Abstract
The homodimeric transmembrane receptor natriuretic peptide receptor B (NPR-B [also known as guanylate cyclase B, GC-B, and GUC2B]; gene name NPR2) produces cytoplasmic cyclic GMP from GTP on binding its extracellular ligand, C-type natriuretic peptide (CNP). CNP has previously been implicated in the regulation of skeletal growth in transgenic and knockout mice. The autosomal recessive skeletal dysplasia known as “acromesomelic dysplasia, type Maroteaux” (AMDM) maps to an interval that contains NPR2. We sequenced DNA from 21 families affected by AMDM and found 4 nonsense mutations, 4 frameshift mutations, 2 splice-site mutations, and 11 missense mutations. Molecular modeling was used to examine the putative protein change brought about by each missense mutation. Three missense mutations were tested in a functional assay and were found to have markedly deficient guanylyl cyclase activity. We also found that obligate carriers of NPR2 mutations have heights that are below the mean for matched controls. We conclude that, although NPR-B is expressed in a number of tissues, its major role is in the regulation of skeletal growth.
Introduction
Endochondral bone growth occurs at growth plates in the axial and appendicular skeleton. Chondrocytes proliferate, differentiate, increase in size, synthesize, calcify matrix, and become apoptotic, ultimately leading to the recruitment of osteoblasts that replace the calcified cartilage matrix with bone. Endochondral growth is regulated by endocrine, paracrine, and autocrine factors (fig. 1), many of which have been identified through the study of individuals who have abnormal growth patterns (van der Eerden et al. 2003; Zelzer and Olsen 2003).
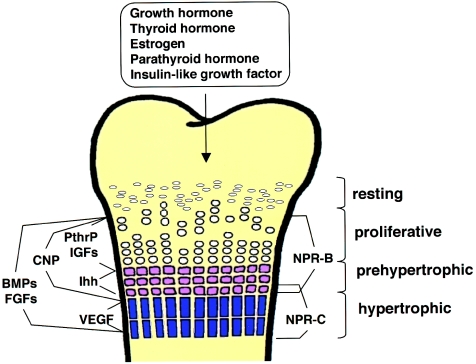
Figure 1.
Schematic depiction of an endochondral growth plate, with resting, proliferative, prehypertrophic, and hypertrophic zones noted. Ligands or ligand families known to regulate endochondral growth (e.g., IGF, FGF, and BMP) are shown at left. Note that CNP is expressed by proliferative and prehypertrophic chondrocytes. Sites of expression of the two CNP receptors are shown at right. NPR-B is expressed in proliferative and prehypertrophic chondrocytes, whereas NPR-C is expressed in prehypertrophic and hypertrophic chondrocytes. Systemic growth factors are noted above. Although some interactions between secreted growth factors are known (see, e.g., Minina et al. 2002), the interplay between most endocrine- and paracrine-signaling systems is still not delineated.
Acromesomelic dysplasia, type Maroteaux (AMDM [MIM 602875]) (Maroteaux et al. 1971), is an autosomal recessive skeletal dysplasia with a prevalence of ∼1/1,000,000 (fig. 2). Birth lengths and weights are normal, although mild shortening of long bones may be detected in some affected infants by clinical and radiographic examination (Langer and Garrett 1980). However, radiographs of newborns do not reveal misshapen bones or abnormal growth plates. In patients with AMDM, skeletal growth falls off sharply after birth, so that a skeletal disorder is strongly suspected in most affected individuals by 1 year of age. By 2 years of age, radiographic skeletal changes are diagnostic for AMDM and include abnormal growth plates and short, misshapen bones in the extremities (fig. 2) and spine. Whereas newborns with AMDM have lengths within 2 SDs of the mean, adults with AMDM have heights that are >5 SDs below the mean. The skeletal system appears to be the only organ system consistently affected among individuals with AMDM. It is interesting that carrier parents of children with AMDM have been noted to be shorter than average (Borrelli et al. 1983).
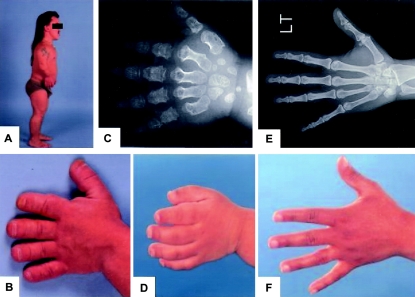
Figure 2.
Clinical and radiographic features of AMDM. A, Side photograph of an adult male with AMDM, whose hand photograph is in panel B. Note the short stature, the shortening of the extremities, and the bowing of the forearm. C, Hand radiograph of a child with AMDM, whose hand photograph is in panel D. Note the shortening and widening of metacarpals and phalanges. E, Hand radiograph of an adult carrier of AMDM, whose height is 145 cm (4 ft, 9 in) and whose hand photograph is in panel F. Note the normal shape and proportion of the skeletal elements.
We used a positional-candidate approach to identify the cause of AMDM. Here we report that AMDM results from loss-of-function mutations in the natriuretic peptide receptor B (NPR-B). This result indicates that, despite a broad pattern of expression during development, a principal function of NPR-B is the regulation of skeletal growth.
Subjects and Methods
Patient Recruitment, Statistical Analysis of Height, and Mutation Detection
This study was approved by the institutional review board at University Hospitals of Cleveland. Informed consent was obtained from all participants who gave blood from which DNA was extracted. The clinical diagnosis of AMDM was based on radiographic criteria (Langer and Garrett 1980). Participating families came from several different geographic and ethnic backgrounds. Only families with available population-control data for height (matched by sex, age group, and geography and/or ethnicity) were used to determine whether there was a heterozygous effect. The difference in height compared with population controls that were matched by sex, age group, and geography and/or ethnicity (James and Schofield 1990) was determined for 30 adult carriers of AMDM. The mean difference in height between carriers and controls was tested for statistical significance by use of the t distribution with 29 df. Human NPR2 genomic DNA sequence was retrieved from GenBank, and PCR primers were designed to amplify coding exons and flanking intronic sequence from patient/parent genomic DNA. PCR amplimers were sequenced by use of BigDye1.1 and an ABI 3100 capillary sequencer. Mutations were confirmed in all available parents. Six missense mutations that alter restriction enzyme sites were evaluated in a sample of at least 50 control individuals from the general population.
Modeling Missense Mutations
The domains of NPR-B were modeled individually (fig. 3) as follows. The extracellular domain (ECD) dimer was modeled on the NPR-A crystal structure (Protein Data Bank [PDB] accession number 1DP4) with the NPR-C crystal structure used as an additional guide (PDB accession number 1JDP). The kinase-homology domain (KHD) of NPR-B was modeled on the lymphocyte-specific kinase Lck structure (PDB accession number 1QPC). The guanylyl cyclase (GC) domain dimer was based on the adenylyl cyclase structure (PDB accession number 1CUL). The structure-based sequence alignment and subsequent modeling was done in an iterative fashion with SWISS-MODELER (Guex and Peitsch 1997) and the program O (Jones et al. 1991), and the model quality was monitored by use of the structure-validation program ERRAT (Colovos and Yeates 1993). The molecular figures were generated by use of MOLSCRIPT (Kraulis 1991) and RASTER 3D (Merritt and Bacon 1997). The composite figure depicting the entire NPR-B receptor is purely for illustrative purposes; the precise orientation of the individual domains with respect to each other is not known.
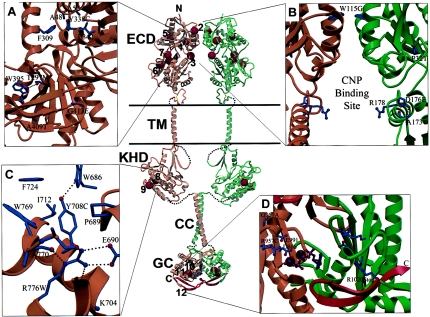
Figure 3.
Schematic diagram of the dimeric NPR-B receptor, highlighting sites containing putative disease-causing missense mutations and a truncation mutation that would not be predicted to cause nonsense-mediated mRNA decay. Also depicted are the ECD, transmembrane helices (TM), the kinase homology domain (KHD), the coiled-coil (CC) region, and the guanylyl cyclase (GC) domain. Mutations are P32T (1), W115G (2), D176E (3), T297M (4), Y338C (5), A409T (6), G413E (7), Y708C (8), R776W (9), R957C (10), G959A (11), and R1020fsX1025 (12). A, Close-up view of four mutation sites (4–7) in the ECD. B, Close-up view of the W115G and D176E mutation sites. C, Close-up view of the Y708C and R776W mutation sites in the KHD. D, Close-up view of the modeled NPR-B GC domain dimer showing R957C, G959A, and R1020fsX1025. The red ribbon indicates the portion of NPR-B that is missing in R1020fsX1025. Note that, for clarity, only one of the nucleotide substrate analogs, derived from the template 1CUL kinase domain structure, is shown (dark blue), including the two magnesium ions (black).
Assaying Wild-Type and Mutant NPR-B Activity
Missense mutations in NPR-B were generated by site-directed mutagenesis using the wild-type rat NPR-B expression construct pRK-NPR-B. Activity in HEK 293 cells was measured as described elsewhere (Abbey and Potter 2003). In brief, cells were seeded to 40%–45% confluency in 10-cm dishes in Dulbecco's modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS). The cells were transfected 2 d later with 4 μg of expression construct with Lipofectamine (Invitrogen) in serum-free DMEM. After transfection, cells were supplemented with 15% FBS and incubated overnight. Transfection efficiency was 40%–50%, as determined by cotransfecting cells with a green fluorescent protein reporter plasmid. Transfected cells were starved for 12 h prior to C-type natriuretic peptide (CNP) exposure (48–72 h after transfection). Cells were then exposed to 1 μM CNP (Bachem) for 3 min. Signaling was terminated by aspirating the CNP-containing media and adding ice-cold 80% ethanol to the cells. This ethanol extract was centrifuged, and the supernatant was collected and vacuum evaporated. The amount of cytoplasmic cyclic GMP (cGMP) in the evaporated samples was measured by use of a cGMP assay kit (Cayman Chemical).
Results
By use of the positional-candidate approach, we identified NPR2, which encodes NPR-B, as the gene responsible for causing AMDM. Eighteen families affected by AMDM were used to map the candidate interval to a 4.7-Mb region of human chromosome 9 (Kant et al. 1998; authors' unpublished data). NPR2 is contained within this interval. NPR-B expression has been reported in growth-plate chondrocytes (Yamashita et al. 2000), in which it is the major receptor for the secreted ligand, CNP. Furthermore, mice with homozygous knockout mutations in CNP are dwarfed (Chusho et al. 2001). We therefore sequenced NPR2 and found putative disease-causing mutations in all affected individuals in our study (table 1). Eight mutations are nonsense or frameshift mutations, seven of which are predicted to cause nonsense-mediated mRNA decay. Two mutations are predicted to affect mRNA splicing. Eleven mutations are missense mutations.
Table 1.
Putative Disease-Causing Mutations in 21 Participating Families and Height of Individuals Heterozygous for AMDM Mutations, Compared with Controls (Matched by Age Group, Sex, and Ethnicity and/or Geography)[Note]
|
Height (in cm) of |
||||||
| FamilyNo. | Consanguineous? | Protein Mutation | cDNA Mutation | Ancestry | Father(Average Matched Control) | Mother(Average Matched Control) |
| 1 | Yes | p.P32T | c.94C→A | Brazilian | 168 (NA) | 162 (NA) |
| 2 | Yes | p.W115G | c.343T→G | Turkish | 170 (175.1) | 160 (158.7) |
| 3 | No | [p.D176E]a+[p.R388X]b | [c.528T→A]a+[c.1162C→T]b | U.S. (white) | 170 (177.0) | 150 (163.3) |
| 4 | Yes | p.Q282X | c.844C→T | East Indian | 168 (NA) | 159 (NA) |
| 5 | Yes | p.T297M | c.890C→T | Pakistani | NA (164.0) | 148 (152.5) |
| 6 | Yes | p.T297M | c.890C→T | Pakistani | 158 (164.0) | 143 (152.5) |
| 7 | Yes | p.T297M | c.890C→T | Pakistani | 154 (164.0) | 146 (152.5) |
| 8 | Yes | p.T297M | c.890C→T | Pakistani | NA (164.0) | 150 (152.5) |
| 9 | Yes | p.Y338C | c.1013A→G | Turkish | 162 (175.1) | 153 (158.7) |
| 10 | No | [p.R371X]b+[p.P438fsX441]a | [c.1111C→T]b+[c.1314_1315delCT]a | U.S. (white) | 162.5 (177.0) | 150 (163.3) |
| 11c | Yes | p.A409T | c.1225G→A | Dutch | NA (179.4) | NA (166.8) |
| 12 | Yes | p.G413E | c.1238G→A | Turkish | 170 (175.1) | 159 (158.7) |
| 13 | Yes | p.P432fsX476 | c.1294delC | NA | NA | NA |
| 14 | Yes | p.Q500X | c.1498C→T | Dutch | 169 (179.4) | NA (166.8) |
| 15 | Yes | p. G630fsX? | c.1887+2T→A | NA | NA | NA |
| 16 | NA | p.Y708C | c.2123A→G | German | 175 (176.0) | 160 (163.7) |
| 17 | Yes | p.W769X | c.2304_2307delTTGG insCTGATGGA | Lebanese | 168 (170.6) | 156 (158.7) |
| 18 | Yes | p.R776W | c.2326C→T | Turkish | 165 (175.1) | 155 (158.7) |
| 19 | Yes | p.R957C | c.2869C→T | Omani | 152 (167.8) | 145 (156.1) |
| 20 | No | [p.G959A]d+[p.K480X]d | [c.2876G→C]d+[c.1436+1G→T]d | NA | NA | NA |
| 21 | Yes | p.R1020fsX1025 | c.3059delG | Lebanese | 167 (170.6) | 152 (158.7) |
Note.— From Centers for Disease Control and Prevention (CDC) growth charts and James and Schofield (1990). NA = not available; p. = protein; c. = cDNA; fs = frameshift; del = deletion; ins = insertion; X = termination. Amino acid and nucleotide numbering starts at the translation initiation Met/ATG codon.
Mother's allele.
Father's allele.
Heights of carrier sisters = 172 cm, 163 cm, 164 cm; height of carrier brother = 175 cm.
Parents not available.
Alignments were made of NPR-B from human, rat, mouse, cow, dogfish, eel, and medaka fish to look for amino acid conservation at missense mutation sites. There was complete conservation of P32, W115, D176, G413, Y708, R776, R957, and G959. In mammals, there was also conservation of T297, Y338, and A409. We did not find any of the six missense mutations testable by restriction-enzyme analysis (W115G, D176E, T297M, A409T, G413E, and R776W) in healthy controls.
We modeled all identified NPR-B missense mutations by use of homology models of both the extra- and intracellular domains (fig. 3). Modeling suggested that each missense mutation would affect receptor function. For example, in the receptor’s ECD, P32T is predicted to disrupt hydrophobic interactions with the side chains of L31, Y38, W40, and A41, as well as with the disulfide bond of C75-C101; W115G is predicted to alter the head-to-head dimer interface (van den Akker et al. 2000; He et al. 2001); and D176E is predicted to disrupt the ligand-binding site. The mutations T297M and Y338C replace small and aromatic side chains with large and polar side chains, respectively, in the protein’s core, and the mutations A409T and G413E affect a β-strand thought to be important for transducing signal across the membrane. Intracellular mutations Y708C and R776W, as well as R957C and G959A, are predicted to affect protein folding, since they alter highly interactive residues within the kinase homology and guanylyl cyclase domains, respectively. As a result, all identified NPR-B mutations are expected to have decreased activity caused by either local (or global) receptor destabilization or by disruption of signal transduction and/or cGMP production. We tested this for three missense muteins (W115G, T297M, and G413E) and one frameshift mutein (R1020FS) by transfecting HEK 293 cells, which do not normally express NPR-B, and measuring cGMP production after stimulation with CNP. The four muteins produced equivalent amounts of protein by western blot, compared with the wild type (data not shown), but had relative guanylyl cyclase activities (±SD), compared with the wild type, of 12.4% (±12.2%), 17.4% (±14.8%), 3.1% (±1.6%), and 3.4% (±3.0%), respectively.
To determine if there was an effect on skeletal growth in obligate carriers of NPR2 mutations, we compared the adult heights of the parents and carrier siblings of patients with AMDM with those of population controls matched by age group, sex, and geography and/or ethnicity. It was not possible to compare carrier heights to those of noncarrier siblings, since there were too few in the participant cohort. We found that the average height among 30 adult carriers of AMDM was 5.7 cm shorter than that of population-matched controls (P<.001) (see table 1).
Discussion
NPR-B is a transmembrane receptor that is expressed in a broad array of tissues, including brain, adrenal gland, and uterus (Tamura and Garbers 2003). However, our results indicate that, in humans, NPR-B is primarily involved in the regulation of skeletal growth. Individuals with AMDM do not have neurologic impairment, abnormal blood pressure, or any other consistent abnormality of an organ system outside the skeleton.
The precise role for NPR-B in endochondral growth is not known, since growth-plate histology is not available from patients with AMDM and a mouse model for NPR-B deficiency has not been described. As a consequence, at present the receptor’s role can only be inferred from studies of its ligand, CNP, or from downstream effectors such as cGMP-dependent protein kinase II (cGKII). Mice lacking CNP are dwarfed (Chusho et al. 2001), and mice overexpressing CNP have longer bones (Chusho et al. 2001; Yasoda et al. 2004). These results and studies of exogenous administration of CNP to in vitro whole-organ cultures of mouse and rat tibias indicate that CNP can stimulate chondrocyte proliferation and increase the size of individual hypertrophic chondrocytes (Yasoda et al. 1998; Mericq et al. 2000). A second role of CNP during endochondral growth involves the regulation of matrix synthesis, since increased matrix production, but not increased cell proliferation, in CNP transgenic mice was shown to mitigate the effect of an Fgfr3 mutation that causes achondroplasia in humans (Yasoda et al. 2004). Since CNP is able to increase chondrocyte proliferation, matrix synthesis, and cell hypertrophy in the growth plate, it is likely that each of these effects is mediated by signaling via NPR-B.
Activation of NPR-B by CNP leads to the production of cGMP, which activates cGMP-dependent protein kinases (cGKs) (Lincoln and Cornwell 1993). In this regard, it is relevant that dwarfism has been observed in mice in which the cGKII gene (PRKG2 in humans) has been disrupted (Pfeifer et al. 1996). cGKII knockout mice have persistent nests of incompletely differentiated chondrocytes within the terminally differentiated hypertrophic chondrocyte zone (Pfeifer et al. 1996). This was not observed in CNP knockout mice, implying that NPR-B affects endochondral growth by both cGKII and non-cGKII pathways (Miyazawa et al. 2002).
NPR-B has a paralogous family member, NPR-A, which has a low affinity for CNP and a high affinity for the natriuretic peptides ANP and BNP. Mice lacking NPR-A, ANP, and BNP have abnormalities of blood pressure, blood volume, and myocyte remodeling, but no skeletal defects (John et al. 1995; Oliver et al. 1997; Tamura et al. 2000). Skeletal overgrowth has been reported in mice lacking a third natriuretic peptide receptor, NPR-C, which is expressed in hypertrophic chondrocytes and is postulated to regulate terminal chondrocyte differentiation (Matsukawa et al. 1999). This receptor is able to bind all three natriuretic peptides with similar affinity, but it lacks guanylyl cyclase activity. As a consequence, NPR-C could effect terminal differentiation by signaling via non-cGMP–dependent pathways or by reducing NPR-B activity through competition for the ligand, CNP (Levin 1993; Pagano and Anand-Srivastava 2001). Skeletal overgrowth has also been observed in transgenic mice that have elevated serum levels of BNP (Suda et al. 1998). BNP in the systemic circulation is likely to reach growth-plate chondrocytes and either directly agonize NPR-B signaling or indirectly increase local CNP levels by saturating the competing receptor NPR-C. The ability of increased levels of BNP in serum to affect skeletal growth suggests that exogenous administration of more-selective agonists (e.g., CNP) may increase skeletal growth in individuals with functioning NPR-B receptors, analogous to the use of exogenously administered recombinant human growth hormone and insulin-like growth factor 1 (Laron 1999; Sandberg and MacGillivray 2000).
Two other observations in individuals and families affected by AMDM warrant comment. First, NPR-B functional haploinsufficiency, as can occur in parents of patients with AMDM, appears to cause shorter stature. A similar haploinsufficiency effect on blood pressure has been observed for the NPR-A gene in mice (Oliver et al. 1998). Given the rarity of AMDM mutations in the general population (estimated carrier frequency 1/500), these loss-of-function alleles will not contribute strongly to the normal population variation in height; however, other NPR2 variants could contribute to this trait, since a height QTL has been placed near NPR2 on human chromosome 9 (Xu et al. 2002). Second, among individuals with AMDM, postnatal skeletal growth is more strongly affected than prenatal growth. A similar effect has been observed in individuals with congenital growth hormone deficiency or mutations in the growth hormone receptor (Pena-Almazan et al. 2001), consistent with the temporally regulated expression of the growth hormone receptor within the growth plate during prenatal and postnatal life (Barnard et al. 1988). Although we do not know yet what accounts for the different effects of NPR-B deficiency on prenatal and postnatal growth, it is intriguing to speculate that it is another example of a temporally regulated paracrine pathway.
Acknowledgments
The authors thank the patients and their families for participating in this study, the Case Western Reserve University Department of Genetics computer resources group for sharing scientific expertise, and Dr. Lincoln Potter for providing the rat NPR-B expression vector. M.L.W. is an assistant investigator with the Howard Hughes Medical Institute and is a recipient of a Clinical Scientist Award in Translational Research from the Burroughs Wellcome Fund.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for NPR2 genomic DNA [accession number NT_008413] and NPR2 cDNA [accession number NM_003995])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for AMDM) [PubMed]
- Protein Data Bank, http://www.rcsb.org/pdb/ (for NPR-A crystal structure [accession number 1DP4], NPR-C crystal structure [accession number 1JDP], lymphocyte-specific kinase Lck structure [accession number 1QPC], and adenylyl cyclase structure [accession number 1CUL])
References
- Abbey SE, Potter LR (2003) Lysophosphatidic acid inhibits C-type natriuretic peptide activation of guanylyl cyclase-B. Endocrinology 144:240–246 10.1210/en.2002-220702 [DOI] [PubMed] [Google Scholar]
- Barnard R, Haynes KM, Werther GA, Waters MJ (1988) The ontogeny of growth hormone receptors in the rabbit tibia. Endocrinology 122:2562–2569 [DOI] [PubMed] [Google Scholar]
- Borrelli P, Fasanelli S, Marini R (1983) Acromesomelic dwarfism in a child with an interesting family history. Pediatr Radiol 13:165–168 [DOI] [PubMed] [Google Scholar]
- Chusho H, Tamura N, Ogawa Y, Yasoda A, Suda M, Miyazawa T, Nakamura K, Nakao K, Kurihara T, Komatsu Y, Itoh H, Tanaka K, Saito Y, Katsuki M (2001) Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc Natl Acad Sci USA 98:4016–4021 10.1073/pnas.071389098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colovos C, Yeates TO (1993) Verification of protein structures: patterns of nonbonded atomic interactions. Protein Sci 2:1511–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714–2723 [DOI] [PubMed] [Google Scholar]
- He X, Chow D, Martick MM, Garcia KC (2001) Allosteric activation of a spring-loaded natriuretic peptide receptor dimer by hormone. Science 293:1657–1662 10.1126/science.1062246 [DOI] [PubMed] [Google Scholar]
- James W, Schofield E (1990) Human energy requirements: a manual for planners and nutritionists. Oxford University Press, New York [Google Scholar]
- John SW, Krege JH, Oliver PM, Hagaman JR, Hodgin JB, Pang SC, Flynn TG, Smithies O (1995) Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science 267:679–681 [DOI] [PubMed] [Google Scholar]
- Jones TA, Zou JY, Cowan SW, Kjeldgaard (1991) Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A 47:110–119 10.1107/S0108767390010224 [DOI] [PubMed] [Google Scholar]
- Kant SG, Polinkovsky A, Mundlos S, Zabel B, Thomeer RT, Zonderland HM, Shih L, van Haeringen A, Warman ML (1998) Acromesomelic dysplasia Maroteaux type maps to human chromosome 9. Am J Hum Genet 63:155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraulis P (1991) MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J Appl Crystallogr 24:946–950 10.1107/S0021889891004399 [DOI] [Google Scholar]
- Langer L, Garrett R (1980) Acromesomelic dysplasia. Radiology 137:349–355 [DOI] [PubMed] [Google Scholar]
- Laron Z (1999) The essential role of IGF-I: lessons from the long-term study and treatment of children and adults with Laron syndrome. J Clin Endocrinol Metab 84:4397–4404 10.1210/jc.84.12.4397 [DOI] [PubMed] [Google Scholar]
- Levin ER (1993) Natriuretic peptide C-receptor: more than a clearance receptor. Am J Physiol 264:E483–E489 [DOI] [PubMed] [Google Scholar]
- Lincoln TM, Cornwell TL (1993) Intracellular cyclic GMP receptor proteins. Faseb J 7:328–338 [DOI] [PubMed] [Google Scholar]
- Maroteaux P, Martinelli B, Campailla E (1971) [Acromesomelic dwarfism]. Presse Med 79:1839–1842 [PubMed] [Google Scholar]
- Matsukawa N, Grzesik WJ, Takahashi N, Pandey KN, Pang S, Yamauchi M, Smithies O (1999) The natriuretic peptide clearance receptor locally modulates the physiological effects of the natriuretic peptide system. Proc Natl Acad Sci USA 96:7403–7408 10.1073/pnas.96.13.7403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mericq V, Uyeda JA, Barnes KM, De Luca F, Baron J (2000) Regulation of fetal rat bone growth by C-type natriuretic peptide and cGMP. Pediatr Res 47:189–193 [DOI] [PubMed] [Google Scholar]
- Merritt E, Bacon D (1997) Raster3D: photorealistic molecular graphics. Meth Enzymol 277:505–524 [DOI] [PubMed] [Google Scholar]
- Minina E, Kreschel C, Naski MC, Ornitz DM, Vortkamp A (2002) Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Dev Cell 3:439–449 10.1016/S1534-5807(02)00261-7 [DOI] [PubMed] [Google Scholar]
- Miyazawa T, Ogawa Y, Chusho H, Yasoda A, Tamura N, Komatsu Y, Pfeifer A, Hofmann F, Nakao K (2002) Cyclic GMP-dependent protein kinase II plays a critical role in C-type natriuretic peptide-mediated endochondral ossification. Endocrinology 143:3604–3610 10.1210/en.2002-220307 [DOI] [PubMed] [Google Scholar]
- Oliver PM, Fox JE, Kim R, Rockman HA, Kim HS, Reddick RL, Pandey KN, Milgram SL, Smithies O, Maeda N (1997) Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc Natl Acad Sci USA 94:14730–14735 10.1073/pnas.94.26.14730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver PM, John SW, Purdy KE, Kim R, Maeda N, Goy MF, Smithies O (1998) Natriuretic peptide receptor 1 expression influences blood pressures of mice in a dose-dependent manner. Proc Natl Acad Sci USA 95:2547–2551 10.1073/pnas.95.5.2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M, Anand-Srivastava MB (2001) Cytoplasmic domain of natriuretic peptide receptor C constitutes Gi activator sequences that inhibit adenylyl cyclase activity. J Biol Chem 276:22064–22070 10.1074/jbc.M101587200 [DOI] [PubMed] [Google Scholar]
- Pena-Almazan S, Buchlis J, Miller S, Shine B, MacGillivray M (2001) Linear growth characteristics of congenitally GH-deficient infants from birth to one year of age. J Clin Endocrinol Metab 86:5691–5694 10.1210/jc.86.12.5691 [DOI] [PubMed] [Google Scholar]
- Pfeifer A, Aszodi A, Seidler U, Ruth P, Hofmann F, Fassler R (1996) Intestinal secretory defects and dwarfism in mice lacking cGMP-dependent protein kinase II. Science 274:2082–2086 10.1126/science.274.5295.2082 [DOI] [PubMed] [Google Scholar]
- Sandberg DE, MacGillivray MH (2000) Growth hormone therapy in childhood-onset growth hormone deficiency: adult anthropometric and psychological outcomes. Endocrine 12:173–182 10.1385/ENDO:12:2:173 [DOI] [PubMed] [Google Scholar]
- Suda M, Ogawa Y, Tanaka K, Tamura N, Yasoda A, Takigawa T, Uehira M, Nishimoto H, Itoh H, Saito Y, Shiota K, Nakao K (1998) Skeletal overgrowth in transgenic mice that overexpress brain natriuretic peptide. Proc Natl Acad Sci USA 95:2337–2342 10.1073/pnas.95.5.2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura N, Garbers DL (2003) Regulation of the guanylyl cyclase-B receptor by alternative splicing. J Biol Chem 278:48880–48889 10.1074/jbc.M308680200 [DOI] [PubMed] [Google Scholar]
- Tamura N, Ogawa Y, Chusho H, Nakamura K, Nakao K, Suda M, Kasahara M, Hashimoto R, Katsuura G, Mukoyama M, Itoh H, Saito Y, Tanaka I, Otani H, Katsuki M (2000) Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Natl Acad Sci USA 97:4239–4244 10.1073/pnas.070371497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Akker F, Zhang X, Miyagi M, Huo X, Misono KS, Yee VC (2000) Structure of the dimerized hormone-binding domain of a guanylyl-cyclase-coupled receptor. Nature 406:101–104 10.1038/35017602 [DOI] [PubMed] [Google Scholar]
- van der Eerden BC, Karperien M, Wit JM (2003) Systemic and local regulation of the growth plate. Endocr Rev 24:782–801 10.1210/er.2002-0033 [DOI] [PubMed] [Google Scholar]
- Xu J, Bleecker ER, Jongepier H, Howard TD, Koppelman GH, Postma DS, Meyers DA (2002) Major recessive gene(s) with considerable residual polygenic effect regulating adult height: confirmation of genomewide scan results for chromosomes 6, 9, and 12. Am J Hum Genet 71:646–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y, Takeshige K, Inoue A, Hirose S, Takamori A, Hagiwara H (2000) Concentration of mRNA for the natriuretic peptide receptor-C in hypertrophic chondrocytes of the fetal mouse tibia. J Biochem (Tokyo) 127:177–179 [DOI] [PubMed] [Google Scholar]
- Yasoda A, Komatsu Y, Chusho H, Miyazawa T, Ozasa A, Miura M, Kurihara T, Rogi T, Tanaka S, Suda M, Tamura N, Ogawa Y, Nakao K (2004) Overexpression of CNP in chondrocytes rescues achondroplasia through a MAPK-dependent pathway. Nat Med 10:80–86 10.1038/nm971 [DOI] [PubMed] [Google Scholar]
- Yasoda A, Ogawa Y, Suda M, Tamura N, Mori K, Sakuma Y, Chusho H, Shiota K, Tanaka K, Nakao K (1998) Natriuretic peptide regulation of endochondral ossification. Evidence for possible roles of the C-type natriuretic peptide/guanylyl cyclase-B pathway. J Biol Chem 273:11695–11700 10.1074/jbc.273.19.11695 [DOI] [PubMed] [Google Scholar]
- Zelzer E, Olsen BR (2003) The genetic basis for skeletal diseases. Nature 423:343–348 10.1038/nature01659 [DOI] [PubMed] [Google Scholar]