Abstract
Defects of lipid-linked oligosaccharide assembly lead to alterations of N-linked glycosylation known as “type I congenital disorders of glycosylation” (CDG). Dysfunctions along this stepwise assembly pathway are characterized by intracellular accumulation of intermediate lipid-linked oligosaccharides, the detection of which contributes to the identification of underlying enzymatic defects. Using this approach, we have found, in a patient with CDG, a deficiency of the ALG9 α1,2 mannosyltransferase enzyme, which causes an accumulation of lipid-linked-GlcNAc2Man6 and -GlcNAc2Man8 structures, which was paralleled by the transfer of incomplete oligosaccharides precursors to protein. A homozygous point-mutation 1567G→A (amino acid substitution E523K) was detected in the ALG9 gene. The functional homology between the human ALG9 and Saccharomyces cerevisiae ALG9, as well as the deleterious effect of the E523K mutation detected in the patient with CDG, were confirmed by a yeast complementation assay lacking the ALG9 gene. The ALG9 defect found in the patient with CDG—who presented with developmental delay, hypotonia, seizures, and hepatomegaly—shows that efficient lipid-linked oligosaccharide synthesis is required for proper human development and physiology. The ALG9 defect presented here defines a novel form of CDG named “CDG-IL.”
Glycosylation is a widespread posttranslational modification that has a considerable impact on numerous biological processes (Varki 1993). The N-linked glycosylation pathway bears the hallmark of first assembling the GlcNAc2Man9Glc3 oligosaccharide core on the lipid carrier dolichylpyrophosphate (DolPP) and then of transferring it en bloc to nascent polypeptides. In recent years, several defects along the N-linked glycosylation pathway have been characterized (Jaeken 2003; Thiel et al. 2003; Wu et al. 2003; Grubenmann et al. 2004; Kranz et al. 2004; Schwarz et al. 2004) and have been defined as types of congenital disorders of glycosylation (CDG). The majority of CDG known to date represent defects of dolichol-linked oligosaccharide assembly, classified as “CDG-I.”
Clinically, CDG are associated with a broad range of symptoms, such as the failure to thrive, axial hypotonia, cerebellar dysfunction, seizures, mental retardation, and coagulopathy (Krasnewich and Gahl 1997). These rather nonspecific features together with a variability in severity of these manifestations render it difficult to make a clear diagnosis and necessitate a genetic characterization to establish the causality of glycosylation disorders.
The study of yeast glycosylation mutants has provided a wealth of knowledge about the mechanisms of endoplasmic reticulum (ER)–associated N-glycosylation (Burda and Aebi 1999), where >20 enzymatic activities and an even greater number of genes are involved (Freeze 2001). In addition, these yeast glycosylation mutants have been instrumental in the characterization of several disorders of glycosylation (Aebi and Hennet 2001). Besides establishing the genetic basis of novel human diseases, the identification of additional defects along the assembly of dolichol-linked oligosaccharides allows an assessment of the biological importance, for humans, of each glycosylation step.
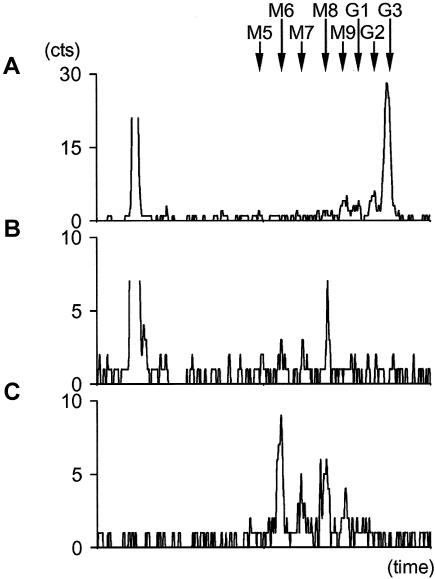
In the present study, we report a novel type of CDG-I identified in a female patient born at term as a twin with a birth weight of 2.75 kg, length of 44 cm, and head circumference of 31.5 cm. As she grew older, her clinical features included severe microcephaly, central hypotonia, seizures, hepatomegaly, developmental delay, and bronchial asthma. This pattern of clinical presentations was compatible with CDG, a diagnosis confirmed by isoelectric focusing analysis of serum transferrin. The patient sample showed significant increase of disialo- and asialotransferrin, indicative of CDG-I (data not shown). Normal values of phosphomannomutase activity measured in patient fibroblast extracts excluded the most frequent CDG type, CDG-Ia (MIM 212065). To detect a possible defect of lipid-linked oligosaccharide (LLO) assembly, fibroblasts from the patient were labeled with [3H]mannose, and the glycans released from the lipid carrier or cleaved from proteins were analyzed by high-performance liquid chromatography, as described elsewhere (Grubenmann et al. 2002). The LLO profile revealed an accumulation of incomplete precursor structures corresponding to DolPP-GlcNAc2Man6 and DolPP-GlcNAc2Man8 (fig. 1B). In comparison, only the complete DolPP-GlcNAc2Man9Glc3 LLO is found in healthy control fibroblasts (fig. 1A).
Figure 1.
[3H]Mannose-labeled lipid- and N-linked oligosaccharide profiles from human primary fibroblasts. A, Separation of LLOs from normal control fibroblasts. B, Separation of LLOs from patient fibroblasts. C, Separation of NLOs from patient fibroblasts. Arrows mark the elution times of standard oligosaccharides from yeast (M5–M9, GlcNAc2Man5–9; G1–G3, GlcNAc2Man9Glc1–3).
The analysis of protein N-linked oligosaccharides (NLO) confirmed the glycosylation alteration detected in the patient’s fibroblasts. The normal NLO profile of human fibroblasts corresponds to three peaks representing the structures GlcNAc2Man8, GlcNAc2Man9, and GlcNAc2Man9Glc1 (data not shown). GlcNAc2Man9 and GlcNAc2Man8 arise from the immediate trimming of NLO by ER resident glucosidase and α-mannosidase enzymes (Kilker et al. 1981; Camirand et al. 1991). In the patient with CDG, an additional peak possibly corresponding to the incomplete GlcNAc2Man6 structure was visible (fig. 1C), suggesting that truncated oligosaccharides were transferred to proteins in this patient.
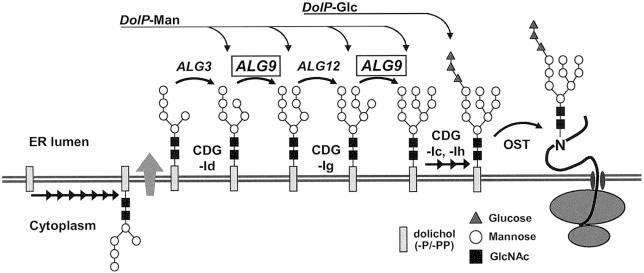
The dual accumulation of DolPP-GlcNAc2Man6 and DolPP-GlcNAc2Man8 suggested a possible defect at the level of the ALG9 α1,2-mannosyltransferase, which, in yeast, catalyzes the addition of the seventh (Burda et al. 1996) and ninth (C. G. Frank and M. Aebi, unpublished data) mannose residues on growing LLOs (fig. 2). A human homolog to the yeast ALG9 gene (MIM 606941) has been described recently (Entrez accession number AF395532) (Baysal et al. 2002). The cDNA encodes a predicted multispanning membrane protein of 611 amino acids, with 43% similarity to the Saccharomyces cerevisiae Alg9 protein. A sequence alignment of several Alg9 proteins showed highly conserved domains, possibly representing regions responsible for the active site of this enzyme (fig. 3). The human ALG9 cDNA was prepared from 4 μg of total fibroblast RNA by use of the specific reverse-transcription primer 5′-TGTTACAGGCGATGACTTGC-3′. The protein-coding region of the ALG9 cDNA was amplified by PCR with 3 μl of reverse transcription product and the primers 5′-CGCGCTCACCGACTTCATA-3′ and 5′-GGAGTCAGGTCACTGGAATCA-3′ through 30 cycles of amplification at 94°C for 45 s, at 53°C for 30 s, and at 72°C for 90 s. The PCR fragments were sequenced directly. In the patient ALG9 cDNA, we detected the point mutation 1567G→A, which introduced the amino acid change E523K in the ALG9 protein (fig. 3). This mutation was not found in the control cDNA samples that were sequenced nor in 108 expressed sequence tags retrieved from GenBank, excluding the possibility of a frequently occurring polymorphism. As a confirmation, the 1567G→A mutation was also detected at the genomic level by sequencing exon 14 of the patient ALG9 gene (data not shown).
Figure 2.
LLO biosynthesis pathway in the ER lumen. Arrows mark the reactions in which dolichol-phosphate (DolP)–Man or DolP-Glc are used as substrates and where the DolP-Man–dependent transferases ALG3, ALG9, and ALG12 are involved. The complete oligosaccharide GlcNAc2Man9Glc3 is transferred from its lipid carrier to nascent polypeptides by the oligosaccharyltransferase complex (OST). Known types of CDG are marked with the respective names CDG-Ic, -Id, -Ig, and -Ih.
Figure 3.
ALG9 protein sequence comparison. Amino acid sequences of ALG9 proteins from Homo sapiens (Hs) (Entrez accession number AAL25798), Caenorhabditis elegans (Ce) (Entrez accession number CAA90107), Drosophila melanogaster (Dm) (Entrez accession number NP_651353), Schizosaccharomyces pombe (Sp) (Entrez accession number CAB75773), and S. cerevisiae (Sc) (Entrez accession number CAA96122) were aligned using the ClustalW program (Thompson et al. 1994). The amino acid change E523K is indicated for the human ALG9 protein.
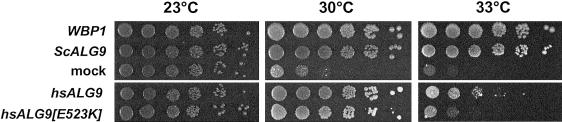
To verify that the E523K mutation impaired ALG9 function, we analyzed the mutant human cDNA in a yeast complementation assay. Deletion of ALG9 alone in yeast does not result in any obvious growth phenotype. We therefore used the synthetic phenotype of a Δalg9 wbp1-2 double mutant. The combination of the deficiency in LLO biosynthesis (Δalg9) and a reduced oligosaccharyltransferase activity (wbp1-2) results in a temperature-sensitive phenotype at 30°C (Burda et al. 1996). The Δalg9 wbp1-2 strain was transformed with control plasmids and multicopy plasmids expressing the normal human ALG9 or the ALG9[E523K] cDNAs, under control of the strong TDH3 promoter. The human ALG9 cDNA included the common polymorphism 865G→A described elsewhere (Baysal et al. 2002). In contrast to the human wild-type ALG9 cDNA, transformation of the mutant ALG9[E523K] allele showed only a minor effect on growth of Δalg9 wbp1-2 yeasts at 33°C (fig. 4). Both versions restored growth at 30°C, indicating a residual activity of ALG9[E523K]. This demonstrates that human ALG9 is functionally homologous to yeast ALG9 and that the E523K mutation has a detrimental effect on ALG9 function. When the biochemical phenotype of the CDG fibroblasts is considered, this study demonstrates that the human ALG9 protein catalyzes, like its yeast counterpart, the transfer of mannose onto the two acceptor substrates DolPP-GlcNAc2Man6 and DolPP-GlcNAc2Man8.
Figure 4.
Complementation of Δalg9 wbp1-2 yeasts with human ALG9 cDNAs. Transformants were spotted in sixfold dilutions on YPD plates and were incubated at 23°C, at 30°C, or at 33°C for 120 h. Transformants carried either the vector alone (“mock”), a plasmid complementing the wbp1-2 mutation (“WBP1”), the pALG9 plasmid expressing the S. cerevisiae ALG9 gene (“ScALG9”), or plasmids expressing the normal human ALG9 cDNA or the CDG patient ALG9[E523K] cDNA.
Clinically, the ALG9 deficiency was accompanied by symptoms that are common for most types of CDG-I; that is, developmental delay, hypotonia, and epilepsy. The fact that a significant residual ALG9 activity was detected, as shown by the presence of normal NLOs in the cells of the patient with CDG and by the yeast complementation assay, underlines the importance of the reactions catalyzed by ALG9, since even a leaky mutation accounts for a severe disease phenotype. As for all types of CDG-I, the disease is caused by protein underglycosylation; that is, the nonoccupancy of critical N-glycosylation sites, which probably accounts for most of the clinical manifestations observed. However, transfer of truncated oligosaccharides to protein may also affect intracellular processes of glycoproteins, such as the chaperone-assisted protein folding, degradation of malfolded proteins in the ER (ERAD), the ER-Golgi trafficking, and the transport to lysosomes (Plemper and Wolf 1999; Parodi 2000; Helenius and Aebi 2001). The monoglucosylation of N-glycans, reciprocally controlled by glucosidase II and the UDP-Glc:glycoprotein glucosyltransferase (GT), regulates the association of glycoproteins with chaperone lectins in the so-called calnexin/calreticulin cycle (Parodi 2000; Helenius and Aebi 2001). In vitro studies showed that truncated N-glycan structures with <9 mannoses are suboptimal substrates for GT (Sousa et al. 1992) or for the lectin chaperones calreticulin and calnexin (Ware et al. 1995; Spiro et al. 1996). Sugar structures also control glycoprotein degradation. For example, in ALG9-null yeast mutants, ERAD of a malfolded glycoprotein was impaired (Jakob et al. 1998). Therefore, part of the phenotype for patients with CDG could result from transfer of shorter-than-normal glycans to protein, which might interfere with cellular protein quality-control mechanisms.
The neurological symptoms found in most types of CDG demonstrate the importance of N-glycosylation for development and function of the nervous system (Krasnewich and Gahl 1997; Jaeken and Matthijs 2001). Recently, Baysal et al. (2002) found carriers of a chromosome translocation interrupting the ALG9 gene in a family showing a high frequency of bipolar affective disorder (BPAD [MIM 125480]). It could be speculated that ALG9 activity, required at two steps along the pathway, becomes rate limiting in cells if only one productive copy of the gene is present. Similarly, mild neurological alterations and behavioral disorders have been described among mild cases of CDG-Ia (Grünewald et al. 2001; Briones et al. 2002). Although involvement of the ALG9 mannosyltransferase activity in BPAD requires further study, it may be relevant to consider testing for alterations of N-glycosylation in patients with BPAD or related psychiatric diseases.
Acknowledgment
This work was supported by TANDEM grant 3238-069471.02 from the Swiss National Science Foundation.
Electronic-Database Information
The accession numbers and URLs for data presented herein are as follows:
- Entrez, http://www.ncbi.nlm.nih.gov/Entrez/ (for ALG9/DIBD1 cDNA for H. sapiens [accession number AF395532], C. elegans [accession number CAA90107], D. melanogaster [accession number NP_651353], S. pombe [accession number CAB75773], and S. cerevisiae [accession number CAA96122])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for CDG-Ia, ALG9/DIBD1, and BPAD)
References
- Aebi M, Hennet T (2001) Congenital disorders of glycosylation: genetic model systems lead the way. Trends Cell Biol 11:136–141 10.1016/S0962-8924(01)01925-0 [DOI] [PubMed] [Google Scholar]
- Baysal BE, Willett-Brozick JE, Badner JA, Corona W, Ferrell RE, Nimgaonkar VL, Detera-Wadleigh SD (2002) A mannosyltransferase gene at 11q23 is disrupted by a translocation breakpoint that co-segregates with bipolar affective disorder in a small family. Neurogenetics 4:43–53 10.1007/s10048-001-0129-x [DOI] [PubMed] [Google Scholar]
- Briones P, Vilaseca MA, Schollen E, Ferrer I, Maties M, Busquets C, Artuch R, Gort L, Marco M, van Schaftingen E, Matthijs G, Jaeken J, Chabas A (2002) Biochemical and molecular studies in 26 Spanish patients with congenital disorder of glycosylation type Ia. J Inherit Metab Dis 25:635–646 10.1023/A:1022825113506 [DOI] [PubMed] [Google Scholar]
- Burda P, Aebi M (1999) The dolichol pathway of N-linked glycosylation. Biochim Biophys Acta 1426:239–257 [DOI] [PubMed] [Google Scholar]
- Burda P, te Heesen S, Brachat A, Wach A, Dusterhoft A, Aebi M (1996) Stepwise assembly of the lipid-linked oligosaccharide in the endoplasmic reticulum of Saccharomyces cerevisiae: identification of the ALG9 gene encoding a putative mannosyl transferase. Proc Natl Acad Sci USA 93:7160–7165 10.1073/pnas.93.14.7160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camirand A, Heysen A, Grondin B, Herscovics A (1991) Glycoprotein biosynthesis in Saccharomyces cerevisiae: isolation and characterization of the gene encoding a specific processing α-mannosidase. J Biol Chem 266:15120–15127 [PubMed] [Google Scholar]
- Freeze HH (2001) Update and perspectives on congenital disorders of glycosylation. Glycobiology 11:129R-143R 10.1093/glycob/11.12.129R [DOI] [PubMed] [Google Scholar]
- Grubenmann CE, Frank CG, Hülsmeier AJ, Schollen E, Matthijs G, Mayatepek E, Berger EG, Aebi M, Hennet T (2004) Deficiency of the first mannosylation step in the N-glycosylation pathway causes congenital disorder of glycosylation type Ik. Hum Mol Genet 13:535–542 10.1093/hmg/ddh050 [DOI] [PubMed] [Google Scholar]
- Grubenmann CE, Frank CG, Kjaergaard S, Berger EG, Aebi M, Hennet T (2002) ALG12 mannosyltransferase defect in congenital disorder of glycosylation type-Ig. Hum Mol Genet 11:2331–2339 10.1093/hmg/11.19.2331 [DOI] [PubMed] [Google Scholar]
- Grünewald S, Schollen E, Van Schaftingen E, Jaeken J, Matthijs G (2001) High residual activity of PMM2 in patients’ fibroblasts: possible pitfall in the diagnosis of CDG-Ia (phosphomannomutase deficiency). Am J Hum Genet 68:347–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A, Aebi M (2001) Intracellular functions of N-linked glycans. Science 291:2364–2369 10.1126/science.291.5512.2364 [DOI] [PubMed] [Google Scholar]
- Jaeken J (2003) Komrower lecture: congenital disorders of glycosylation (CDG): it’s all in it! J Inherit Metab Dis 26:99–118 10.1023/A:1024431131208 [DOI] [PubMed] [Google Scholar]
- Jaeken J, Matthijs G (2001) Congenital disorders of glycosylation. Annu Rev Genomics Hum Genet 2:129–151 10.1146/annurev.genom.2.1.129 [DOI] [PubMed] [Google Scholar]
- Jakob CA, Burda P, Roth J, Aebi M (1998) Degradation of misfolded endoplasmic reticulum glycoproteins in Saccharomyces cerevisiae is determined by a specific oligosaccharide structure. J Cell Biol 142:1223–1233 10.1083/jcb.142.5.1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilker RD Jr, Saunier B, Tkacz JS, Herscovics A (1981) Partial purification from Saccharomyces cerevisiae of a soluble glucosidase which removes the terminal glucose from the oligosaccharide Glc3Man9GlcNAc2. J Biol Chem 256:5299–5603 [PubMed] [Google Scholar]
- Kranz C, Denecke J, Lehle L, Sohlbach K, Jeske S, Meinhardt F, Rossi R, Gudowius S, Marquardt T (2004) Congenital disorder of glycosylation type Ik (CDG-Ik): a defect of mannosyltransferase I. Am J Hum Genet 74:545–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnewich D, Gahl WA (1997) Carbohydrate-deficient glycoprotein syndrome. Adv Pediatr 44:109–140 [PubMed] [Google Scholar]
- Parodi AJ (2000) Protein glucosylation and its role in protein folding. Annu Rev Biochem 69:69–93 10.1146/annurev.biochem.69.1.69 [DOI] [PubMed] [Google Scholar]
- Plemper RK, Wolf DH (1999) Retrograde protein translocation: ERADication of secretory proteins in health and disease. Trends Biochem Sci 24:266–270 10.1016/S0968-0004(99)01420-6 [DOI] [PubMed] [Google Scholar]
- Schwarz M, Thiel C, Lübbehusen J, Dorland B, de Koning T, von Figura K, Lehle L, Körner C (2004) Deficiency of GDP-Man:GlcNAc2-PP-dolichol mannosyltransferase causes congenital disorder of glycosylation type Ik. Am J Hum Genet 74:472–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa MC, Ferrero-Garcia MA, Parodi AJ (1992) Recognition of the oligosaccharide and protein moieties of glycoproteins by the UDP-Glc:glycoprotein glucosyltransferase. Biochemistry 31:97–105 [DOI] [PubMed] [Google Scholar]
- Spiro RG, Zhu Q, Bhoyroo V, Soling HD (1996) Definition of the lectin-like properties of the molecular chaperone, calreticulin, and demonstration of its copurification with endomannosidase from rat liver Golgi. J Biol Chem 271:11588–11594 10.1074/jbc.271.19.11588 [DOI] [PubMed] [Google Scholar]
- Thiel C, Schwarz M, Peng J, Grzmil M, Hasilik M, Braulke T, Kohlschutter A, von Figura K, Lehle L, Korner C (2003) A new type of congenital disorders of glycosylation (CDG-Ii) provides new insights into the early steps of dolichol-linked oligosaccharide biosynthesis. J Biol Chem 278:22498–22505 10.1074/jbc.M302850200 [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ (1994) ClustalW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucl Acids Res 22:4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A (1993) Biological roles of oligosaccharides: all of the theories are correct. Glycobiology 3:97–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware FE, Vassilakos A, Peterson PA, Jackson MR, Lehrman MA, Williams DB (1995) The molecular chaperone calnexin binds Glc1Man9GlcNAc2 oligosaccharide as an initial step in recognizing unfolded glycoproteins. J Biol Chem 270:4697–4704 10.1074/jbc.270.9.4697 [DOI] [PubMed] [Google Scholar]
- Wu X, Rush JS, Karaoglu D, Krasnewich D, Lubinsky MS, Waechter CJ, Gilmore R, Freeze HH (2003) Deficiency of UDP-GlcNAc:dolichol phosphate N-acetylglucosamine-1 phosphate transferase (DPAGT1) causes a novel congenital disorder of glycosylation type Ij. Hum Mutat 22:144–150 10.1002/humu.10239 [DOI] [PubMed] [Google Scholar]