Abstract
Metaphase karyotype analysis of fetal cells obtained by amniocentesis or chorionic villus sampling is the current standard for prenatal cytogenetic diagnosis, particularly for the detection of trisomy 21. We previously demonstrated that large quantities of cell-free fetal DNA (cffDNA) are easily extracted from amniotic fluid (AF). In this study, we explored potential clinical applications of AF cffDNA by testing its ability to hybridize to DNA microarrays for comparative genomic hybridization (CGH) analysis. cffDNA isolated from 11 male fetuses showed significantly increased hybridization signals on SRY and decreased signals on X-chromosome markers, compared with female reference DNA. cffDNA isolated from six female fetuses showed the reverse when compared with male reference DNA. cffDNA from three fetuses with trisomy 21 had increased hybridization signals on the majority of the chromosome 21 markers, and cffDNA from a fetus with monosomy X (Turner syndrome) had decreased hybridization signals on most X-chromosome markers, compared with euploid female reference DNA. These results indicate that cffDNA extracted from AF can be analyzed using CGH microarrays to correctly identify fetal sex and aneuploidy. This technology facilitates rapid screening of samples for whole-chromosome changes and may augment standard karyotyping techniques by providing additional molecular information.
Definitive prenatal cytogenetic diagnosis is currently limited to metaphase karyotype analysis of cultured cells obtained by amniocentesis or chorionic villus sampling. Because amniotic fluid (AF) samples contain predominantly dying cells, ∼1–2 wk are required to promote expansion of the generally low number of viable cells for metaphase analysis. When particular fetal genetic abnormalities are suspected, additional aberrations—such as deletions, duplications or translocations—can be evaluated using FISH analysis with specific DNA probes (Pergament 2000; Hulten et al. 2003). Less routinely, the gene or chromosome in question can be targeted with PCR on DNA extracted from cultured or uncultured amniocytes (Fredrickson et al. 1999). Newer molecular techniques, such as comparative genomic hybridization (CGH) microarray analysis, have potential clinical applications for rapid and detailed high-resolution genomic analysis of uncultured fetal genetic material. However, experimentation on primary amniocytes is not practical, because, in normal pregnancies, only ∼10–30 ml can safely be removed from the fetal sac, and the small numbers of amniocytes obtained are required for the indicated cytogenetic testing and are therefore not available for research purposes. Typically, after removal of cells from the sample, several milliliters of AF supernatant are analyzed for the levels of cell-free proteins, which can serve as biomarkers for genetic abnormalities. The remaining supernatant is normally discarded.
Cell-free fetal DNA (cffDNA) is a source of nucleic acids that is readily available in the AF supernatant (Bianchi et al. 2001). We hypothesized that extraction, fluorescent labeling, hybridization, and analysis of AF cffDNA could be used to simultaneously screen every chromosome for aneuploidy, as well as any selected set of genetic loci for subtle aberrations, such as gains or deletions, on CGH microarrays. Thus, this microarray-based approach could provide higher resolution, higher sensitivity, and more specific localization (within 100–200 kb) of abnormalities in the fetal genome than the standard metaphase karyotype obtained from cultured amniocytes, which is generally limited to the pattern recognition of ∼450 Giemsa-stained bands. In the present feasibility study, we aimed to detect whole-chromosome differences between AF cffDNA samples by analysis of differential hybridization patterns of markers on chromosomes X, Y, and 21 in female, male, euploid, and aneuploid fetuses. Approval for this study was obtained from the institutional review boards of Tufts–New England Medical Center and Women and Infants' Hospital to anonymously use discarded AF supernatant samples and amniocytes from the clinical cytogenetics laboratories.
Frozen residual AF supernatant samples were obtained from 46 second-trimester pregnant women carrying euploid fetuses and 26 women carrying aneuploid fetuses with known cytogenetic karyotypes. Residual amniocytes for eight of the euploid samples were also obtained from the clinical cytogenetics laboratory, after culture and routine karyotyping were complete. cffDNA was extracted from each sample, by use of the Blood and Body Fluid Vacuum Protocol (Qiagen), which was modified for large volumes. For each 10 ml of AF, the following were used: 1 ml of protease, 10 ml of buffer AL (Qiagen lysis buffer), and 10 ml of 100% ethanol. The QIAvac vacuum manifold (Qiagen) was fitted with 60 ml syringes to accommodate the large volumes. DNA was extracted from the cultured residual amniocytes using the “Protocol for Cultured Cells Appendix” of the Blood and Body Fluid Spin Protocol (Qiagen). Quantitation of all samples prior to hybridization to GenoSensor microarrays (Vysis) was performed using Hoechst H 33258 dye (Molecular Probes), according to the manufacturer’s protocol. Samples containing at least 100 ng of DNA were selected for hybridization to arrays, including 28 total cffDNA samples (19 euploid and 9 aneuploid) and the 8 corresponding euploid amniocyte DNA samples. Study design, sample selection by known karyotype, and DNA extractions were performed at Tufts–New England Medical Center. DNA samples were then sent in a blinded fashion to Vysis for hybridization to arrays and analysis of data without knowledge of fetal karyotype.
For microarray CGH analysis, 100 ng each of AF “test” DNA sample and normal reference DNA were labeled with Cyanine 3-dCTP and Cyanine 5-dCTP (Perkin Elmer), respectively, by direct incorporation methods with the GenoSensor Random Priming DNA labeling kit (Vysis). Samples were digested using DNase I to reduce the size of the fragments to ∼200–800 bp. DNA was purified with MicroSpin S-200 Columns (Amersham Biosciences) to remove unincorporated nucleotides and primers and then was precipitated with ethanol. The quality and quantity of labeled, fragmented DNA was assessed by electrophoresis of a small fraction of the labeled material on a 2% agarose gel. Next, each sample of labeled test and reference DNA was combined with the preformulated Microarray Hybridization Buffer (Vysis), was denatured, and was hybridized to a GenoSensor Array 300 according to the manufacturer’s protocol. After hybridization, microarrays were subjected to three 50% Formamide 2× SSC washes and four 1× SSC washes to remove unhybridized and nonspecifically bound probe. A cover slip with 4′,6-diamidino-2-phenylindole (DAPI) mounting solution was then applied to each microarray. DAPI stain was used for segmentation by the GenoSensor software, while aqueous mounting solution protected fluorescent signal from degradation. The GenoSensor Reader (Vysis) was utilized to capture the resulting fluorescence, and the GenoSensor analysis software (Vysis) was used for data acquisition, preprocessing, and analysis.
The GenoSensor Array 300 is a genomic array with 287 targets, spotted in triplicate, which includes subtelomeric regions, microdeletions, and other loci of interest, allowing for rapid fine mapping of regions of gained or lost DNA sequence. All samples were hybridized with female reference DNA isolated from a normal female donor. In early feasibility experiments, all test specimens were also run against male reference DNA samples. In later experiments, as techniques improved, test samples were run against female reference DNA only to identify male fetuses, under the assumption that samples from female fetuses would show no sex-chromosome differences compared to euploid female reference DNA.
The GenoSensor software segmented and identified each target by use of the blue (DAPI) image plane. Mean intensities were measured from the green and red image planes, background was subtracted, a mean ratio of green/red signal was determined, and the ratios were normalized. The normalized ratio for each target was calculated relative to the modal DNA copy number, and the statistical significance of each change was reported as a P value (Piper et al. 2002). A P value of <.01 indicated a significant difference between the copy numbers of a target and the modal clones. The data were exported from GenoSensor software as Excel (Microsoft) files, and JMP 5.0.1.2 software (SAS Institute) was used to process and summarize the data (see table A [online only], a tab-delimited ASCII file that can be imported into a spreadsheet, for raw array data, and the associated note). All data analysis was performed blindly, without knowledge of fetal karyotype.
Data are presented for the informative 17 of 28 microarrays hybridized with cffDNA extracted from AF and for 7 of 8 microarrays hybridized with DNA extracted from residual cultured amniocytes. Eleven cffDNA arrays were uninformative, but the success of hybridization improved during the course of the study. The karyotypes for the 17 cffDNA samples were 46,XX (4), 46,XY (9), 47,XY,+21 (2), 47,XX,+21 (1), and 45,X (1). Of the 17 samples in this group, 7 had corresponding cellular samples. Figures 1, 2, and 3 show data from all 17 cffDNA samples, representing chromosomes X, Y, and 21 for each of these microarrays. All nine samples from euploid male fetuses had increased hybridization-signal intensity for SRY and decreased signal intensity for X-chromosome markers compared with female reference DNA, represented by statistically significant changes in Cyanine 3 (test) and Cyanine 5 (reference) signal intensity ratios (P<.01). Samples from all four euploid female fetuses had significantly decreased hybridization signals for SRY and increased hybridization signals for X chromosome markers compared with male reference DNA (P<.01). As expected, when samples were compared with reference DNA of the same sex, there was no significant difference in hybridization-signal intensity for either the X or Y chromosome.
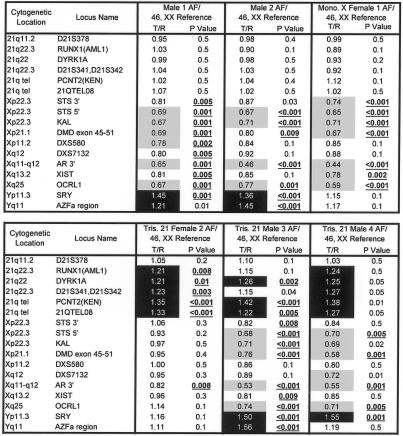
Figure 1.
Microarray data from two euploid and four aneuploid cffDNA AF samples. Data show the expected ratio differences for clones from chromosomes X, Y, and 21, when sample genomes are compared with a normal female genome. Samples are labeled by sex and number, followed by the karyotype of the reference DNA used for hybridization. All samples were hybridized with normal female reference DNA. Female 1 had monosomy X (Turner syndrome). Female 2 and males 3 and 4 had trisomy 21. A subset of GenoSensor Array 300 clones (Vysis), including markers on chromosomes 21, X, and Y, is shown for each array result. T/R = target DNA to reference euploid DNA ratio of Cyanine 3 (test) and Cyanine 5 (reference) fluorescent intensities (background corrected and normalized). Markers with increased copy numbers (>1.2) are highlighted in black, and markers with decreased copy numbers (<0.8) are highlighted in gray. Copy number changes with P values of <.01 are considered significant and are underlined and shown in bold.
Figure 2.
Comparison data for four euploid cffDNA AF samples, each hybridized separately with male and female reference DNA. Data show the expected ratio differences for clones from chromosomes X, Y, and 21, when sample genomes are compared with both a normal male genome and a normal female genome. Samples are labeled by sex and number, followed by the karyotype of the reference DNA used for hybridization. A subset of GenoSensor Array 300 (Vysis) clones, including markers on chromosomes 21, X, and Y, is shown for each array result. T/R = target DNA to reference euploid DNA ratio of fluorescent intensities (background corrected and normalized). Markers with increased copy numbers (>1.2) are highlighted in black, and markers with decreased copy numbers (<0.8) are highlighted in gray. Copy number changes with P values of <.01 are considered significant and are underlined and shown in bold.
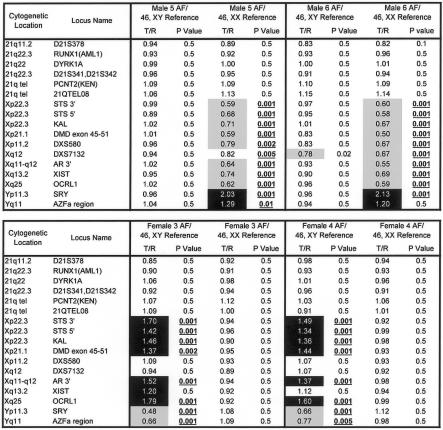
Figure 3.
Comparison data for seven euploid cffDNA AF samples and their corresponding amniocyte (cellular) DNA. Data show the expected ratio differences for clones from chromosomes X, Y, and 21, when genomes from cffDNA and genomes from cellular DNA are compared with a normal female genome. cffDNA hybridized to the arrays nearly as well as did the DNA extracted from whole cells. Samples are labeled by sex and number, followed by the karyotype of the reference DNA used for hybridization. All samples were hybridized with normal female reference DNA. A subset of GenoSensor Array 300 (Vysis) clones, including markers on chromosomes 21, X, and Y, is shown for each array result. T/R = target DNA to reference euploid DNA ratio of fluorescent intensities (background corrected and normalized). Markers with increased copy numbers (>1.2) are highlighted in black, and markers with decreased copy numbers (<0.8) are highlighted in gray. Copy number changes with P values of <.01 are considered significant and are underlined and shown in bold.
Figure 1 shows data from two euploid and four aneuploid cffDNA samples. For all 13 euploid fetal samples (11 others shown in figs. 2 and 3), markers on chromosome 21 were not significantly different from euploid reference DNA. However, the three fetal samples with trisomy 21 had increased ratios of target-to-reference intensities on most chromosome 21 markers (fig. 1). The fetal sample with monosomy X had decreased hybridization signals on seven of nine X-chromosome markers compared with euploid female reference DNA (fig. 2). Figure 2 shows array data obtained when four euploid cffDNA samples were hybridized separately with either male or female reference DNA. Figure 3 shows comparison data from euploid samples in which both AF cffDNA and DNA from the corresponding amniocytes were hybridized separately to the arrays.
When the hybridization performance of cffDNA samples was compared with samples of DNA isolated from their corresponding amniocytes, the cffDNA and cellular DNA samples were all informative for sex, but cffDNA samples had higher clone-clone variability (noise). Noise in the samples was assessed using the median adjacent-clone ratio difference (MACRD) criterion, calculated by determining the median of the absolute Cyanine 3–to–Cyanine 5 fluorescent intensity-ratio difference between cytogenetically adjacent clones. This measure should normally be small. Currently, the “desirable” MACRD recommended by GenoSensor analysis software for a high-quality assay is <0.065 (Vysis, unpublished data). Higher MACRDs indicate poor-quality hybridization, since adjacent clone pairs have similar ratios in the vast majority of cases. On average, the MACRDs for DNA isolated from amniocytes were ⩽0.065, whereas cffDNA samples exhibited values of 0.05–0.084. Although MACRDs were higher for some cffDNA samples than for cellular DNA, in cffDNA samples, the sensitivity of detection of chromosome-21, -X, and -Y markers, measured by normalized target/reference ratios of fluorescent intensities and P values, was similar, and quality values of array parameters, including mean intratarget coefficient of variation and modal distribution of SD, were at or below the acceptable cutoffs established from multiple sets of hybridizations done at Vysis for quality criteria development.
Our results indicate that cffDNA extracted from AF can be analyzed using CGH microarrays to correctly identify fetal sex and whole-chromosome gains or losses, such as trisomy 21 and monosomy X. To date, no other study has utilized DNA from the cell-free fraction of AF for prenatal molecular diagnosis. cffDNA has the advantage of being readily available from the portion of AF that is normally discarded. Thus, it can be used in conjunction with standard karyotyping and will not interfere with the current standard of care or compromise fetal health. In addition, it does not require the time-consuming expansion of cultured cells but can be performed immediately after the specimen is received, providing a more rapid diagnosis.
Microarray CGH analysis has several advantages over traditional cytogenetic karyotyping. It is more sensitive for the detection of small genomic changes not revealed by standard G-banding methods, such as microdeletions and microduplications, and can be used to screen large panels of selected genes of interest (Pinkel et al. 1998; Pollack et al. 1999; Veltman et al 2002; Vissers et al. 2003). Array CGH analysis also reduces subjectivity inherent in G-banding and provides statistical proof of the likelihood of the presence of an abnormality in the fetus being tested.
The potential for molecular genetic screening of fetuses is large. Ultimately, this technology could be used for genomewide screening of submicroscopic DNA copy number changes, including rearrangements of subtelomeres, which are a recently recognized cause of mental retardation (Flint et al. 1995; Knight et al. 1999; Biesecker 2002; deVries et al 2003). Array CGH technology has been shown elsewhere to be robust for detection of microdeletions and microduplications in patients with an apparently normal metaphase karyotype, by use of DNA from blood lymphocytes (Vissers et al. 2003). Prenatal array CGH analysis of AF could thus provide more rapid and comprehensive information about the fetal genome than is currently available from standard metaphase karyotyping.
The performance of cffDNA for microarray analysis was compared with DNA isolated from cultured amniocytes from some of the same fetuses, to assess hybridization efficiency of this novel source of fetal DNA. cffDNA showed similar performance in hybridization to microarrays compared with cellular DNA, but it did have more clone-clone variability (MACRD, or noise)—which could be due to inherent degradation of cffDNA causing inefficient labeling—making these samples less reliable than cellular DNA.
cffDNA appears to have some different properties from DNA from whole cells. One study demonstrated that cell-free DNA in plasma is made up of very short fragments, and 79% of cffDNA in maternal plasma is <313 bp in length (Chan et al. 2004). The size distribution of cffDNA in AF is unknown but likely also comprises small fragments due to apoptosis. DNA degradation is also a potential problem because of freezer storage time. However, an earlier study showed no freezer effect on degradation of cffDNA in AF, whereas cffDNA in maternal plasma degraded by 0.6 genome equivalents/ml/month (Lee et al. 2002). In the current study, the samples of DNA isolated from whole cells were also frozen prior to use, and they still hybridized well to the arrays. Therefore, degradation from freezing is probably not a major factor affecting performance of cffDNA.
In summary, molecular analysis of cffDNA from AF by use of CGH microarray technology is a promising technique that allows for rapid screening of samples for whole-chromosome changes, including aneuploidy, and may augment standard karyotyping techniques for prenatal genetic diagnosis. This technology may aid the discovery and description of minor genetic aberrations, such as microdeletions and microduplications, which will potentially enhance future prenatal genetic diagnostic applications. Further investigation is warranted to explore the clinical significance of the detection of submicroscopic genetic rearrangements in the developing fetus.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grant R01 HD42053 (to D.W.B.).
Electronic-Database Information
The URL for data presented herein is as follows:
- Vysis, Inc., http://www.vysis.com/GenoSensorArray300_6615.asp?bhcp=1 (for GenoSensor Array 300)
References
- Bianchi DW, LeShane ES, Cowan JM (2001) Large amounts of cell-free fetal DNA are present in amniotic fluid. Clin Chem 47:1867–1869 [PubMed] [Google Scholar]
- Biesecker LG (2002) The end of the beginning of chromosome ends. Am J Med Genet 107:263–266 10.1002/ajmg.10160.abs [DOI] [PubMed] [Google Scholar]
- Chan KCA, Zhang J, Hui ABY, Wong N, Lau TK, Leung TN, Lo KW, Huang DWS, Lo YMD (2004) Size distributions of maternal and fetal DNA in maternal plasma. Clin Chem 50:88–92 10.1373/clinchem.2003.024893 [DOI] [PubMed] [Google Scholar]
- de Vries BBA, Winter R, Schinzel A, Van Ravenswaaij-Arts C (2003) Telomeres: a diagnosis at the end of the chromosomes. J Med Genet 40:385–398 10.1136/jmg.40.6.385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint J, Wilkie AO, Buckle VJ, Winter RM, Holland AJ, McDermid HE (1995) The detection of subtelomeric chromosomal rearrangements in idiopathic mental retardation. Nat Genet 9:132–140 [DOI] [PubMed] [Google Scholar]
- Fredrickson RM, Wang HS, Surh LC (1999) Some caveats in PCR-based prenatal diagnosis on direct amniotic fluid versus cultured amniocytes. Prenat Diagn 19:113–117 [DOI] [PubMed] [Google Scholar]
- Hulten MA, Dhanjal S, Pertl B (2003) Rapid and simple prenatal diagnosis of common chromosome disorders: advantages and disadvantages of the molecular methods FISH and QF-PCR. Reproduction 126:279–297 10.1530/rep.0.1260279 [DOI] [PubMed] [Google Scholar]
- Knight SJL, Regan R, Nicod A, Horsley SW, Kearney L, Homfray T, Winter RM, Bolton P, Flint J (1999) Subtle chromosomal rearrangements in children with unexplained mental retardation. Lancet 354:1676–1681 10.1016/S0140-6736(99)03070-6 [DOI] [PubMed] [Google Scholar]
- Lee T, LeShane ES, Messerlian GM, Canick JA, Farina A, Heber WW, Bianchi DW (2002) Down syndrome and cell-free fetal DNA in archived maternal serum. Am J Obstet Gynecol 187:1217–1221 10.1067/mob.2002.127462 [DOI] [PubMed] [Google Scholar]
- Pergament E (2000) The application of fluorescence in-situ hybridization to prenatal diagnosis. Curr Opin Obstet Gynecol 12:73–76 10.1097/00001703-200004000-00003 [DOI] [PubMed] [Google Scholar]
- Pinkel D, Segraves R, Sudar D, Clark S, Poole I, Kowbel D, Collins C, Kuo WL, Chen C, Zhai Y, Dairkee SH, Ljung BM, Gray JW, Albertson DG (1998) High resolution analysis of DNA copy number variation using comparative genomic hybridization to microarrays. Nat Genet 20:207–211 10.1038/2524 [DOI] [PubMed] [Google Scholar]
- Piper J, Stegenga S, Pestova E, Marble H, Lucas M, Wilber K, King W (2002) An objective method for detecting copy-number changes in CGH microarray experiments. Paper presented at Proceedings of the Third Euroconference on Quantitative Molecular Cytogenetics, Rosenon, Sweden, July 4–6 [Google Scholar]
- Pollack JR, Perou CM, Alizadeh AA, Eisen MB, Pergamenschikov A, Williams CF, Jeffrey SS, Botstein D, Brown PO (1999) Genome-wide analysis of DNA copy-number changes using cDNA microarrays. Nat Genet 23:41–46 [DOI] [PubMed] [Google Scholar]
- Veltman JA, Schoenmakers EFPM, Eussen BH, Janssen I, Merkx G, van Cleef B, van Ravenswaaij CM, Brunner HG, Smeets D, van Kessel AG (2002) High-throughput analysis of subtelomeric chromosome rearrangements by use of array-based comparative genomic hybridization. Am J Hum Genet 70:1269–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers LELM, de Vries BBA, Osoegawa K, Janssen IM, Feuth T, Choy CO, Straatman H, van der Vliet W, Huys EHLPG, van Rijk A, Smeets D, van Ravenswaaij-Arts CMA, Knoers NV, van der Burgt I, de Jong PJ, Brunner HG, van Kessel AG, Schoenmakers EFPM, Veltman JA (2003) Array-based comparative genomic hybridization for the genomewide detection of submicroscopic chromosomal abnormalities. Am J Hum Genet 73:1261–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.