Abstract
Neuroblastoma (NB) is a frequent pediatric tumor for which recurrent somatic rearrangements are known. Germline mutations of predisposing gene(s) are suspected on the basis of rare familial cases and the association of NB with other genetically determined congenital malformations of neural crest–derived cells—namely, Hirschsprung disease (HSCR) and/or congenital central hypoventilation syndrome (CCHS). We recently identified the paired–like homeobox 2B (PHOX2B) gene as the major disease-causing gene in isolated and syndromic CCHS, which prompted us to regard it as a candidate gene in NB. Here, we report on germline mutations of PHOX2B in both a familial case of NB and a patient with the HSCR-NB association. PHOX2B, therefore, stands as the first gene for which germline mutations predispose to NB.
Neuroblastoma (NB [MIM 256700]) is a tumor of the sympathetic nervous system that accounts for ∼10% of all cancers in childhood. Although no predisposing gene(s) have been identified thus far, several lines of evidence support the involvement of genetic factors in NB, namely, rare familial cases with vertical transmission and multifocality (Chatten and Voorhess 1967; Clausen et al. 1989; Maris et al. 1997, 2002) and the association of NB with other genetically determined congenital malformations of neural-crest origin, such as Hirschsprung disease (HSCR [MIM 142623]) and/or congenital central hypoventilation syndrome (CCHS, also known as “Ondine's curse” [MIM 209880]) (Rohrer et al. 2002). In particular, patients with CCHS have a high predisposing risk of developing a tumor of the sympathetic nervous system (i.e., 5%–10% occurrence of NB, ganglioneuroblastoma, and ganglioneuroma—versus 1/10,000 in the general population) (Rohrer et al. 2002). We recently identified the paired–like homeobox 2B (PHOX2B [MIM 603851]) gene as the major disease-causing gene of CCHS, with an autosomal dominant mode of inheritance and de novo mutation in the first generation (Amiel et al. 2003). Moreover, we showed that isolated CCHS and CCHS associated with HSCR (Haddad syndrome), whatever the presence or absence of NB, are allelic conditions. We therefore regarded PHOX2B as a candidate gene for both familial and syndromic NB. Here, we report on heterozygous missense mutations located in the homeodomain of PHOX2B in both a familial case of NB (mutation R100L) and an isolated case of NB associated with HSCR (mutation R141G). These data suggest that germline mutation at the PHOX2B locus predispose to hereditary NB.
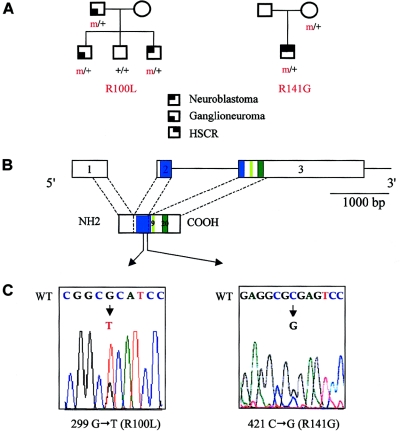
Patient 1, a male, was the first child born to nonconsanguineous parents. A multifocal abdominal ganglioneuroma was surgically removed from the patient at age 10 years. His younger brother presented with an abdominal NB, at age 6 years, which was surgically removed, and experienced local recurrences 18 mo and 30 mo later. No MYCN amplification was detected. His sister is 10 years old and healthy. The father had a ganglioneuroma of the adrenal medulla, which was surgically removed at age 44 years (fig. 1A).
Figure 1.
PHOX2B mutations in familial and syndromic neuroblastoma. A, Pedigree of patients 1 and 2. B, Genomic organization of the PHOX2B protein. The homeobox domain and the 9- and 20-residue polyalanine tracts are indicated by blue and green boxes, respectively. C, DNA sequence electrophoregrams of exon 2 of the PHOX2B gene, showing missense heterozygous mutations in the homeobox. Wild-type (WT) (top) and mutant (bottom) sequences are indicated.
Patient 2, a male, was born to nonconsanguineous parents after an uneventful pregnancy. He received the diagnosis of HSCR in the neonatal period and was treated surgically, with a good result. Multifocal tumors, both thoracic and abdominal, were diagnosed at age 9 mo and were surgically removed. The histological examination confirmed the diagnosis of multifocal NB. No MYCN amplification could be detected. A simple survey, with a 10-year follow-up, showed no recurrence (fig. 1A).
Patient 3, a male, presented with an NB at age 3 years, whereas his maternal half-sister presented with a ganglioneuroblastoma at age 1 year. The mother is asymptomatic, now aged 39 years (pedigree not shown).
Blood samples were obtained, after receiving informed consent from patients, for DNA analyses, and DNA was extracted from peripheral leukocytes following standard protocols. We screened the entire coding sequence of the PHOX2B gene by direct DNA sequencing. Primers used for PCR amplification of PHOX2B are as follows: exon 1 (F 5′-GCCACCTTCTCCATATCC-3′; R 5′-GAAAGGCGGCTTCCTCCG-3′), exon 2 (F 5′-GCTCCACGGCCGGCGAGCTG-3′; R 5′-CTCCCCGGACCAGTGCGGCG-3′), exon 3A (F 5′-GGCCACCCTAACCGGTGC-3′; R 5′-GGCCACCCTAACCGGTGC-3′), and exon 3B (F 5′-CCAGCTGCGGGGCGAATG-3′; R 5′-CTGGCTCGCCCGCTGTC-3′). The PCR reaction mixture (25 μl) contained 100 ng of leukocyte DNA, 20 pmol of each primer, 0.1 μM dNTP, 0.07 μl of 33PdCTP, and 1 U Taq DNA polymerase (Taq [Invitrogen] or Taq Expand [Roche]). DNA sequencing was performed by the fluorometric method on both strands (Big DyeTerminator Cycle Sequencing kit [Applied Biosystems]).
In patient 1, we identified a heterozygous G→T transversion at nucleotide 299 in exon 2 of the PHOX2B gene, presumably changing an arginine into a leucine in the homeodomain of the protein (R100L) (fig. 1C). The mutation was inherited from the father; it segregated to his younger affected son but not to his healthy daughter (fig. 1A). A tumoral DNA sample was available for patient 1, and PCR amplification showed no loss of heterozygosity (LOH) at the PHOX2B locus (data not shown).
In patient 2, we identified a heterozygous C→G transversion at nucleotide 421 in exon 2 of the PHOX2B gene, changing an arginine into a glycine in the homeodomain (R141G) (fig. 1C). The mutation was inherited from the mother. It is unfortunate that no tumoral sample for patient 2 was available for study.
Both nucleotidic changes were absent from a panel of 220 control chromosomes and involved extremely conserved amino acids within the homeodomain family proteins among different species. No nucleotide change was detected in the PHOX2B coding sequence for patient 3.
The human PHOX2B gene maps to chromosome 4p12 and encodes a highly conserved 314–amino acid homeobox transcription factor (fig. 1B) (Yokoyama et al. 1999). The knockout of the mouse ortholog demonstrated that Phox2b is the master gene for both central and peripheral autonomic nervous-system development, since all neurons of the autonomic circuit are absent in Phox2b−/− mice (Pattyn et al. 2000; Brunet and Pattyn 2002). Moreover, Phox2b has been shown to regulate neuronal cell cycle (Dubreuil et al. 2000). Mutation and expression studies in humans showed that PHOX2B is involved in a wide spectrum of autonomic nervous system disorders, ranging from dysgenetic malformations (CCHS and HSCR) to tumor predisposition (NB) (Amiel et al. 2003). However, PHOX2B gene mutations identified thus far in patients with isolated or syndromic CCHS have been either in-frame–polyalanine expansions or frameshift mutations, in contrast with the missense mutations identified in patients showing tumor predisposition without the CCHS phenotype. In the latter case, either loss-of-function or dominant negative effects could be speculated. Indeed, on the one hand, the fact that both missense mutations of the homeodomain and frameshift mutations predispose to NB (with or without CCHS) would favor haploinsufficiency. On the other hand, since the R100 residue of the homeodomain has been shown to contact DNA of target genes (Banerjee-Basu et al. 2003), its substitution could result in loss of DNA binding, whereas its ability to dimerize with the wild-type PHOX2B or other proteins could be retained, resulting in a dominant negative effect.
Several recurrent somatic rearrangements have been described in NB, namely, amplification of the MYCN proto-oncogene, duplication of the 17q13-qter chromosome bands, and deletions of the 1p36, 11q23, and 14q23-qter chromosomal regions (Maris and Matthay 1999). Amplification of MYCN and the 1p36 deletion are both associated with a poorer outcome (Weinstein et al. 2003). Here we show that missense mutations lying in the homeodomain of the protein predispose to NB. No LOH could be found in the tumoral sample of patient 1. Although this result argues against the two-hit model proposed for NB (Knudson and Meadows 1976), no conclusion can be drawn from this single case. In particular, LOH may have been underestimated owing to a sample contamination by nontumoral cells.
The R141G mutation identified in patient 2 is inherited from the healthy mother (fig. 1A and 1C). Several hypotheses could be proposed to explain this observation: (i) the tumor may develop in adulthood, as in family 1 (the father was diagnosed with ganglioneuroma in his 40s), (ii) spontaneous tumor regression is a well-known phenomenon for the sympathetic nervous system (Beckwith and Perrin 1963), and (iii) incomplete penetrance, higher for tumor predisposition than for HSCR, was already suspected in pedigrees with NB (Maris et al. 1997, 2002) like that described in families with retinoblastoma or Wilms tumor. It is interesting that heterozygous germline gain-of-function mutations of the RET proto-oncogene predispose to both multiple-endocrine neoplasia type 2A, with a high penetrance, and HSCR, with a lower one (Mulligan et al. 1994). The underlying mechanism accounting for this dual effect (congenital malformation and tumor predisposition) has been characterized in vitro; missense mutations at codons C609, C618, and C620 are responsible for both an altered translocation of the RET tyrosine kinase receptor to the plasma membrane, leading to haploinsufficiency during enteric–nervous system development, whereas constitutive activation of the RET molecules that reached the cell surface results in cellular transformation (Chappuis-Flament et al. 1998; Hansford and Mulligan 2000).
Recent results suggest that hereditary NB is heterogeneous. Indeed, linkage analysis of seven families with two or more first-degree relatives demonstrated cosegregation of NB with 16p markers (Maris et al. 2002). Another report has recently suggested cosegregation with 4p markers in a large pedigree with NB (Perri et al. 2002). It is interesting that this last study also demonstrated loss of heterozygosity at 4p loci, suggesting a two-hit mechanism for tumorigenesis. It will be of interest to test whether PHOX2B, the gene of which is mapping to 4p12, could be the susceptibility gene involved in the family reported by Perri et al. (2002), although the linked region is telomeric to the PHOX2B locus. Moreover, genes known to be involved in the Mash1-Phox-Ret pathway (required in the development of sympathetic and enteric nervous systems) (Pattyn et al. 1999) are not located in those candidate intervals. Finally, the RET locus, at 10q11.2, has already been excluded by linkage analysis in familial NB (Maris et al. 1997). Further studies should enable us to determine whether dominant negative effect or haploinsufficiency account for the role of PHOX2B in the development of NB.
A high level of expression of most 3–amino acid loop extension (TALE)–family-member genes is found in NB cell lines (Geerts et al. 2003). TALE genes are homeobox genes known to play a role in the development of the nervous system and implicated in tumorigenesis. TALE proteins bind other homeobox proteins via their TALE insertion in the homeodomain, whereas heterodimerization enhances the affinity and specificity of DNA binding. The question of whether PHOX2B is a partner of one (or several) of this gene-family members remains open, a hypothesis that could well establish a link between predisposing PHOX2B mutation and other molecular events observed in NB.
Thus, PHOX2B is the first gene predisposing to NB for which germline mutations have been identified. Further studies are needed to investigate its putative role in sporadic NB.
Acknowledgments
This work was supported by European Union grant 2001-01646 and grants from Maladies Rares (INSERM-AFM), the Association pour la Recherche sur le Cancer, the Ligue Nationale Contre le Cancer–Comité de Paris (laboratoire associé INSERM U-509), and Action Concertée Incitative–development.
Electronic-Database Information
The URL for data presented herein is as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for NB, HSCR, Ondine's curse, and PHOX2B)
References
- Amiel J, Laudier B, Attie-Bitach T, Trang H, de Pontual L, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C, Lyonnet S (2003) Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet 33:459–461 10.1038/ng1130 [DOI] [PubMed] [Google Scholar]
- Banerjee-Basu S, Moreland T, Hsu BJ, Trout KL, Baxevanis AD (2003) The homeodomain resource: 2003 update. Nucleic Acids Res 31:304–306 12520008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith JB, Perrin EV (1963) In situ neuroblastomas: a contribution to the natural history of neural crest tumors. Am J Pathol 43:1089–1104 [PMC free article] [PubMed] [Google Scholar]
- Brunet JF, Pattyn A (2002) Phox2 genes: from patterning to connectivity. Curr Opin Genet Dev 12:435–440 10.1016/S0959-437X(02)00322-2 [DOI] [PubMed] [Google Scholar]
- Chappuis-Flament S, Pasini A, De Vita G, Segouffin-Cariou C, Fusco A, Attie T, Lenoir GM, Santoro M, Billaud M (1998) Dual effect on the RET receptor of MEN 2 mutations affecting specific extracytoplasmic cysteines. Oncogene 17:2851–2861 9879991 [DOI] [PubMed] [Google Scholar]
- Chatten J, Voorhess ML (1967) Familial neuroblastoma: report of a kindred with multiple disorders, including neuroblastomas in four siblings. N Engl J Med 277:1230–1236 [DOI] [PubMed] [Google Scholar]
- Clausen N, Andersson P, Tommerup N (1989) Familial occurrence of neuroblastoma, von Recklinghausen’s neurofibromatosis, Hirschsprung’s agangliosis and jaw-winking syndrome. Acta Paediatr Scand 78:736–741 [DOI] [PubMed] [Google Scholar]
- Dubreuil V, Hirsch MR, Pattyn A, Brunet JF, Goridis C (2000) The Phox2b transcription factor coordinately regulates neuronal cell cycle exit and identity. Development 127:5191–5201 [DOI] [PubMed] [Google Scholar]
- Geerts D, Schilderink N, Jorritsma G, Versteeg R (2003) The role of the MEIS homeobox genes in neuroblastoma. Cancer Lett 197:87–92 10.1016/S0304-3835(03)00087-9 [DOI] [PubMed] [Google Scholar]
- Hansford JR, Mulligan LM (2000) Multiple endocrine neoplasia type 2 and RET: from neoplasia to neurogenesis. J Med Genet 37:817–827 10.1136/jmg.37.11.817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson AG Jr, Meadows AT (1976) Developmental genetics of neuroblastoma. J Natl Cancer Inst 57:675–682 [DOI] [PubMed] [Google Scholar]
- Maris JM, Kyemba SM, Rebbeck TR, White PS, Sulman EP, Jensen SJ, Allen C, Biegel JA, Brodeur GM (1997) Molecular genetic analysis of familial neuroblastoma. Eur J Cancer 33:1923–1928 10.1016/S0959-8049(97)00265-7 [DOI] [PubMed] [Google Scholar]
- Maris JM, Matthay KK (1999) Molecular biology of neuroblastoma. J Clin Oncol 17:2264–2279 [DOI] [PubMed] [Google Scholar]
- Maris JM, Weiss MJ, Mosse Y, Hii G, Guo C, White PS, Hogarty MD, Mirensky T, Brodeur GM, Rebbeck TR, Urbanek M, Shusterman S (2002) Evidence for a hereditary neuroblastoma predisposition locus at chromosome 16p12-13. Cancer Res 62:6651–6658 [PubMed] [Google Scholar]
- Mulligan LM, Eng C, Attie T, Lyonnet S, Marsh DJ, Hyland VJ, Robinson BG, Frilling A, Verellen-Dumoulin C, Safar A, Venter DJ, Munnich A, Ponder BAJ (1994) Diverse phenotypes associated with exon 10 mutations of the RET proto-oncogene. Hum Mol Genet 3:2163–2167 [DOI] [PubMed] [Google Scholar]
- Pattyn A, Goridis C, Brunet JF (2000) Specification of the central noradrenergic phenotype by the homeobox gene Phox2b. Mol Cell Neurosci 15:235–243 10.1006/mcne.1999.0826 [DOI] [PubMed] [Google Scholar]
- Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF (1999) The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature 399:366–370 10.1038/20700 [DOI] [PubMed] [Google Scholar]
- Perri P, Longo L, Cusano R, McConville CM, Rees SA, Devoto M, Conte M, Ferrara GB, Seri M, Romeo G, Tonini GP (2002) Weak linkage at 4p16 to predisposition for human neuroblastoma. Oncogene 21:8356–8360 10.1038/sj.onc.1206009 [DOI] [PubMed] [Google Scholar]
- Rohrer T, Trachsel D, Engelcke G, Hammer J (2002) Congenital central hypoventilation syndrome associated with Hirschsprung’s disease and neuroblastoma: case of multiple neurocristopathies. Pediatr Pulmonol 33:71–76 10.1002/ppul.10031 [DOI] [PubMed] [Google Scholar]
- Yokoyama M, Watanabe H, Nakamura M (1999) Genomic structure and functional characterization of NBPhox (PMX2B), a homeodomain protein specific to catecholaminergic cells that is involved in second messenger-mediated transcriptional activation. Genomics 59:40–50 10.1006/geno.1999.5845 [DOI] [PubMed] [Google Scholar]
- Weinstein JL, Katzenstein HM, Cohn SL (2003) Advances in the diagnosis and treatment of neuroblastoma. Oncologist 8:278–292 [DOI] [PubMed] [Google Scholar]