amplification (Saiki et al. 1985) products, followed by sequencing (Sanger et al. 1977) on a Prism 377 DNA Sequencer (Applied Biosystems) by the Center for Gene Therapy, Tulane University, New Orleans. The mutation introduces a DdeI restriction site, which was detected in a PCR amplification product spanning COL1A1 introns 15–19 of the mosaic carrier’s dermal fibroblast and leukocyte genomic DNA. The mutation causes skipping of exon 19 at the cDNA level (fig. 1A and 1B); RT-PCR amplification of total fibroblast RNA (Chomczynski and Sacchi 1987) of one of the affected sons showed that about half of the transcripts of the mutant allele have exon 19 skipping (data not shown).
Figure 1.
Collagen mutation and clonal lines of the mosaic carrier of OI type IV. A, Sequence of the normal and mutant cDNA of son of the mosaic carrier, showing skipping of COL1A1 exon 19 sequences. B, Sequence of COL1A1 gene, showing the splice-donor–site mutation IVS 19 G+1→C. C, Demonstration that the mosaic carrier has two distinct cell lines. The PCR fragment containing the exon 19/intron 19 junction was amplified from DNA of dermal fibroblast clonal lines by PCR, by use of a forward primer in exon 19 (5′-TGCTCCTGGTATTGCTGGTGCTCCTGGCTTC-3′) and a reverse primer in intron 19 (5′-gcgtcttcctgctccccagatgagagccgc-3′) (lowercase letters denote intron nucleotides; uppercase letters denote exon nucleotides). PCR conditions were 94°C for 5 min; 30 cycles of 1 min at 94°C, 30 s at 67°C, and 30 s at 72°C; and, finally, 7 min at 72°C. Amplification products were digested with DdeI and electrophoresed on a 6% acrylamide gel. Five clonal lines (2, 3, 6, 7, 8) have undigested product (174 nt) from normal allele sequence and 6 clonal lines (1, 4, 5, 9, 10, 11) are heterozygous for normal and mutant allele products (DdeI digestion products, 97 nt and 77 nt). S is the 50-bp ladder; N and H are normal and heterozygous control cells.
Skin fibroblast cultures of the mosaic carrier and her son were established from dermal punch biopsies. Osteoblast primary cultures were established from surgical bone chips (Robey and Termine 1985). Bone chips from the mosaic carrier of OI type IV were obtained from a left patella repair and a right iliac crest biopsy; bone chips from her son were obtained at osteotomy. All samples were obtained with informed consent of the carrier and the assent of her son.
The collagen mutation was estimated to be present in 72% of maternal leukocytes, by densitometry of DdeI digestion products of a 32P-labeled PCR fragment that included the exon 19/intron 19 junction. The existence of two distinct cell populations demonstrated that the individual was a true mosaic carrier for the mutation. From the dermal fibroblasts of the mosaic carrier, 11 clonal lines were generated. Dilute low-passage fibroblasts were distributed in 96-well plates; wells with single cells were identified by microscopic inspection. When sufficient numbers of cell divisions had occurred, the clonal cells were transferred successively to 24-well, 12-well, and 6-well plates, at which time their DNA was isolated using the PureGene DNA Isolation Kit (Gentra Systems). PCR of clonal genomic DNA followed by DdeI digestion identified six lines with the IVS19 G+1→C mutation and with five normal collagen sequences (fig. 1C).
The proportion of fibroblasts and osteoblasts heterozygous for the collagen mutation was quantitated by both (1) a radiolabeled PCR assay using total genomic DNA and (2) a single-cell PCR amplification of the region surrounding the point mutation. The accuracy of both assays was validated by use of DNA and cells from the heterozygous son of the carrier. For the radiolabeled PCR assay, a 174-bp region of COL1A1 genomic DNA was amplified using primers located in exon 19 and intron 19 and 5 μCi of 111 TBq/mmol [α-32P] dCTP. PCR products were digested with DdeI. Digestion products were quantitated by densitometry of autoradiograms. Allele proportions from the mosaic-carrier samples were normalized to allele detection in heterozygous samples. Triplicate PCR reactions were analyzed for each person at each passage. The labeled PCR yielded estimates of 90%–96% heterozygous cells, compared with the expected 100% heterozygous cells in the son.
The assay for quantitating the proportion of heterozygous cells in the mosaic carrier by single-cell PCR was modified from the procedure used by Leeflang et al. (1995). Cells were plated in 96-well plates at an average of 1 cell/well, with ∼25%–30% of wells containing a single cell. Wells with individual cells were identified by microscopic examination the following morning, lysed in the well, and the entire single-cell lysate was used for a heminested PCR. First-round amplifications used 100 mM 7-deaza-dGTP, 10% glycerol, 3% DMSO, 100 μg/ml gelatin, and 1 U rTth DNA polymerase (Perkin Elmer). The sense primer for first-round amplification spanned the COL1A1 intron 18/exon 19 junction (5′-acagGGTGCTCCTGGTATTGCTGG-3′ [lowercase letters denote intron nucleotides; uppercase letters denote exon nucleotides]) and the antisense primer was in intron 19. Second-round amplifications used 10% glycerol, 3% DMSO, 100 μg/ml gelatin, 1 U of Taq DNA polymerase, and 5 μl of first-round PCR product. The sense primer for the second round was in exon 19 (5′-GTGCCCGAGGCCCCCTCTGGA-3′), and the antisense primer was identical to first round amplification. All PCR conditions are available from the authors upon request. The entire second-round PCR reaction was concentrated in Microcon YM-30 spin columns (Millipore), digested with DdeI, electrophoresed on a 6% polyacrylamide gel, and visualized by ethidium bromide staining. Heterozygosity of an individual cell was determined by the presence of both alleles in the PCR product. The proportion of heterozygous cells in the mosaic carrier was normalized for the low percentage of false-normal PCRs of heterozygous control cells in which only the normal allele was amplified. In the affected heterozygous son of the mosaic carrier, single-cell PCR detected 93% of heterozygous fibroblasts and 88% of heterozygous osteoblasts. The small false-normal background occurs in the assays of single heterozygous cells because of physical loss of the single copy of the normal allele or its failure to amplify in the early cycles of the PCR. The raw values of the mosaic carrier assays were corrected for the detection level in the heterozygous samples.
The use of these assays on carrier fibroblast DNA (tables 1 and 2) yielded consistent estimates of 86% mutant fibroblasts by labeled PCR and 85% mutant fibroblasts by single-cell PCR, persisting in passages 2–17. The proportion was similar on both sides of the carrier's body, with only mild selection against mutant cells at high passage. Cultured osteoblasts (tables 3 and 4) from the right and left sides of her body had different proportions of mutant cells. On the left, she had 73% mutant cells, as determined by both labeled PCR and single-cell PCR. In the right iliac crest sample, 50% mutant cells were detected by labeled PCR, in agreement with 48% mutant cells detected by single-cell PCR. The proportion of mutant osteoblasts was stable through medium passage numbers and fell only at high passage numbers (P10). We are unable to comment on any selection against mutant osteoblasts that might occur in vivo at high passages, because osteoblasts in culture dedifferentiate to a fibroblast phenotype at medium passages.
Table 1.
Fibroblast Results from 32P-Labeled PCR Assay of Samples from the Mosaic Carrier of OI Type IV and Affected Son[Note]
|
% of Fibroblasts Heterozygous for Mutation ± SD (Normalized) for |
|||
| Mosaic Carrier |
|||
| PassageNo. | Heterozygous Affected Sona | Left Side | Right Side |
| P2–P7 | 96 ± 4 | 83 ± 8 (86) | 82 ± 6 (85) |
| P13 | 95 ± 4 | 81 ± 13 (85) | 82 ± 6 (86) |
| P17 | 95 ± 3 | 80 ± 4 (84) | 84 ± 5 (88) |
| P20 | 92 ± 5 | 74 ± 4 (80) | 70 ± 7 (76) |
Genomic DNA of COL1A1 was amplified for exon 19–intron 19 (174 bp).
Value calculated by densitometry of DdeI-digested PCR fragments as follows: digested fragments/total fragments × 2 × 100.
Table 2.
Fibroblast Results from Single-Cell PCR Assay of Samples from the Mosaic Carrier of OI Type IV and Affected Son
|
No. of Cells Detected in |
|||||||||||||||
| Mosaic Carrier |
|||||||||||||||
| Heterozygous Affected Son |
Left Side |
Right Side |
|||||||||||||
| Passage No. | Normal | Mutant | Heterozygous | Total | %a | Normal | Mutant | Heterozygous | Total | % | Normal | Mutant | Heterozygous | Total | % |
| P6–10 | 6 | 6 | 93 | 105 | 94.3 | 25 | 2 | 81 | 108 | 82.4 | 23 | 2 | 88 | 113 | 85.0 |
| P 20 | 8 | 4 | 94 | 106 | 92.5 | 37 | 3 | 74 | 114 | 75.4 | 39 | 0 | 61 | 100 | 69.0 |
Percentage of heterogeneous cells plus mutant cells, divided by the total number of cells. (In the mosaic carrier, this number has been normalized for false-normal cells in the affected son.)
Table 3.
Osteoblast Results from 32P-Labeled PCR Assay of Samples from the Mosaic Carrier of OI Type IV and Affected Son[Note]
|
% of Osteoblasts Heterozygous for Mutation ± SD (Normalized) for |
|||
| Mosaic Carrier |
|||
| PassageNo. | Heterozygous Affected Sona | Left Side | Right Side |
| P1 | 96 ± 2 b | … | 49 ± 4 (50) |
| P2 | … | … | 40 ± 3 (41) |
| P3 | 97 ± 1 | 71 ± 2 (73) | 37 ± 5 (39) |
| P5 | 97 ± 3 | 71 ± 7 (73) | … |
| P7 | 90 ± 2 | 65 ± 12 (73) | … |
| P10 | 95 ± 7 | 39 ± 6 (41) | … |
Note.— Genomic DNA of COL1A1 was amplified for exon 19–intron 19 (174 bp).
Value calculated by densitometry of DdeI-digested PCR fragments as follows: digested fragments/total fragments × 2 × 100.
P1 Osteoblast DNA was used to normalize P1–P2 mosaic carrier cells.
Table 4.
Osteoblast Results from Single-Cell PCR Assay of Samples from the Mosaic Carrier of OI Type IV and Affected Son
|
No. of Cells Detected in |
|||||||||||||||
| Mosaic Carrier |
|||||||||||||||
| Heterozygous Affected Son |
Left Side |
Right Side |
|||||||||||||
| Passage No. | Normal | Mutant | Heterozygous | Total | %a | Normal | Mutant | Heterozygous | Total | %a | Normal | Mutant | Heterozygous | Total | %a |
| P1 | … | … | … | … | … | … | … | … | … | … | 67 | 0 | 39 | 106 | 48.1 |
| P3 | 12 | 0 | 93 | 105 | 88.6 | 36 | 0 | 66 | 102 | 73.0 | 69 | 0 | 39 | 108 | 47.2 |
| P10 | 12 | 0 | 90 | 102 | 88.2 | 52 | 1 | 50 | 103 | 61.2 | … | … | … | … | … |
Percentage of heterogeneous cells plus mutant cells, divided by the total number of cells. (In the mosaic carrier, this number has been normalized for false-normal cells in the affected son.)
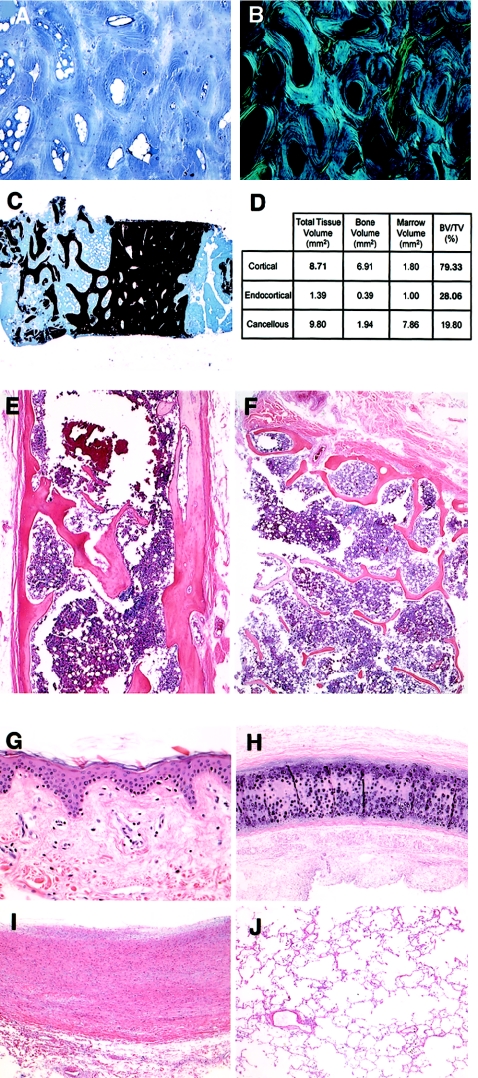
Bone from the right iliac crest biopsy had normal histology (fig. 2A–2D). Under polarized light, the toluidine-blue–stained specimen showed well-organized lamellae around their respective osteocytes. Bone volume and bone volume per total volume were normal.
Figure 2.
Histology of tissues from both mosaic carriers. A–C, Biopsy of the iliac crest sample from the mosaic carrier of OI type IV. A and B, Toluidine-blue–stained section under normal and polarized light. C, von Kossa stained section, with normal bone volume. D, Quantitative histomorphometry of sample in panel C. E and F, Sections of calvarial and rib bone, respectively, of the mosaic carrier of OI type III, stained with hematoxylin-eosin. G–J, Sections of dermis, tracheal ring, aorta wall, and lung, respectively, of the mosaic carrier of OI type III, stained with hematoxylin-eosin.
The second mosaic carrier analyzed was a 67-year-old woman. Two of her seven children had severe OI type III. One affected son died of pneumonia as a child. The second affected son is now 46 years old and has a 17-year-old daughter with severe OI. On physical exam, the carrier had normal height (161 cm; 50th percentile for adult women) and well-proportioned span (158 cm). Her upper-to-lower segment ratio was 0.90, which is within the normal range (−1.5 SDs for adult women). The only manifestations of a connective tissue disorder were blue sclerae and a triangular-shaped facies. She had never sustained a fracture. She died of pneumonia after an intracranial hemorrhage at age 67 years; tissues taken at autopsy and embedded in paraffin for histology analysis were available.
The type I collagen mutation causing OI type III in this family has been published (Wang et al. 1996). It is a gene deletion of 562 bp in the genomic DNA of one COL1A1 allele, which results in the deletion of exons 33–36. PCR screening of family members by Wang and colleagues revealed that the mosaic carrier had a high proportion of leukocytes heterozygous for the mutant allele.
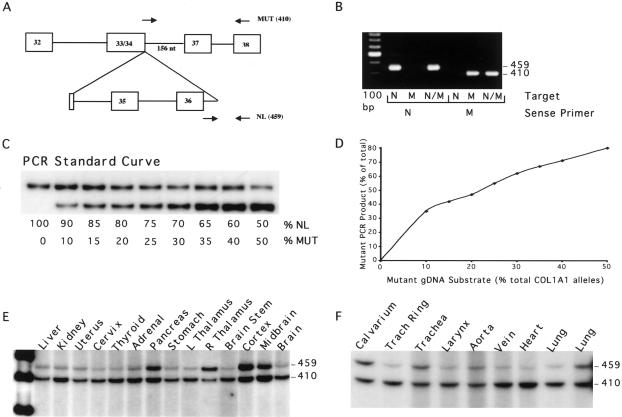
DNA was extracted from paraffin-embedded autopsy tissues, cultured P5 fibroblasts, and leukocytes of the mosaic carrier of OI type III, with informed consent of her family, by use of the Puregene DNA Purification Kit. A 32P-labeled multiplex PCR assay (fig. 3) was developed, which allowed quantitation of the proportions of the two COL1A1 alleles. Allele ratios were calculated and then doubled for the proportion of heterozygous cells.
Figure 3.
Multiplex PCR of autopsy tissues of the mosaic carrier of OI type III. A, Diagram of multiplex PCR amplification of DNA from autopsy tissues of the mosaic carrier. The normal allele sense primer spanned the exon 36/intron 36 junction (5′-TGGCCCCCCTgtgagtaccaagacccccat-3′ [lowercase letters denote intron nucleotides; uppercase letters denote exon nucleotides]), and the mutant allele sense primer spanned the novel junction between exon 34 and the retained portion of intron 36 (5′-CCTGCTGGTGCCCCTGGTGACggaa-3′).The common antisense primer (5′-CGAGCACCTTTGGCTCCAGGAGCACCAACA-3′) is in exon 38. B, Allele specificity of PCR primers, verified using subcloned mutant (M) and normal (N) proband DNA. Only the specific normal COL1A1 allele (459 bp) and mutant allele (410 bp) products were detected by the respective sense primers. C, A 32P-labeled standard curve (200 ng DNA/sample), prepared by mixing genomic DNA from normal leukocytes with DNA from the heterozygous leukocytes of the carrier’s affected son. PCR reactions used 200 ng genomic DNA, 1 U Platinum Taq DNA Polymerase High Fidelity (Invitrogen), and 5 μCi of 111 TBq/mmol [α-32P] dCTP. Normal and mutant allele products have similar numbers of C nucleotides, 132 and 120, respectively; preferential amplification of the mutant allele product is presumably due to differences in secondary structure, as well as smaller size. D, The PCR standard curve, quantitated by densitometry, and the proportion of mutant amplification product for each allele ratio in the standard curve. E and F, PCR amplification products of DNA from various autopsy tissues of the mosaic carrier. The normal allele product (459 bp) and the mutant allele product (410 bp) are indicated. Allele ratios were quantitated by densitometry and normalized to the standard curve.
The mosaic carrier had ∼60% mutant leukocytes and ∼45% mutant fibroblasts (data not shown). By use of the autopsy samples of type-I-collagen–rich tissues (fig. 3), we determined that 65% of cells were heterozygous for the collagen deletion in tracheal ring, aorta, and lung. In the skeletal system, ∼40% of calvarial cells (∼20% of alleles) carried the collagen mutation. We assumed for our calculations that the DNA was derived from osteoblasts and osteocytes in the calvarial sample, but the presence of other cells in the autopsy tissue cannot be excluded.
Histology of bone from rib and calvarium was normal for a woman of the carrier's age (fig. 2E and 2F). The trabeculae were of normal number and width, with well-organized lamellae. Likewise, histology of skin, tracheal ring, and aorta showed well-organized connective tissues (fig. 2G–2I). An aggressive infiltrative process compatible with her pneumonia distorts the lung histology (fig. 2J).
Mosaic carriers occur in a wide range of dominant genetic disorders, including skeletal dysplasias (Gottlieb et al. 2001; Youssoufian and Pyeritz 2002). These carriers have distinct mutant and normal cell lines in somatic and/or germline tissues. In OI, mosaic-carrier individuals are most commonly identified when they have more than one child with clinically significant disease. The mosaic carriers themselves do not usually come to medical attention before they have affected children, because their phenotypes range from clinically normal to minimally affected. Some mosaic carriers have physical findings, such as blue sclerae, dentinogenesis imperfecta, shorter stature than same-gender siblings, or a medical history that includes one to several fractures. Some mosaic carriers have a clinical course similar to the mildest end of the OI severity range. However, mosaic carriers do not have an intermediate form of the severe skeletal disorder seen in their own children. The mild clinical condition of mosaic carriers might be due to a low proportion of cells carrying the collagen mutation in skeletal tissues, resulting from the fortuitous distribution of the daughter cells of the original mutant cell line or from slower growth of mutant cells. Alternatively, the mild clinical condition of mosaic carriers might result from a high threshold for the structural effects of mutant collagen in the extracellular matrix. The proportion of mutant osteoblasts compatible with the mild or normal clinical status of OI mosaic carriers has not been determined.
Mosaic carriers are medically significant because they are the natural model for mesenchymal cell therapy. Mesenchymal stem cells (MSCs) are multipotential cells isolated from bone marrow that can differentiate into osteoblasts, chondrocytes, myoblasts, and adipocytes (Prockop 1997). Patients treated with cell therapy would become somatic mosaics of their own heterozygous cells and normal donor cells. Transplantation of MSCs into mice (Pereira et al. 1998; Oyama et al. 1999), fetal lambs (Mackenzie and Flake 2001), and baboons (Devine et al. 2001) has resulted in the uptake of low levels of donor cells into bone marrow or tissue, which is detectable by PCR, GFP, or CAT assays. MSC therapy of five children with severe OI resulted in a 1%–2% donor cell skeletal engraftment (Horwitz et al. 1999) and reports of transient clinical improvement (Marini 1999; Horwitz et al. 2001). Because some mosaic carriers of OI have heterozygosity of virtually all fibroblasts, it was speculated that only a very small percentage of normal-cell engraftment would be required for successful therapy (Gerson 1999).
We have determined the proportion of mutant osteoblasts in two mosaic carriers for OI and correlated this data with the clinical exam and bone histology. We demonstrated that normal or mildly decreased skeletal function is compatible with a substantial burden of mutant cells. The mildly affected carrier of OI type IV has 50%–75% mutant osteoblasts, depending on body side. The clinically normal carrier of severe OI type III had 40% mutant osteoblasts in her calvarium and ∼65% mutant cells in collagen-rich connective tissues, such as aorta, tracheal ring, dermis, and lung. To approximate the growth competition experienced by the mutant cells in vivo, the cultured cells of the type IV carrier were examined over multiple passages. In both fibroblasts and osteoblasts, the proportion of mutant cells was stable over multiple passages, with only modest decline in the proportion of mutant cells at high passages, showing that these mutant cells have normal rates of cell division in culture. Previously, the proportion of mutant cells in the cultured fibroblasts of two mosaic carriers for lethal OI type II (Constantinou et al. 1989; Cohen-Solal et al. 1996) was reported to decrease by one or two orders of magnitude at high passage numbers compared with low passage numbers. Despite the high proportion of cells synthesizing mutant collagen, both women had skeletal growth within the normal population range. The carrier of OI type IV had moderately shorter stature than predicted by parental heights, but she had a short trunk, which is less typical of OI than are short legs. She also had normal lumbar spine (L1–L4) results, as measured by DEXA. Furthermore, bone histology was normal for her age.
We infer from our data that mutant collagen can be incorporated to a high level without detriment to histology and density and with only modest detriment to bone growth and strength. These data suggest that there is a threshold, rather than a linear effect, for the mutant matrix components and that features of mild OI are not noted until the threshold level of mutant collagen is exceeded. Since the carrier of OI type IV has a moderately short stature, radiographic dentinogenesis imperfecta, hearing loss, and two minor fractures, her mutation burden may be at or near the functional threshold.
Our data provide the first quantitation of the proportion of heterozygous cells in the skeletal system of mosaic carriers for moderate and severe types of OI. Other mosaic carriers may have a lower level of mutant osteoblasts because the embryonic mutation was distributed to only a few skeletal precursors. These low-percentage mosaic carriers are not informative for the maximum level of osteoblasts compatible with normal skeletal functioning. Still other mosaic carriers may have a higher level of mutant osteoblasts, supporting the conclusion that transplantation of only a small percentage of normal osteoblast precursors would be clinically beneficial. Until data from additional rare mosaic-carrier bone samples are available, the data from the carriers presented in this study can be used to tentatively set the goal for gene therapy by MSC transplant at 30%–60% normal osteoblasts. It should be noted that we have examined mosaic carriers for the severe and moderate (types III and IV, respectively) forms of OI. A higher proportion of uptake of normal donor cells might be required if the recipient were heterozygous for a collagen mutation that caused the lethal (type II) form of OI, since the threshold of functional expression might be lower with increased disease severity. Furthermore, in our cases we found minimal selection against fibroblasts or osteoblasts carrying the collagen mutations. For some collagen mutations, normal cells might have a selective advantage. In these cases, uptake of 5% normal cells could gradually result in normal cells comprising ⩾30% of the bone cell population. Thus, the proportion of normal osteoblasts needed for proper skeletal functioning could be achieved either directly, by uptake of transplanted stem cells, or gradually, by selective advantage of normal cells.
The distribution of mutant and normal cells within mosaic-carrier tissue may contribute to normal function. The proportion of mutant osteoblasts in the carrier of OI type IV differs by 25% between body sides. This difference may result from clustering of the daughter cells of normal and mutant osteoblasts. Daughter cells from an MSC transplant might also have a patchy distribution. CAT-tagged MSCs were distributed in isolated lacunae in mouse bones 1 mo after transplantation (Hou et al. 1999). This pattern could provide a functional advantage because fibrils are assembled in the pericellular matrix. Local collections of normal cells in mosaic-carrier bone would result in regional pockets of almost-normal matrix that could increase the overall mechanical strength. Nonetheless, transplantation of even modest levels of normal osteoblast precursors remains a significant challenge. Selection of osteoblast precursors at a particular developmental stage or elucidation of physiological homing signals for bone precursor cells may enhance the percentage of transplanted cells taken up by bone tissue. Furthermore, normal cells need to be distributed throughout bone to enhance mechanical properties. This factor favors transplantation of osteoblast precursors during the higher–bone-turnover period of early childhood.
Acknowledgments
We are indebted to the mosaic carriers and their families, for their longstanding support of OI research; to C. Michael Reing, M.D., and Elizabeth Hopkins, B.S.N., for the iliac crest biopsy of the mosaic carrier of OI type IV; to Laura Tosi, M.D., for osteotomy bone chips from the son of the mosaic carrier of OI type IV; to Lawrence Loesel, M.D., for preparation of paraffin blocks and autopsy notes; and to Norman Arnheim and Raji Grewal, for guidance in adapting single-cell PCR techniques to our cases.
Footnotes
Presented as a platform presentation at the National Meeting of the American Society of Bone and Mineral Research (Late-Breaking Research Session), San Antonio, TX, September 2002.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- Database of Type I and III Collagen Mutations, http://www.le.ac.uk/genetics/collagen/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for OI type I, OI type II, OI type III, and OI type IV)
References
- Byers PH, Cole WG (2002) Osteogenesis imperfecta. In: Royce P, Steinmann B (ed) Connective tissue and its heritable disorders, 2nd ed. Wiley-Liss, New York, pp 385–430 [Google Scholar]
- Byers PH, Tsipouras P, Bonadio JF, Starman BJ, Schwartz RC (1988) Perinatal lethal osteogenesis imperfecta (OI type II): a biochemically heterogeneous disorder usually due to new mutations in the genes for type I collagen. Am J Hum Genet 42:237–248 [PMC free article] [PubMed] [Google Scholar]
- Byers PH, Wallis GA, Willing MC (1991) Osteogenesis imperfecta: translation of mutation to phenotype. J Med Genet 28:433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159 10.1006/abio.1987.9999 [DOI] [PubMed] [Google Scholar]
- Cohen-Solal L, Zolezzi F, Pignatti PF, Mottes M (1996) Intrafamilial variable expressivity of osteogenesis imperfecta due to mosaicism for lethal G382R substitution in the COL1A1 gene. Mol Cell Probes 10:219–225 10.1006/mcpr.1996.0030 [DOI] [PubMed] [Google Scholar]
- Constantinou CD, Nielsen KB, Prockop DJ (1989) A lethal variant of osteogenesis imperfecta has a single base mutation that substitutes cysteine for glycine 904 of the alpha 1(I) chain of type I procollagen. The asymptomatic mother has an unidentified mutation producing an overmodified and unstable type I procollagen. J Clin Invest 83:574–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish R (1997) The human type I collagen mutation database. Nucleic Acids Res 25:181–187 10.1093/nar/25.1.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ——— (1998) The human collagen mutation database. Nucleic Acids Res 26:253–255 10.1093/nar/26.1.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine SM, Bartholomew AM, Mahmud N, Nelson M, Patil S, Hardy W, Sturgeon C, Hewett T, Chung T, Stock W, Sher D, Weissman S, Ferrer K, Mosca J, Deans R, Moseley A, Hoffman R (2001) Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp Hematol 29:244–255 10.1016/S0301-472X(00)00635-4 [DOI] [PubMed] [Google Scholar]
- Edwards MJ, Wenstrup RJ, Byers PH, Cohn DH (1992) Recurrence of lethal osteogenesis imperfecta due to parental mosaicism for a mutation in the COL1A2 gene of type I collagen. The mosaic parent exhibits phenotypic features of a mild form of the disease. Hum Mutat 1:47–54 [DOI] [PubMed] [Google Scholar]
- Gerson SL (1999) Mesenchymal stem cells: no longer second class marrow citizens. Nat Med 5:262–264 10.1038/6470 [DOI] [PubMed] [Google Scholar]
- Gottlieb B, Beitel LK, Trifiro MA (2001) Somatic mosaicism and variable expressivity. Trends Genet 17:79–82 10.1016/S0168-9525(00)02178-8 [DOI] [PubMed] [Google Scholar]
- Hall JG (1988) Review and hypotheses: somatic mosaicism: observations related to clinical genetics. Am J Hum Genet 43:355–363 [PMC free article] [PubMed] [Google Scholar]
- Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK (1999) Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med 5:309–313 10.1038/6529 [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Prockop DJ, Gordon PL, Koo WW, Fitzpatrick LA, Neel MD, McCarville ME, Orchard PJ, Pyeritz RE, Brenner MK (2001) Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood 97:1227–1231 10.1182/blood.V97.5.1227 [DOI] [PubMed] [Google Scholar]
- Hou Z, Nguyen Q, Frenkel B, Nilsson SK, Milne M, van Wijnen AJ, Stein JL, Quesenberry P, Lian JB, Stein GS (1999) Osteoblast-specific gene expression after transplantation of marrow cells: implications for skeletal gene therapy. Proc Natl Acad Sci USA 96:7294–7299 10.1073/pnas.96.13.7294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkko J, Ala-Kokko L, De Paepe A, Nuytinck L, Earley J, Prockop DJ (1998) Analysis of the COL1A1 and COL1A2 genes by conformation-sensitive gel electrophoresis identifies only COL1A1 mutations in 15 patients with osteogenesis imperfecta type I: identification of common sequences of null-allele mutations. Am J Hum Genet 62:98–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeflang, EP, Zhang L, Tavare S, Hubert R, Srinidhi J, MacDonald ME, Myers RH, de Young M, Wexler NS, Gusella JF, Arnheim N (1995) Single sperm analysis of the trinucleotide repeats in the Huntington’s disease gene: quantification of the mutation frequency spectrum. Hum Mol Genet 4:1519–1526 [DOI] [PubMed] [Google Scholar]
- Lund AM, Astrom E, Soderhall S, Schwartz M, Skovby F (1999) Osteogenesis imperfecta: mosaicism and refinement of the genotype-phenotype map in OI type III. Hum Mutat 13:503 [DOI] [PubMed] [Google Scholar]
- Lund AM, Nicholls AC, Schwartz M, Skovby F (1997) Parental mosaicism and autosomal dominant mutations causing structural abnormalities of collagen I are frequent in families with osteogenesis imperfecta type III/IV. Acta Paediatr 86:711–718 [DOI] [PubMed] [Google Scholar]
- Lund AM, Schwartz M, Raghunath M, Steinmann B, Skovby F (1996) Gly802Asp substitution in the pro alpha 2(I) collagen chain in a family with recurrent osteogenesis imperfecta due to paternal mosaicism. Eur J Hum Genet 4:39–45 [DOI] [PubMed] [Google Scholar]
- Mackenzie TC, Flake AW (2001) Human mesenchymal stem cells persist, demonstrate site-specific multipotential differentiation, and are present in sites of wound healing and tissue regeneration after transplantation into fetal sheep. Blood Cells Mol Dis 27:601–604 10.1006/bcmd.2001.0424 [DOI] [PubMed] [Google Scholar]
- Marini JC (1999) Osteogenesis imperfecta calls for caution. Nat Med 5:466–467 10.1038/8326 [DOI] [PubMed] [Google Scholar]
- Marini JC, Chernoff EJ (2001) Osteogenesis imperfecta. In: Cassidy SB, Allanson JE (eds) Management of genetic syndromes. Wiley-Liss, New York, pp 281–300 [Google Scholar]
- Marini JC, Gerber NL (1997) Osteogenesis imperfecta: rehabilitation and prospects for gene therapy. JAMA 277:746–750 [DOI] [PubMed] [Google Scholar]
- Niyibizi C, Wallach CJ, Mi Z, Robbins PD (2002) Approaches for skeletal gene therapy. Crit Rev Eukaryot Gene Expr 12:163–173 [DOI] [PubMed] [Google Scholar]
- Oyama M, Tatlock A, Fukuta S, Kavalkovich K, Nishimura K, Johnstone B, Robbins PD, Evans CH, Niyibizi C (1999) Retrovirally transduced bone marrow stromal cells isolated from a mouse model of human osteogenesis imperfecta (OIM) persist in bone and retain the ability to form cartilage and bone after extended passaging. Gene Ther 6:321–329 10.1038/sj.gt.3300839 [DOI] [PubMed] [Google Scholar]
- Pereira RF, O’Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, Simon D, Livezey K, Prockop DJ (1998) Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci USA 95:1142–1147 10.1073/pnas.95.3.1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ (1997) Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276:71–74 10.1126/science.276.5309.71 [DOI] [PubMed] [Google Scholar]
- Robey PG, Termine JD (1985) Human bone cells in vitro. Calcif Tissue Int 37:453–460 [PubMed] [Google Scholar]
- Saiki RK, Scharf S, Faloona F, Mullis KB, Horn GT, Erlich HA, Arnheim N (1985) Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 230:1350–1354 [DOI] [PubMed] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis GA, Starman BJ, Zinn AB, Byers PH (1990) Variable expression of osteogenesis imperfecta in a nuclear family is explained by somatic mosaicism for a lethal point mutation in the alpha (I) gene (COL1A1) of type I collagen in a parent. Am J Hum Genet 46:1034–1040 [PMC free article] [PubMed] [Google Scholar]
- Wang QA, Forlino A, Marini JC (1996) Alternative splicing in COL1A1 mRNA leads to a partial null allele and two in-frame forms with structural defects in non-lethal osteogenesis imperfecta. J Biol Chem 271:28617–28623 10.1074/jbc.271.45.28617 [DOI] [PubMed] [Google Scholar]
- Willing MC, Deschenes SP, Scott DA, Byers PH, Slayton RL, Pitts SH, Arikat H, Roberts EJ (1994) Osteogenesis imperfecta type I: molecular heterogeneity for COL1A1 null alleles of type I collagen. Am J Hum Genet 55:638–647 [PMC free article] [PubMed] [Google Scholar]
- Youssoufian H, Pyeritz RE (2002) Mechanisms and consequences of somatic mosaicism in humans. Nat Rev Genet 3:748–758 10.1038/nrg906 [DOI] [PubMed] [Google Scholar]