To the Editor:
Although several families with X-linked mental retardation (XLMR) (Martin and Bell 1943; Allan et al. 1944; Bickers and Adams 1949; Losowsky 1961) had been reported prior to the Renpenning study (Renpenning et al. 1962), the term “Renpenning syndrome” (MIM 309500) came into general use for XLMR, encompassing both syndromic and nonsyndromic forms (Richards 1970; Gerrard and Renpenning 1974; Steele and Chorazy 1974; Howard-Peebles et al. 1979; Jennings et al. 1980; McLaughlin and Kriegsmann 1980; Proops and Webb 1981; Archidiacono et al. 1987). Renpenning et al. (1962) described a Mennonite family in which 20 males in three generations had mental retardation (MR). Manifestations in affected males included microcephaly, short stature, and small testes; carrier females appeared normal.
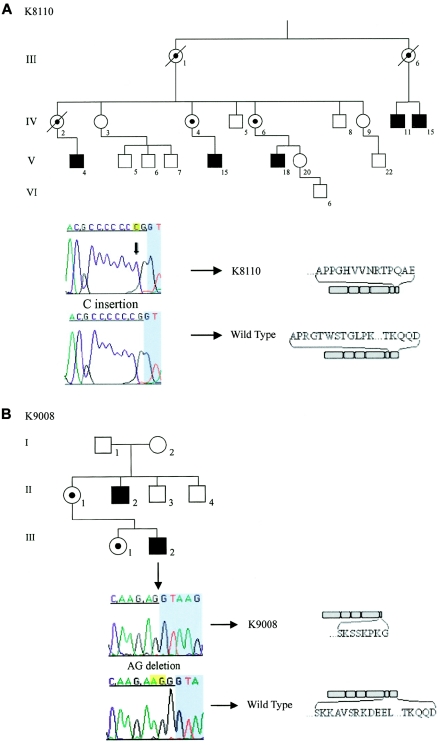
Previously, we reported linkage of the family described by Renpenning and colleagues (1962) to Xp11.2–p11.4 (Stevenson et al. 1998) (fig. 1A). In addition, we identified another family exhibiting microcephaly and MR (fig. 1B). Herein, we report truncating mutations in the polyglutamine tract binding protein 1 (PQBP1) gene (MIM 300463) in both families.
Figure 1.
Families with novel mutations in the PQBP1 gene. A, Partial pedigree of the family described by Renpenning and colleagues (1962) (family K8110), with the c.641insC mutation. Numbering of the pedigree is consistent with that published elsewhere (Stevenson et al. 1998). The c.641insC mutation, highlighted and indicated by the arrow, is present in exon 5 and is very close to the exon/intron boundary. The shaded region shows the intronic sequence. This mutation causes a frameshift in the C2 domain, resulting in the premature truncation of the protein. B, Pedigree of family K9008, with the c.575_576delAG mutation in the NLS (nuclear localization signal) domain. This deletion is in exon 4 (highlighted in yellow) and occurs very close to the exon/intron boundary (sequence contributed from the intron is shaded). This mutation causes a frameshift and results in a premature truncation of the PQBP1 protein, which lacks the C2 domain.
Mutations in the gene that codes for the polyglutamine tract binding protein 1, located in Xp11.2, have been found in patients with Sutherland-Haan syndrome (MIM 309470), cerebropalatocardiac (Hamel) syndrome, and MRX55, as well as in two other families (Kalscheuer et al. 2003). PQBP1 consists of six exons that code for a protein of 265 amino acids. A WW domain is encoded by the amino acid positions 47–78, which has been shown to play an important role in regulation of transcription activity by interacting with the carboxy-terminal domain of the RNA polymerase II (Sudol et al. 2001). Additionally, a polyglutamine-binding region (in the polar-amino-acid–rich domain [PRD]), a DR/ER stretch, is encoded by exon 4 and is involved in transcriptional control by binding to the polyQ region of the transcription factor BRN2 that silences transcription (Sudol et al. 2001). The unifying findings in these patients with XLMR are microcephaly and short stature. A variety of other manifestations, including ocular colobomas, cleft palate, cardiac defects, small testes, anal anomalies, and spasticity, have occurred in a minority of patients. So far, all mutations have been found in exon 4 (GenBank accession number NM_005710), where they are predicted to disrupt the polyglutamine-binding PRD and to shorten the protein (Kalscheuer et al. 2003).
The mutation in the family described by Renpenning and colleagues (1962) is an insertion of one cytosine residue in exon 5 (c.641insC). This frameshift insertion causes a premature stop codon at amino acid position 226 (fig. 1A). The observed mutation was found in all available males with MR in the pedigree, including one male who was found to have milder cognitive impairment (IQ=70) (individual V-18) (fig. 1A). X-chromosome inactivation in carrier females was not skewed. In the other family presenting with MR and microcephaly (K9008), we detected a novel AG dinucleotide deletion at the end of exon 4 (c.575_576delAG), causing a frameshift and introducing a new stop codon at amino acid position 198 (fig. 1B). Neither of these truncating alterations was found in 200 X chromosomes from unaffected males.
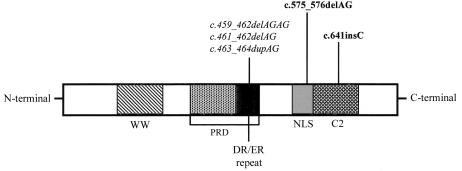
In contrast to the previously found mutations in the PQBP1 gene that are all located in the middle of exon 4, these mutations, at either the end of exon 4 or exon 5, do not affect the PRD (fig. 2). Taking into account all mutations in the PQBP1 gene that have been described here and by Kalscheuer et al. (2003), two groups of mutations can be classified: (1) deletions or insertions of AG nucleotides affecting the DR/ER repeat in the PRD and (2) frameshift aberrations leaving the PRD undisturbed (fig. 2). However, the common clinical manifestations, MR, microcephaly, and short stature, are present in all families. Therefore, it might be of interest to determine whether the PRD in the truncated proteins from the family described by Renpenning and colleagues (1962) and the K9008 family still interacts with BRN2 or whether the loss of the last 67 or 40 amino acids disturbs this function. Alternatively, the interaction with other, yet-unknown proteins might be disrupted.
Figure 2.
Truncating mutations in the PQBP1 protein. This figure shows the structure of the PQBP1 protein with the WW domain, the PRD with DR/ER repeats, the NLS domain, and the C2 domain (modified from Waragai et al. 1999). The novel mutations reported in this study (c.575_576delAG and c.641insC) are in boldface. The three different mutations in the DR/ER domain reported by Kalscheuer et al. (2003) are in italics.
Advances in clinical delineation and in molecular understandings of XLMR have now identified 120 syndromic forms of XLMR and 81 families with nonsyndromic XLMR (Stevenson et al. 2003). To date, mutations in 47 genes have been linked to XLMR. Of these 47 genes, 29 have been linked exclusively to syndromic XLMR, 11 exclusively to nonsyndromic XLMR, and 7 to both (Stevenson and Schwartz 2002).
With the exception of Allan-Herndon syndrome [MIM 309600], all XLMR syndromes reported prior to the discovery of the fragile X syndrome [MIM 309550] have now been linked to mutations of a specific gene. Identification of a PQBP1 mutation in Renpenning syndrome and other XLMR syndromes (Kalscheuer et al. 2003) exemplifies the lumping of XLMR syndromes that has become justified on the basis of molecular studies (Stevenson 2000). As MR, microcephaly, and short stature seem to be consistent findings among individuals with PQBP1 mutations, patients with these findings should be included in any testing scheme. In a South Carolina study of mental retardation, among 4,008 males with MR of unknown cause, 486 (12%) have microcephaly, 350 (9%) have short stature, and 128 (3%) have both (R.E.S., unpublished data). Hence, microcephaly is the most common physical finding among males with MR of unknown cause (Stevenson et al. 2003) and is a reliably ascertained finding that may be useful in the selection of cases for PQBP1 mutation testing.
Acknowledgments
The authors express their gratitude to the families involved in this research and to Julianne Collins, Ph.D., Sharon Cardwell, Celeste Krauss, M.D., Martha MacMillin, and Lynda Holloway for clinical information and assistance. This work was supported by the German Ministry for Research and Education (BMBF, 01KW9974), the European Community (QLG2-CT-1999-00791) (A.M.), grants from the National Institutes of Health (HD26208 [C.E.S.] and MH57840 [R.E.S.]), a grant from the Belgian National Fund for Scientific Research—Flanders (FWO), and the Interuniversity Attraction Poles Program (IUAP-V) (R.F.K.). This study is dedicated to the memory of Ethan Francis Schwartz (1996–1998).
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for PQBP1 [accession number NM_005710])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for Renpenning syndrome, PQBP1, Sutherland-Haan syndrome, Allan-Herndon syndrome, and fragile X syndrome)
References
- Allan W, Herndon CN, Dudley FC (1944) Some examples of the inheritance of mental deficiency: apparently sex-linked idiocy and microcephaly. Am J Ment Defic 48:325–334 [Google Scholar]
- Archidiacono N, Rocchi M, Rinaldi A, Filippi G (1987) X-linked mental retardation. II. Renpenning syndrome and other types (report of 14 families). J Genet Hum 35:381–398 [PubMed] [Google Scholar]
- Bickers DA, Adams RD (1949) Hereditary stenosis of the aqueduct of Sylvius as a cause of congenital hydrocephalus. Brain 72:246–262 [DOI] [PubMed] [Google Scholar]
- Gerrard JW, Renpenning HJ (1974) Sex-linked mental retardation. Lancet 1:1346 10.1016/S0140-6736(74)90714-4 [DOI] [PubMed] [Google Scholar]
- Howard-Peebles PN, Stoddard GR, Mims MG (1979) Familial X-linked mental retardation, verbal disability, and marker X chromosomes. Am J Hum Genet 31:214–222 [PMC free article] [PubMed] [Google Scholar]
- Jennings M, Hall JG, Hoehn H (1980) Significance of phenotypic and chromosomal abnormalities in X-linked mental retardation (Martin-Bell or Renpenning syndrome). Am J Med Genet 7:417–432 [DOI] [PubMed] [Google Scholar]
- Kalscheuer VM, Freude K, Musante L, Jensen LJ, Yntema HG, Gécz J, Sefiani A, et al (2003) Mutations in the polyglutamine-binding protein 1 gene cause X-linked mental retardation. Nat Genet 35:313–315 10.1038/ng1264 [DOI] [PubMed] [Google Scholar]
- Losowsky MS (1961) Hereditary mental defect showing the pattern of sex influence. J Ment Defic Res 5:60–62 [DOI] [PubMed] [Google Scholar]
- Martin JP, Bell J (1943) A pedigree of mental defect showing sex-linkage. J Neurol Psychiatry 6:154–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JF, Kriegsmann E (1980) Developmental dyspraxias in a family with X-linked mental retardation (Renpenning syndrome). Dev Med Child Neurol 22:84–92 [DOI] [PubMed] [Google Scholar]
- Proops R, Webb T (1981) The fragile X chromosome in the Martin-Bell-Renpenning syndrome and in males with other forms of familial mental retardation. J Med Genet 18:366–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renpenning H, Gerrard JW, Zaleski WA, Tabata T (1962) Familial sex-linked mental retardation. Can Med Assoc J 87:954–957 [PMC free article] [PubMed] [Google Scholar]
- Richards BW (1970) “Renpenning” syndrome. Lancet 2:520 [DOI] [PubMed] [Google Scholar]
- Steele MW, Chorazy AL (1974) Renpenning’s syndrome. Lancet 1:752–753 10.1016/S0140-6736(74)92978-X [DOI] [PubMed] [Google Scholar]
- Stevenson RE (2000) Splitting and lumping in the nosology of XLMR. Am J Med Genet 97:174–182 [DOI] [PubMed] [Google Scholar]
- Stevenson RE, Arena JF, Ouzts E, Gibson A, Shokeir MHK, Vnencak-Jones C, Lubs HA, May M, Schwartz CE (1998) Renpenning syndrome maps to Xp11. Am J Hum Genet 62:1092–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RE, Procopio-Allen AM, Schroer RJ, Collins JS (2003) Genetic syndromes among individuals with mental retardation. Am J Med Genet 123A:29–32 [DOI] [PubMed] [Google Scholar]
- Stevenson RE, Schwartz CE (2002) Clinical and molecular contributions to the understanding of X-linked mental retardation. Cytogenet Genome Res 99:265–275 10.1159/000071603 [DOI] [PubMed] [Google Scholar]
- Sudol M, Sliwa K, Russo T (2001) Functions of WW domains in the nucleus. FEBS Lett 490:190–195 10.1016/S0014-5793(01)02122-6 [DOI] [PubMed] [Google Scholar]
- Waragai M, Lammers CH, Takeuchi S, Imafuku I, Udagawa Y, Kanazawa I, Kawabata M, Mouradian MM, Okazawa H (1999) PQBP-1, a novel polyglutamine tract-binding protein, inhibits transcription activation by Brn-2 and affects cell survival. Hum Mol Genet 8:977–987 10.1093/hmg/8.6.977 [DOI] [PubMed] [Google Scholar]