Abstract
Joubert syndrome (JS) is an autosomal recessive multisystem disease characterized by cerebellar vermis hypoplasia with prominent superior cerebellar peduncles (the “molar tooth sign” [MTS] on axial magnetic resonance imaging), mental retardation, hypotonia, irregular breathing pattern, and eye-movement abnormalities. Some individuals with JS have retinal dystrophy and/or progressive renal failure characterized by nephronophthisis (NPHP). Thus far, no mutations in the known NPHP genes, particularly the homozygous deletion of NPHP1 at 2q13, have been identified in subjects with JS. A cohort of 25 subjects with JS and either renal and/or retinal complications and 2 subjects with only juvenile NPHP were screened for mutations in the NPHP1 gene by standard methods. Two siblings affected with a mild form of JS were found to have a homozygous deletion of the NPHP1 gene identical, by mapping, to that in subjects with NPHP alone. A control subject with NPHP and with a homozygous NPHP1 deletion was also identified, retrospectively, as having a mild MTS and borderline intelligence. The NPHP1 deletion represents the first molecular defect associated with JS in a subset of mildly affected subjects. Cerebellar malformations consistent with the MTS may be relatively common in patients with juvenile NPHP without classic symptoms of JS.
Joubert syndrome (JS [MIM 213300]) is an autosomal recessive disorder clinically characterized by congenital hypotonia evolving into ataxia, developmental delay, and either an abnormal breathing pattern (alternating tachypnea and/or apnea), unusual eye movements, or both (Joubert et al. 1969; Boltshauser and Isler 1977; Saraiva and Baraitser 1992; Maria et al. 1999a; Parisi and Glass 2003). The abnormal eye movements often comprise oculomotor apraxia (OMA) or difficulty with smooth eye pursuits and horizontal saccades, with jerking head thrusting and nystagmus (Vassella et al. 1972; Tusa and Hove 1999). JS is characterized by the “molar tooth sign” (MTS), a distinctive radiological finding that reflects a complex malformation of the midbrain and hindbrain, consisting of increased interpeduncular distance at the pontomesencephalic junction; cerebellar vermis hypoplasia; and thickened, straight, and elongated superior cerebellar peduncles that resemble a molar tooth on axial magnetic resonance imaging (MRI) (Maria et al. 1997, 1999b). No genes have been identified as causative in JS, although genetic heterogeneity is known to occur (Bennett et al. 2003), and two loci have been mapped—to 9q34.3 (Saar et al. 1999) and to the pericentromeric region of chromosome 11 (Keeler et al. 2003; Valente et al. 2003).
There is clinical heterogeneity in conditions such as JS that are associated with the MTS, sometimes collectively termed “cerebello-oculo-renal syndromes (CORS)” or “Joubert syndrome-related disorders” (table 1). Some children exhibit ocular colobomas, polydactyly, and liver fibrosis (Saraiva and Baraitser 1992; Chance et al. 1999). A subset of children with JS has retinal dystrophy, sometimes described as Leber amaurosis or congenital retinal blindness, and an association with renal disease has been observed in this subgroup (JS type B) (King et al. 1984; Saraiva and Baraitser 1992). A severe form of JS, with congenital cystic dysplastic kidneys in conjunction with retinal blindness, has been termed “Dekaban-Arima syndrome” (Dekaban 1969; Satran et al. 1999; Gleeson et al. 2004). Juvenile-onset nephronophthisis (NPHP [MIM 256100]) has been described in some individuals with JS (Hildebrandt et al. 1998; Apostolou et al. 2001) and is another renal manifestation occurring in this disorder. NPHP, or tubulointerstitial medullary cystic kidney disease, presents with concentration defects and leads to renal failure (i.e., end-stage renal disease [ESRD]) within the first 2 decades of life (Hildebrandt et al. 1997b). Of individuals with NPHP, ∼15% also have retinal dystrophy, a combination known as “Senior-Løken syndrome” (SLS [MIM 266900]) (Løken et al. 1961; Senior et al. 1961; Caridi et al. 1998), and a few individuals with SLS also demonstrate the MTS accompanied by mental retardation (Satran et al. 1999; Gleeson et al. 2004). Four genes have been identified as causative in individuals with NPHP or SLS (Hildebrandt et al. 1997a; Otto et al. 2002, 2003; Olbrich et al. 2003), and most have a familial form with autosomal recessive inheritance (Otto et al. 2002), with ∼80% of patients demonstrating an ∼290-kb homozygous deletion that encompasses the NPHP1 gene at 2q13 (Konrad et al. 1996; Hildebrandt et al. 1997b, 2001; Saunier et al. 2000).
Table 1.
Features in CORS with the MTS
|
Presence in Disordera |
||||||
| Clinical Features | JSb,c | Dekaban-Arima Syndromed | COACHd,e | JuvenileNPHPf | SLSg | CoganSyndromeh |
| MTS | + | (+) | (+) | (+)i | (+) | (+) |
| Hypotonia | + | + | + | (+) | (+) | (+) |
| Mental retardation | + | + | + | (+) | (+) | (+) |
| OMA | + | (+) | (+) | (+) | (+) | + |
| Breathing abnormalitiesj | (+) | − | (+) | ? | (+) | − |
| NPHP | (+) | − | (+) | + | + | (+) |
| Cystic dysplastic kidneys | (+) | + | − | − | − | − |
| Retinal dystrophyk | (+) | + | ? | − | + | − |
| Coloboma | (+) | − | + | − | − | − |
| Hepatic fibrosis | − | (+) | + | (+) | (+) | − |
+ = present; − = absent; (+) = sometimes present; ? = unknown or not described.
JS with retinal dystrophy and renal symptoms is sometimes designated type B.
Genetic loci JBTS1, CORS2, NPHP1 (present results), and others.
Genetic loci unknown or not described.
COACH = cerebellar vermis hypoplasia, oligophrenia, ataxia, colobomas, and hepatic fibrosis.
Genetic loci NPHP1, 2, 3, 4, and others.
Genetic loci NPHP1, 3, 4, and others.
Genetic loci NPHP1 and others unknown or not described.
Present results.
Episodic tachynpnea and/or apnea.
May include severe congenital blindness defined as Leber congenital amaurosis.
Given the finding of NPHP in a subset of patients with JS, we sought to determine if the NPHP1 gene, implicated as causative in the majority of familial cases of NPHP, may also be involved in JS. Three groups (W.B.D., J.G.G., and M.A.P.) evaluated their total cohort of subjects with JS, as defined by clinical criteria (Saraiva and Baraitser 1992; Maria et al. 1999a; Parisi and Glass 2003), for those with either renal involvement, retinal changes, or both. Fulfilling these criteria were 25 probands, with features summarized in table 2. Informed consent for genetic studies from each patient or legal guardian was obtained under protocols approved by the authors’ respective institutional review boards. Of the 25 JS probands studied, 32% had both retinal and renal involvement, whereas 24% had renal involvement alone, and 44% had only retinal dysplasia. Five probands had documented NPHP. For the three subjects without MRI confirmation of the MTS, cerebellar vermis hypoplasia was reported on computed tomography scans, prior to the description of the MTS (Maria et al. 1997). A presumptive diagnosis of SLS with the MTS was made in three subjects.
Table 2.
Summary of Clinical Characteristics of Subjects
| Characteristic | No. of Subjectsa | % |
| Sex: | ||
| Female | 14 | 56 |
| Male | 11 | 44 |
| Consanguinity | 4 | 16 |
| MTS | 22 | 88 |
| OMA | 12 | 48 |
| Renal involvement: | 14 | 56 |
| Cystic kidney disease | 7 | 28 |
| NPHP | 5 | 20 |
| Other renal pathology | 2 | 8 |
| Retinal dystrophy: | 19 | 76 |
| LCAb | 4 | 16 |
| Colobomas | 2 | 8 |
| Combined retinal and renal involvement | 8 | 32 |
N=25.
LCA = Leber congenital amaurosis.
Clinical details of the subjects with positive results for NPHP1 analysis are summarized in table 3. Of special interest, subject K76-1, who exhibited mild gross motor delay, hypotonia, and mild cognitive delay, has a history of a congenital head tilt and unusual eye movements. She was diagnosed with NPHP at the age of 10 years and is being treated with chronic peritoneal dialysis at age 11 years 8 mo. Her MRI scan at the age of 3 years showed cerebellar vermis hypoplasia with long, mildly thickened superior cerebellar peduncles, consistent with a mild MTS (fig. 1A and 1B and table 3). At 10 years of age, she manifested cyclorotatory nystagmus with hypometric saccades, consistent with a mild form of OMA. The retinal examination was unremarkable, with normal visual evoked potential and electroretinogram studies. Her sister, subject K76-2, has a history of a head tilt and mild gross motor delay but normal cognitive function. She has difficulty with smooth pursuits on visual tracking, mild ataxia, and impaired balance. Her renal function, including renal ultrasound, blood urea nitrogen, creatinine, and urine concentrating ability, were normal at age 8 years. She has not had an MRI or recentophthalmologic examination. There is no history of breathing abnormalities, tongue thrusting, colobomas, or polydactyly in either sibling. Neither parent has cognitive impairment, ataxia, retinal dystrophy, or renal disease, and there is no known consanguinity.
Table 3.
Clinical Characteristics of Subjects with NPHP1 Mutations
| Subject | Age(years) | Sex | Alteration in NPHP1 | NPHPa | Age at ESRD (years)b | RetinalDystrophya | OMAa | MTSa | DevelopmentalDelaya |
| K76-1 | 11 | F | Homozygous deletion | + | 10 | − | + | + (Mild) | + |
| K76-2 | 8 | F | Homozygous deletion | − | NA | − | + | NE | + (Mild motor) |
| K84-1 | 17 | M | Homozygous deletion | + | 9 | − | − | + (Mild) | + (Mild) |
| K89-1 | 15 | M | Deletion/W490fsX507 | + | 12 | − | − | − | − |
+ = present; − = absent; NE = not examined.
NA = not applicable.
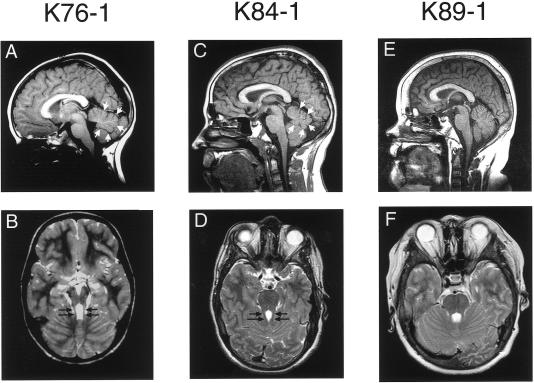
Figure 1.
Midline sagittal T1 images (A, C, and E) and associated axial T2 images through the level of the superior cerebellar peduncles (SCPs) (B, D, and F) in subjects indicated above each column. Note the superiorly positioned fourth ventricle with moderate inferior vermis hypoplasia (small white arrows) on the sagittal images of subjects K76-1 (A) and K84-1 (C), compared with the normal fourth ventricle with mild inferior vermis deficiency in subject K89-1 (E). Elongated SCPs are demonstrated on axial images (B and D, black arrows), with associated mild MTS, compared with normal SCPs (F).
Two subjects with NPHP and known homozygous and heterozygous NPHP1 deletions, respectively, were recruited from the nephrology registry at the University of Washington, to serve as controls for molecular analyses. As summarized in table 3, subject K84-1 was diagnosed with NPHP at age 8 years and progressed to ESRD and transplantation at age 9 years. He was identified elsewhere as having a homozygous deletion of NPHP1 on genetic testing (R.M., unpublished data). He had mild motor and learning delay, with an IQ of 76 at the age of 6.5 years. He lacks OMA, retinal dystrophy, and cerebellar signs, such as ataxia, but has mild incoordination. However, an MRI revealed mild cerebellar vermis hypoplasia with a mild MTS (fig. 1C and 1D). Subject K89-1 was diagnosed with NPHP at the age of 8 years and received a kidney transplant at age 14 years. He had a heterozygous deletion of NPHP1, identified by FISH analysis, with an unidentified second mutation (R.M., unpublished data). He has normal development, with neither ocular abnormalities nor retinal changes. His MRI was normal, without the MTS (fig. 1E and 1F).
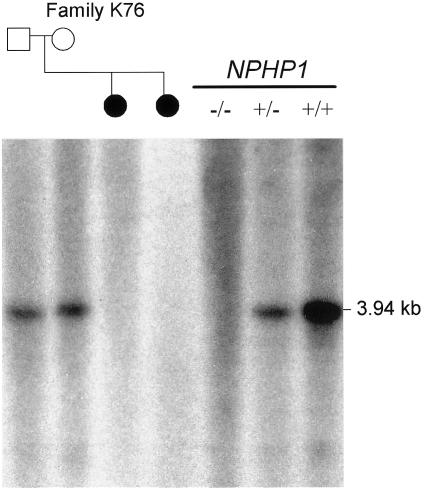
Analysis of NPHP1 was performed on genomic DNA isolated from peripheral blood or transformed cell lines from affected individuals. The 20 exons and flanking 30–50 nt at each intron-exon boundary of the NPHP1 gene were amplified by PCR with the use of standard protocols, and the products were sequenced bidirectionally (primers and PCR conditions are available upon request). No deleterious point mutations were identified in any of the 25 probands included in the analysis. One SNP described elsewhere was identified in exon 7 and did not alter the encoded amino acid (Caridi et al. 2001). Four additional intronic SNPs were identified in our cohort, as described in the SNP database (dbSNP). In one proband and her sibling (K76-1 and K76-2), we were unable to amplify any of the exons, suggesting a homozygous deletion of NPHP1. This finding was confirmed by Southern analysis, demonstrating the failure of an internal NPHP1 probe to hybridize to DNA from the sisters (fig. 2) (Konrad et al. 1996). We also identified the second mutation in control subject K89-1 with NPHP alone and a heterozygous NPHP1 deletion (table 3).
Figure 2.
Southern analysis of EcoRI-digested genomic DNA from members of family K76 and from control subjects, confirming the presence of a homozygous deletion of NPHP1 in the two affected daughters (blackened circles). The probe is a 405-bp PCR fragment, including exons 9–10 (forward primer: 5′-TGGAAAGCAAGTTCTTAGTAAGCAGCG-3′, reverse primer: 5′-GGCAGAATTTGGACTTGCTACCTTGA-3′). The number of wild-type alleles in control samples is indicated by the genotype symbols underneath the black line (below “NPHP1”). Dosage is apparent by intensity of the 3.94-kb band that hybridizes to the intragenic NPHP1 probe. A ß-actin control probe detected a band of almost equal intensity for all samples, confirming equal loading of DNA (data not shown).
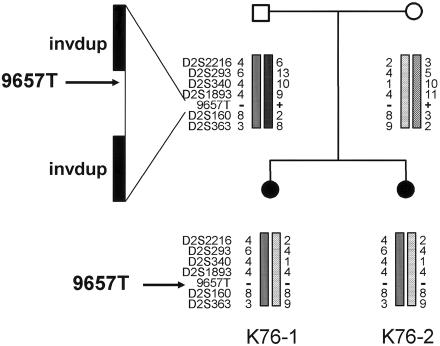
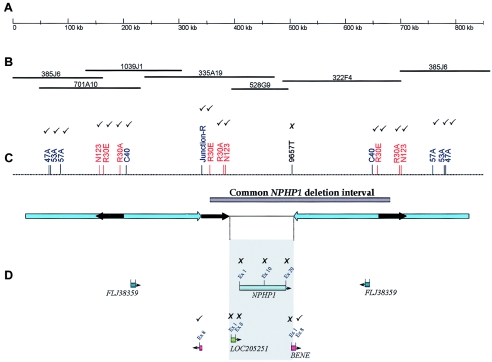
Homozygous deletions in the NPHP1 gene were identified by evaluating the presence or loss of an STS marker (9657T) 3′ to the NPHP1 gene and within the common deleted region (fig. 3), with the use of an elsewhere described procedure with minor modifications (Hildebrandt et al. 1998; Heninger et al. 2001). Subjects K76-1, K76-2, and K84-1 (known to have a homozygous deletion of NPHP1) had this marker deleted. The haplotype analysis of family K76 is consistent with inheritance by each affected daughter of the same two alleles from both parents, who each carry an NPHP1 deletion. Several markers within the NPHP1 commonly deleted region and within the 330-kb inverted repeat regions (Saunier et al. 1997a, 2000; Nothwang et al. 1998) were tested to map the extent of the homozygous NPHP1 deletion in subjects K76-1, K76-2, and K84-1 (fig. 4). We generated a junction fragment marker (junction-R), at the boundary of the proximal 330-kb repeat and the 45-kb direct repeat, and new STS markers at the 5′ and 3′ regions of the genes within the commonly deleted region (LOC205251, NPHP1, and BENE), by inspection of the UCSC human genome browser. Of note, a full copy of the BENE gene was deleted, but the final exon that is known to be duplicated at the distal end of the proximal 330-kb inverted repeat, outside the commonly deleted interval (Saunier et al. 1997a), was present in the deletion control subject K84-1. Characterization of the deletion in these subjects with JS by STS mapping showed concordance with the “classic” NPHP1 deletion, seen in control subject K84-1 (fig. 4). All STS markers located in the 330-kb inverted repeat region (47A, 53A, 57A, N123, R30E, R30A, and C40) were present in subjects K76-1 and K76-2, suggesting that a homozygous deletion encompassing a larger interval than that seen in patients with classic NPHP can be excluded. However, the presence of a larger-than-normal heterozygous deletion in the affected members of family K76 cannot be excluded.
Figure 3.
Haplotype analysis in family K76, showing markers compatible with linkage to the 2q13 region surrounding NPHP1. The deleted STS marker 9657T is indicated in affected subjects K76-1 and K76-2. The 290-kb deletion is mediated by two inverted duplicated segments (invdup) of ∼330 kb each, via a complex mechanism.
Figure 4.
Comparison of the NPHP1 deletion in subjects with JS (K76-1 and K76-2) and in a control subject with the classic deletion (K84-1). The results of STS deletion mapping are indicated in C and D as ticks (to indicate the marker was present in these three subjects) and crosses (to indicate the marker was absent in all three subjects). A, Scale bar indicating 850 kb of contiguous DNA sequence at 2q13, isolated from the UCSC genome browser. B, BAC clone contig of the interval. (Note that clone 385J6 flanks both ends, demonstrating the difficulty presented by genomic duplications in generating accurate contigs.) C, Map of STS markers in the region that was used for deletion mapping. The NPHP1 deletion interval is indicated by a gray bar, and arrow diagrams outline the repeat regions: large blue arrows indicate the 330-kb inverted repeat, and short black arrows indicate the three copies of the 45-kb repeat, of which the two direct repeats are known to mediate unequal crossover. D, Gene locations included within the region encompassing the 45-kb repeats, mapped with orientation indicated by arrowheads. The shaded gray box indicates the only unique DNA sequence of the deleted region, which includes LOC205251, NPHP1, and all but the 3′ portion of the BENE gene.
To exclude the presence of a compound heterozygous mutation consisting of one deleted NPHP1 allele and a cryptic point mutation in the other allele, we analyzed a set of 10 polymorphic markers, including 5 microsatellite markers described elsewhere (Heninger et al. 2001) and the 5 intragenic SNPs within the common NPHP1 deletion region, in each of the 25 probands. The presence of at least one heterozygous marker would indicate that the patient does not harbor a full NPHP1 gene deletion. For subjects in which parental and/or sibling DNA samples were available, haplotypes of the region encompassing the common 2q13 deletion were constructed, to determine if the segregation pattern was suggestive of a heterozygous deletion. For 21 of the subjects, excluding K89-1, K84-1, and affected members of family K76, at least one polymorphic marker or SNP within the common NPHP1 deletion region could be identified. In several cases, familial haplotype segregation analysis provided further evidence against inheritance of a deleted allele (data not shown). For three subjects, there was insufficient DNA available to complete the analysis. For the three subjects with homozygous deletions, these markers could not be amplified. As anticipated, analysis of polymorphic markers within the deleted region did not uncover any heterozygous alleles from control subject K89-1. These results provide evidence that none of the subjects with JS is a heterozygous deletion carrierin whom the second mutation was not identified bymutational analysis.
The 18 families with JS that were suitable for haplotype analysis (either multiplex families with at least two affected children or those with consanguinity) were analyzed for evidence of linkage, with the use of six polymorphic microsatellite markers and one sequence-tagged site (D2S2216, D2S293, D2S340, D2S1893, STS 9657T, D2S160, and D2S363) within a 27-Mb region on chromosome 2q13 surrounding the NPHP1 gene. Four of the probands in these families were part of the set of 25 subjects included in the comprehensive NPHP1 sequence analysis. Of the 18 families, all but one (K76) were incompatible with linkage to 2q13 (data not shown).
From these investigations, we have identified the first molecular defect associated with JS—a homozygous deletion of the NPHP1 gene—in two siblings with mild JS and a documented MTS. However, on the basis of these results, we believe that the frequency of this mutation in the renal-retinal subtypes of JS, often termed “type B,” is low, as it was only identified in 1 (4%) of 25 families in our cohort, which was selected for retinal and/or renal involvement. Even in multiplex, unselected families with JS, haplotype segregation analysis excludes this locus in all but one family (K76), providing further evidence that NPHP1 is not likely to be a major locus associated with JS. In addition, other surveys, which included a total of 12 families with 15 affected individuals, have failed to identify the NPHP1 deletion in patients with JS type B (Hildebrandt et al. 1998; Apostolou et al. 2001).
What, then, differentiates our subjects with JS and NPHP1 deletions from other subjects with JS who do not have the NPHP1 deletion? Our subjects with JS and an NPHP1 deletion have a form of JS at the mild end of the clinical spectrum for this disorder, as they are not severely mentally retarded and their presenting symptoms in infancy were head tilt and mild hypotonia with gross motor delay, with a lack of the respiratory disturbance often seen in this disorder. In fact, the younger sibling (K76-2) has not yet demonstrated cognitive delay. Although the MTS was, in retrospect, visible on the MRI of the older child (K76-1), this scan was interpreted initially as normal. Comprehensive ophthalmologic evaluation failed to identify any evidence of retinal dystrophy in the proband, indicating that these subjects are not examples of JS type B, nor do they have SLS. It is also intriguing that our NPHP deletion control subject, K84-1, was identified as having a mild MTS, and we established, retrospectively, the presence of nonspecific delays prior to the onset of NPHP. Although this subject has the MTS, he does not fulfill all clinical criteria for JS (Saraiva and Baraitser 1992; Maria et al. 1999a; Parisi and Glass 2003). Thus, the severity of the pleiotropic features in JS appears to be variable.
Extrarenal findings have been described in some individuals with NPHP (Antignac et al. 1998). There are several reports of families with congenital OMA (Cogan syndrome) accompanied by NPHP who have documented deletions and/or point mutations in the NPHP1 gene (Saunier et al. 1997b; Betz et al. 2000). Some individuals with Cogan syndrome have abnormalities of the cerebellar vermis (Whitsel et al. 1995; Sargent et al. 1997). One recent case report showed hypoplasia of the brainstem and cerebellar vermis in a boy with NPHP and homozygous deletion of NPHP1 (Takano et al. 2003); in fact, this child may have a JS phenotype that was not recognized, but confirmation from the provided MRIs is impossible. We suspect that several of these patients with NPHP or Cogan syndrome with cerebellar involvement and NPHP1 mutations may have an MTS not identified explicitly (Saunier et al. 1997b; Betz et al. 2000; Takano et al. 2003); however, since MRI details are not included in these reports, this assertion remains speculative. In two separate series of MRI findings in patients with Cogan syndrome (n=21), at least three of the images show the MTS, but these cases were published before the MTS was recognized and the association with NPHP1 mutations was established (Whitsel et al. 1995; Sargent et al. 1997). Systematic evaluations by cranial MRI studies of the cerebellum in individuals with NPHP to identify the MTS have not been described. Presumably, other children with NPHP and either SLS or learning delays have been screened for NPHP1 deletions, and some may have had this molecular abnormality without being considered as affected with JS. This lack of diagnostic assignment to JS may be owing to either lack of MRI studies or failure to identify the MTS even if an MRI scan was obtained. In our NPHP heterozygous control subject (K89-1), with a known intragenic frameshift mutation of NPHP1 in association with the classic deletion, an essentially normal cerebellum is present on MRI without the MTS. Thus, there is likely to be a spectrum of cerebellar findings on MRI in subjects with NPHP1-associated NPHP, with the majority not exhibiting a typical MTS. Until comprehensive surveys of brain imaging studies of patients with NPHP are undertaken, the question of what proportion of subjects with only NPHP manifests an MTS will remain unresolved.
One of the questions that arises from these results is whether the NPHP1 deletion in JS patients is different from that described in patients with NPHP alone. On the basis of the available evidence, given the current limitations of mapping breakpoints within a complex repetitive region of the human genome, the deletion appears to be identical to the classic deletion in patients with NPHP. Although three genes reside within the unique deleted NPHP1 locus (LOC205251, NPHP1, and BENE), only NPHP1 has been implicated in the etiology of NPHP, and no differences in the extent of the deletion between subjects were identified by STS mapping. A more subtle or complex rearrangement, such as a second larger heterozygous deletion, cannot be excluded by the current analysis. Such a heterozygous deletion in combination with the common deletion may expand the disease phenotype because of the deletion of one (or more) dosage-sensitive gene(s). However, support for a common shared deletion that results in a broad clinical phenotype is provided by identification of the identical deletion in subjects who have NPHP with and without OMA (in both cases, some subjects are heterozygous for a deletion and a point mutation) (Saunier et al. 1997b; Betz et al. 2000) and by large genomic deletions mediated by duplicated repetitive elements that cause disorders, such as Charcot-Marie-Tooth syndrome and neurofibromatosis type 1, with highly variable phenotypes despite a common mechanism (Stankiewicz and Lupski 2002).
If the NPHP1 deletion is the same in NPHP, JS, and other CORS, what accounts for the widely divergent phenotypes? One possibility is that the other ORFs within the NPHP1 deleted region may contribute to the CORS phenotypes through unknown genetic or epigenetic factors, as their functions have not yet been completely elucidated. One of these genes, BENE, appears to encode a protein involved in membrane traffickingin apical cells of various tissues, including the renalepithelium (de Marco et al. 2001).
Alternatively, other as-yet unknown genes outside of the 2q13 deletion may work in concert with NPHP1 to contribute to OMA or to the MTS brain malformation and other clinical features that typify JS and related disorders. The nephrocystin protein encoded by NPHP1 is hypothesized to be important in mediating cell adhesion and signal transduction in the ciliated kidney epithelium (Konrad et al. 1996; Hildebrandt et al. 1997a; Otto et al. 2003) and interacts with the NPHP2, NPHP3, and NPHP4 proteins in a multimeric complex (Mollet et al. 2002; Olbrich et al. 2003; Otto et al. 2003). These other NPHP genes may contribute to the CORS phenotype via rare polymorphisms, thereby acting as potential genetic modifiers. Alternatively, JS, like Bardet-Biedl syndrome, may be due to a complex genetic segregation pattern, known as triallelic inheritance, in which some affected individuals must inherit three, rather than two, separate disease-associated alleles in order to manifest the complete disorder (Katsanis et al. 2001; Beales et al. 2003). Under this hypothesis, another JS locus, in addition to NPHP1, may harbor a mutation that modulates the phenotype of the NPHP1 deletion, generating the JS phenotype. Indirect evidence for this hypothesis is provided by nonconsanguineous pedigrees linked to the NPHP3 locus, in that only one NPHP3 mutation was identified in six of seven families with NPHP(Olbrich et al. 2003), suggesting that other genes (including other NPHP genes) may be providing a second or third mutation necessary to cause disease. In addition, the development of renal disease in mice deficient for the murine ortholog of NPHP3 (pcy) has been shown to be highly dependent on modifier loci, with 37% of the variance in renal cyst phenotype owing to a single locus in the region of the mouse NPHP2 gene (Woo et al. 1997; Otto et al. 2003). Thus, it is highly likely that modifier loci influence the known phenotypic variability in human renal cystic disorders. Since there is known genetic heterogeneity in JS, complex inheritance remains a formal possibility but is not testable until additional causative genes for JS are identified. An obvious next step is to examine the other recently identified NPHP genes for mutations in patients with JS.
The evidence for a role of NPHP1 in brain development remains unexplored. Although known to be expressed in a wide variety of tissues that includes skeletal muscle, heart, pancreas, and brain in addition to kidney, the temporospatial and developmental expression in the cerebellum has not been documented. It is possible that NPHP1, in addition to its hypothesized roles in cell adhesion and primary ciliary function in the epithelia of the kidney and retina (Caridi et al. 1998; Otto et al. 2002, 2003; Olbrich et al. 2003), may also have a similar function in cells important in embryonic brain development. Other examples of proteins with related functions are those whose genes cause conditions, such as Bardet-Biedl syndrome and other hepatorenal fibrocystic syndromes, for which common genetic mechanisms are being clarified (reviewed in Johnson et al. [2003] and in Watnick and Germino [2003]).
Finally, any child with the MTS and either JS, Cogan syndrome, SLS, or NPHP should be considered for deletion analysis of the NPHP1 gene. Subjects with OMA or JS are at risk for the development of NPHP, and routine screening for renal compromise is recommended in this cohort (Parisi and Glass 2003). It is hoped that as additional causative genes for JS and related CORS are identified, the association of specific phenotypes with known genotypes will be elucidated.
Acknowledgments
We thank the patients and their families, for generously donating DNA samples and clinical information, and the Joubert Syndrome Foundation, for support of this research. M.A.P. is supported by National Institutes of Health grant K23 NS45832. We acknowledge Richard Peet and Huy Huynh,for cell-culture expertise and technical assistance, and KarenBarnett, for coordinating the genetic studies.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- dbSNP, http://www.ncbi.nlm.nih.gov/SNP/ (for SNP numbers rs2271245, rs2271244, rs3817140, rs1509417, and ss22970415)
- GeneReviews at GeneTests-GeneClinics, http://www.geneclinics.org or http://www.genetests.org
- Joubert Syndrome Foundation, http://www.joubertsyndrome.org
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/
- UCSC human genome browser, http://genome.ucsc.edu/cgi-bin/hgGateway?org=human
References
- Antignac C, Kleinknecht C, Habib R (1998) Nephronophthisis. In: Cameron D, Davison AM, Cameron JS, Grunfeld J-P, Kerr DNS, Ritz E, Winearls G (eds) Clinical nephrology. Oxford University Press, Oxford and New York, pp 2417–2426 [Google Scholar]
- Apostolou T, Nikolopoulou N, Theodoridis M, Koumoustiotis V, Pavlopoulou E, Chondros D, Billis A (2001) Late onset of renal disease in nephronophthisis with features of Joubert syndrome type B. Nephrol Dial Transplant 16:2412–2415 10.1093/ndt/16.12.2412 [DOI] [PubMed] [Google Scholar]
- Beales PL, Badano JL, Ross AJ, Ansley SJ, Hoskins BE, Kirsten B, Mein CA, Froguel P, Scambler PJ, Lewis RA, Lupski JR, Katsanis N (2003) Genetic interaction of BBS1 mutations with alleles at other BBS loci can result in non-Mendelian Bardet-Biedl syndrome. Am J Hum Genet 72:1187–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CL, Meuleman J, Chance PF, Glass IA (2003) Clinical and genetic aspects of the Joubert syndrome: a disorder characterised by cerebellar vermian hypoplasia and accompanying brainstem malformations. Curr Genomics 4:123–129 [Google Scholar]
- Betz R, Rensing C, Otto E, Mincheva A, Zehnder D, Lichter P, Hildebrandt F (2000) Children with ocular motor apraxia type Cogan carry deletions in the gene (NPHP1) for juvenile nephronophthisis. J Pediatr 136:828–831 10.1067/mpd.2000.106225 [DOI] [PubMed] [Google Scholar]
- Boltshauser E, Isler W (1977) Joubert syndrome: episodic hyperpnea, abnormal eye movements, retardation and ataxia, associated with dysplasia of the cerebellar vermis. Neuropadiatrie 8:57–66 [DOI] [PubMed] [Google Scholar]
- Caridi G, Dagnino M, Miglietti N, Carrea A, Perfumo F, Gusmano R, Ghiggeri GM (2001) Juvenile nephronophthisis and related variants: clinical features and molecular approach. In: Schieppati A, Daina E, Sessa A, Remuzzi G (eds) Rare kidney diseases, vol 136. Karger, Basel, pp 57–67 [DOI] [PubMed] [Google Scholar]
- Caridi G, Murer L, Bellantuono R, Sorino P, Caringella DA, Gusmano R, Ghiggeri GM (1998) Renal-retinal syndromes: association of retinal anomalies and recessive nephronophthisis in patients with homozygous deletion of the NPH1 locus. Am J Kidney Dis 32:1059–1062 [DOI] [PubMed] [Google Scholar]
- Chance PF, Cavalier L, Satran D, Pellegrino JE, Koenig M, Dobyns WB (1999) Clinical nosologic and genetic aspects of Joubert and related syndromes. J Child Neurol 14:660–666 [DOI] [PubMed] [Google Scholar]
- de Marco MC, Kremer L, Albar JP, Martinez-Menarguez JA, Ballesta J, Garcia-Lopez MA, Marazuela M, Puertollano R, Alonso MA (2001) BENE, a novel raft-associated protein of the MAL proteolipid family, interacts with caveolin-1 in human endothelial-like ECV304 cells. J Biol Chem 276:23009–23017 10.1074/jbc.M009739200 [DOI] [PubMed] [Google Scholar]
- Dekaban AS (1969) Hereditary syndrome of congenital retinal blindness (Leber), polycystic kidneys and maldevelopment of the brain. Am J Ophthalmol 68:1029–1037 [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Keeler LC, Parisi MA, Marsh SE, Chance PF, Glass IA, Graham JM Jr, Maria BL, Barkovich AJ, Dobyns WB (2004) Molar tooth sign of the midbrain-hindbrain junction: occurrence in multiple distinct syndromes. Am J Med Genet 125A:125–134 [DOI] [PubMed] [Google Scholar]
- Heninger E, Otto E, Imm A, Caridi G, Hildebrandt F (2001) Improved strategy for molecular genetic diagnostics in juvenile nephronophthisis. Am J Kidney Dis 37:1131–1139 [DOI] [PubMed] [Google Scholar]
- Hildebrandt F, Nothwang HG, Vossmerbaumer U, Springer C, Strahm B, Hoppe B, Keuth B, Fuchshuber A, Querfeld U, Neuhaus TJ, Brandis M (1998) Lack of large, homozygous deletions of the nephronophthisis 1 region in Joubert syndrome type B. Pediatr Nephrol 12:16–19 10.1007/s004670050394 [DOI] [PubMed] [Google Scholar]
- Hildebrandt F, Otto E, Rensing C, Nothwang HG, Vollmer M, Adolphs J, Hanusch H, Brandis M (1997a) A novel gene encoding an SH3 domain protein is mutated in nephronophthisis type 1. Nat Genet 17:149–153 [DOI] [PubMed] [Google Scholar]
- Hildebrandt F, Rensing C, Betz R, Sommer U, Birnbaum S, Imm A, Omran H, Leipoldt M, Otto E, APN Study Group (2001) Establishing an algorithm for molecular genetic diagnostics in 127 families with juvenile nephronophthisis. Kidney Int 59:434–445 10.1046/j.1523-1755.2001.059002434.x [DOI] [PubMed] [Google Scholar]
- Hildebrandt F, Strahm B, Nothwang H-G, Gretz N, Schnieders B, Singh-Sawhney I, Kutt R, Vollmer M, Brandis M (1997b) Molecular genetic identification of families with juvenile nephronophthisis type 1: rate of progression to renal failure. APN Study Group. Kidney Int 51:261–269 [DOI] [PubMed] [Google Scholar]
- Johnson CA, Gissen P, Sergi C (2003) Molecular pathology and genetics of congenital hepatorenal fibrocystic syndromes. J Med Genet 40:311–319 10.1136/jmg.40.5.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert M, Eisenring JJ, Robb JP, Andermann F (1969) Familial agenesis of the cerebellar vermis: a syndrome ofepisodic hyperpnea, abnormal eye movements, ataxia, and retardation. Neurology 19:813–825 [DOI] [PubMed] [Google Scholar]
- Katsanis N, Ansley SJ, Badano JL, Eichers ER, Lewis RA, Hoskins BE, Scambler PJ, Davidson WS, Beales PL, Lupski JR (2001) Triallelic inheritance in Bardet-Biedl syndrome, a Mendelian recessive disorder. Science 293:2256–2259 10.1126/science.1063525 [DOI] [PubMed] [Google Scholar]
- Keeler LC, Marsh SE, Leeflang EP, Woods CG, Sztriha L, Al-Gazali L, Gururaj A, Gleeson JG (2003) Linkage analysis in families with Joubert syndrome plus oculo-renal involvement identifies the CORS2 locus on chromosome 11p12-q13.3. Am J Hum Genet 73:656–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MD, Dudgeon J, Stephenson JB (1984) Joubert’s syndrome with retinal dysplasia: neonatal tachypnoea as the clue to a genetic brain-eye malformation. Arch Dis Child 59:709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad M, Saunier S, Heidet L, Silbermann F, Benessy F,Calado J, Le Paslier D, Broyer M, Gubler M-C, Antignac C (1996) Large homozygous deletions of the 2q13 region are a major cause of juvenile nephronophthisis. Hum Mol Genet 5:367–371 10.1093/hmg/5.3.367 [DOI] [PubMed] [Google Scholar]
- Løken AC, Hanssen O, Halvorsen S, Jolster NJ (1961) Hereditary renal dysplasia and blindness. Acta Paediatr 50:177–184 [DOI] [PubMed] [Google Scholar]
- Maria BL, Boltshauser E, Palmer SC, Tran TX (1999a) Clinical features and revised diagnostic criteria in Joubert syndrome. J Child Neurol 14:583–590 [DOI] [PubMed] [Google Scholar]
- Maria BL, Hoang KB, Tusa RJ, Mancuso AA, Hamed LM, Quisling RG, Hove MT, Fennell EB, Booth-Jones M, Ringdahl DM, Yachnis AT, Creel G, Frerking B (1997) “Joubert syndrome” revisited: key ocular motor signs with magnetic resonance imaging correlation. J Child Neurol 12:423–430 [DOI] [PubMed] [Google Scholar]
- Maria BL, Quisling RG, Rosainz LC, Yachnis AT, Gitten JC, Dede DE, Fennell E (1999b) Molar tooth sign in Joubert syndrome: clinical, radiologic, and pathologic significance. J Child Neurol 14:368–376 [DOI] [PubMed] [Google Scholar]
- Mollet G, Salomon R, Gribouval O, Silbermann F, Bacq D, Landthaler G, Milford D, Nayir A, Rizzoni G, Antignac C, Saunier S (2002) The gene mutated in juvenile nephronophthisis type 4 encodes a novel protein that interacts with nephrocystin. Nat Genet 32:300–305 10.1038/ng996 [DOI] [PubMed] [Google Scholar]
- Nothwang HG, Stubanus M, Adolphs J, Hanusch H, Vossmerbaumer U, Denich D, Kubler M, Mincheva A, Lichter P, Hildebrandt F (1998) Construction of a gene map of the nephronophthisis type 1 (NPHP1) region on human chromosome 2q12-q13. Genomics 47:276–285 10.1006/geno.1997.5102 [DOI] [PubMed] [Google Scholar]
- Olbrich H, Fliegauf M, Hoefele J, Kispert A, Otto E, Volz A, Wolf MT, Sasmaz G, Trauer U, Reinhardt R, Sudbrak R, Antignac C, Gretz N, Walz G, Schermer B, Benzing T, Hildebrandt F, Omran H (2003) Mutations in a novel gene, NPHP3, cause adolescent nephronophthisis, tapeto-retinal degeneration and hepatic fibrosis. Nat Genet 34:455–459 10.1038/ng1216 [DOI] [PubMed] [Google Scholar]
- Otto E, Hoefele J, Ruf R, Mueller AM, Hiller KS, Wolf MT, Schuermann MJ, Becker A, Birkenhager R, Sudbrak R, Hennies HC, Nurnberg P, Hildebrandt F (2002) A gene mutated in nephronophthisis and retinitis pigmentosa encodes a novel protein, nephroretinin, conserved in evolution. Am J Hum Genet 71:1161–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto EA, Schermer B, Obara T, O’Toole JF, Hiller KS, Mueller AM, Ruf RG, Hoefele J, Beekmann F, Landau D, Foreman JW, Goodship JA, Strachan T, Kispert A, Wolf MT, Gagnadoux MF, Nivet H, Antignac C, Walz G, Drummond IA, Benzing T, Hildebrandt F (2003) Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet 34:413–420 10.1038/ng1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi MA, Glass IA (2003) Joubert syndrome. In: GeneReviews at GeneTests-GeneClinics: medical genetics information resource [online database]. University of Washington, Seattle [PubMed] [Google Scholar]
- Saar K, Al-Gazali L, Sztriha L, Rueschendorf F, Nur E, Kamal M, Reis A, Bayoumi R (1999) Homozygosity mapping in families with Joubert syndrome identifies a locus on chromosome 9q34.3 and evidence for genetic heterogeneity. Am J Hum Genet 65:1666–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva JM, Baraitser M (1992) Joubert syndrome: a review. Am J Med Genet 43:726–731 [DOI] [PubMed] [Google Scholar]
- Sargent MA, Poskitt KJ, Jan JE (1997) Congential ocular motor apraxia: imaging findings. Am J Neuroradiol 18:1915–1922 [PMC free article] [PubMed] [Google Scholar]
- Satran D, Pierpont ME, Dobyns WB (1999) Cerebello-oculo-renal syndromes including Arima, Senior-Loken and COACH syndromes: more than just variants of Joubert syndrome. Am J Med Genet 86:459–469 [DOI] [PubMed] [Google Scholar]
- Saunier S, Calado J, Benessy F, Silbermann F, Heilig R, Weissenbach J, Antignac C (2000) Characterization of the NPHP1 locus: mutational mechanism involved in deletions in familial juvenile nephronophthisis. Am J Hum Genet 66:778–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunier S, Calado J, Heilig R, Silbermann F, Benessy F, Morin G, Konrad M, Broyer M, Gubler M-C, Weissenbach J, Antignac C (1997a) A novel gene that encodes a protein with a putative src homology 3 domain is a candidate gene for familial juvenile nephronophthisis. Hum Mol Genet 6:2317–2323 10.1093/hmg/6.13.2317 [DOI] [PubMed] [Google Scholar]
- Saunier S, Morin G, Calado J, Benessy F, Silbermann F,Antignac C (1997b) Large deletions of the NPH1 region in Cogan syndrome (CS) associated with familial juvenile nephronophthisis (NPH). Am J Hum Genet 61A:346 [Google Scholar]
- Senior B, Friedmann AI, Braudo JL (1961) Juvenile familial nephropathy with tapetoretinal degeneration. Am J Ophthalmol 52:625–633 [DOI] [PubMed] [Google Scholar]
- Stankiewicz P, Lupski JR (2002) Genome architecture, rearrangements and genomic disorders. Trends Genet 18:74–82 10.1016/S0168-9525(02)02592-1 [DOI] [PubMed] [Google Scholar]
- Takano K, Nakamoto T, Okajima M, Sudo A, Uetake K, Saitoh S (2003) Cerebellar and brainstem involvement in familial juvenile nephronophthisis type I. Pediatr Neurol 28:142–144 10.1016/S0887-8994(02)00619-7 [DOI] [PubMed] [Google Scholar]
- Tusa RJ, Hove MT (1999) Ocular and oculomotor signs in Joubert syndrome. J Child Neurol 14:621–627 [DOI] [PubMed] [Google Scholar]
- Valente EM, Salpietro DC, Brancati F, Bertini E, Galluccio T, Tortorella G, Briuglia S, Dallapiccola B (2003) Description, nomenclature, and mapping of a novel cerebello-renal syndrome with the molar tooth malformation. Am J Hum Genet 73:663–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassella F, Lutschg J, Mumenthaler M (1972) Cogan’s congenital ocular motor apraxia in two successive generations. Dev Med Child Neurol 14:788–803 [DOI] [PubMed] [Google Scholar]
- Watnick T, Germino G (2003) From cilia to cyst. Nat Genet 34:355–356 10.1038/ng0803-355 [DOI] [PubMed] [Google Scholar]
- Whitsel EA, Castillo M, D’Cruz O (1995) Cerebellar vermis and midbrain dysgenesis in oculomotor apraxia: MR findings. Am J Neuroradiol 16:831–834 [PMC free article] [PubMed] [Google Scholar]
- Woo DD, Nguyen DK, Khatibi N, Olsen P (1997) Genetic identification of two major modifier loci of polycystic kidney disease progression in pcy mice. J Clin Invest 100:1934–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]