Abstract
Gilles de la Tourette syndrome (GTS) is characterized by multiple motor and phonic tics and high comorbidity rates with other neurobehavioral disorders. It is hypothesized that frontal-subcortical pathways and a complex genetic background are involved in the etiopathogenesis of the disorder. The genetic basis of GTS remains elusive. However, several genomic regions have been implicated. Among them, 17q25 appears to be of special interest, as suggested by various independent investigators. In the present study, we explored the possibility that 17q25 contributes to the genetic component of GTS. The initial scan of chromosome 17 performed on two large pedigrees provided a nonparametric LOD score of 2.41 near D17S928. Fine mapping with 17 additional microsatellite markers increased the peak to 2.61 (P=.002). The original families, as well as two additional pedigrees, were genotyped for 25 single-nucleotide polymorphisms (SNPs), with a focus on three genes in the indicated region that could play a role in the development of GTS, on the basis of their function and expression profile. Multiple three-marker haplotypes spanning all three genes studied provided highly significant association results (P<.001). An independent sample of 96 small families with one or two children affected with GTS was also studied. Of the 25 SNPs, 3 were associated with GTS at a statistically significant level. The transmission/disequilibrium test for a three-site haplotype moving window again provided multiple positive results. The background linkage disequilibrium (LD) of the region was studied in eight populations of European origin. A complicated pattern was revealed, with the pairwise tests producing unexpectedly high LD values at the telomeric TBCD gene. In conclusion, our findings warrant the further investigation of 17q25 as a candidate susceptibility region for GTS.
Introduction
Gilles de la Tourette syndrome (GTS [MIM %137580]) is a neurodevelopmental disorder with onset in childhood. The phenotype includes the presence of multiple motor and phonic tics that occur in bouts and that wax and wane in severity over a period of days, weeks, or months (Leckman 2002). Tics are sudden habitual movements or vocalizations that typically mimic some fragment of normal behavior and involve discrete muscle groups (Leckman and Riddle 2000). The mean age at onset of the disorder is 7 years (range 2–15 years), and, in uncomplicated cases, the severity of tics peaks early in the 2nd decade of life, with symptoms often showing a striking decline in frequency and severity by age 19 years (Leckman et al. 1998). This suggests that the substrate for GTS is not neurodegeneration; rather, the disorder may be due to features of the developing brain that are present to a lesser degree in the mature nervous system.
Once thought to be as rare as 1–10/10,000, GTS is now considered much more common, with estimated prevalence in the range of 1%–3.8% (Singer 2000; Robertson 2003). This variation among studies can probably be attributed to selection of the target population and ascertainment bias. Tics have the greatest effect on a patient’s self-esteem and peer and family relationships during ages 7–12 years. The high comorbidity of GTS with other behavioral disorders detracts even more from the patient’s quality of life (Spencer et al. 1998; Carter et al. 2000; Elstner et al. 2001; Peterson et al. 2001). Indeed, the behavioral spectrum of GTS and related tic disorders includes obsessive-compulsive (OC) symptoms or even formal obsessive-compulsive disorder (OCD [MIM %164230]), other anxiety disorders, mood disorders, and attention-deficit and disruptive behavior disorders (Kurlan et al. 2002). It seems likely that these disorders share a common or overlapping neurobiological basis.
The neuroanatomic localization of GTS is unknown, but it is currently thought that the pathophysiology of the disorder involves the cortico-striatal-thalamocortical circuits (Singer and Minzer 2003). A popular model of basal ganglia functional anatomy suggests that involuntary movements are associated with decreased inhibitory output from the basal ganglia resulting in excessive activity in fronto-cortical areas (Mink 2001). This model has been invoked to explain several “hyperkinetic” movement disorders (tics, chorea, and dystonia), as well as many psychiatric disorders (schizophrenia, OCD, and depression). It has been hypothesized that the same mechanisms that are involved in habit formation are also involved in tics (Leckman and Riddle 2000).
Several lines of evidence suggest that GTS is an inherited disorder. In twin studies, the concordance rate was 53%–56% in MZ twins versus 8% in DZ twins, indicating a genetic basis of the disorder (Price et al. 1985; Hyde et al. 1992). At the same time, the fact that the concordance rate between MZ twins is not 100% demonstrates the importance of environmental or other nongenetic factors in the pathogenesis of the disorder. First-degree relatives of individuals with GTS have a 10- to 100-fold increased risk of developing the disorder, compared with individuals in the general population (Pauls et al. 1991). Early segregation-analysis studies reported either a pattern consistent with autosomal dominant inheritance (Pauls and Leckman 1986; Eapen et al. 1993) or a model in which the penetrance of heterozygous individuals was intermediate between those of the homozygotes (Hasstedt et al. 1995). More recent studies, however, were interpreted as showing evidence for a significant multifactorial (polygenic) background (Walkup et al. 1996). On the other hand, Seuchter et al. (2000) could not support Mendelian transmission of GTS and related conditions. Nevertheless, the majority of studies suggest that the etiology of GTS has a strong and, most likely, complex genetic component.
Despite the numerous genetic studies undertaken by various groups (reviewed by Pauls [2003] and Singer [2000]) targeting a large number of both large pedigrees and small nuclear families, the genetic basis of GTS has so far remained elusive, accentuating the likelihood of the heterogeneity of the disorder. To date, neuroleptics are the main treatment for GTS, suggesting that a dysfunction in dopaminergic pathways might be implicated in the development of the disorder. Consequently, several genes involved in these pathways have been studied, but their role in the etiopathogenesis of GTS still remains unclear. A positive-association result between the dopamine receptor gene DRD4 (MIM 126452) and GTS has been reported (Grice et al. 1996; Cruz et al. 1997; Díaz-Anzaldúa et al. 2004), but other studies have failed to replicate this result (Brett et al. 1995; Hebebrand et al. 1997; Comings et al. 1999). Positive association was also found between the monoamine oxidase A gene (MAOA [MIM 309850]) and GTS (Gade et al. 1998; Díaz-Anzaldúa et al. 2004). Chromosomal abnormalities in individuals and families with GTS have also been studied in the hope of identifying a gene or genes of major effect that would be disrupted by the rearrangement (Brett et al. 1996; Kroisel et al. 2001; Petek et al. 2001; Crawford et al. 2003; and State et al. 2003, among others). Using this approach, Verkerk et al. (2003) hypothesized that disruption of the contactin-associated protein 2 gene (CNTNAP2 [MIM 604569]) could lead to the GTS phenotype. Simonic et al. (1998), in a genomewide search using a case-control strategy, reported positive associations, with markers in seven regions and, in a subsequent study, provided additional evidence for loci on chromosomes 2, 8, and 11 (Simonic et al. 2001). It is interesting that linkage was found with the same marker on chromosome 11 reported by Simonic et al. in a large French Canadian pedigree that was analyzed by use of a multipoint approach (Mérette et al. 2000).
Whole-genome scans performed either on large families or on families with two affected sibs have provided indications for linkage of GTS with several genomic regions. The results between studies are, however, quite inconsistent. This is believed to be due to uncertainties in the definition of the phenotype, diagnostic assessment, and family ascertainment schemes, as well as a misspecified genetic model used for the data analysis. A partial genome scan in 1991 excluded 50% of the genome, under the assumption of an autosomal dominant gene in all of the families studied (Pakstis et al. 1991). Barr et al. (1999) reported genomewide significant linkage with eight markers, using the affected-pedigree method, a nonparametric approach. In the same study, two of those markers on chromosomes 5 and 19 also gave weak indications of linkage by use of the parametric LOD score. A genome screen of 110 affected sib pairs performed by the Tourette Syndrome Association International Consortium for Genetics (TSAICG [1999]) provided suggestive positive-linkage results with markers on chromosomes 4 and 8. When affected individuals in this study were stratified according to OC symptoms, significant allele sharing was noted for hoarding phenotypes for markers at 4q34-35, 5q35, and 17q25.4 (Zhang et al. 2002).
Among the regions providing indications for linkage to GTS so far, we considered 17q25 to be of particular interest, as suggested by various independent studies and investigators. A genomewide linkage study performed on a large pedigree from Utah gave the highest LOD score (2.2) at marker D17S802 (106 cM from 17pter) (Leppert et al. 1996). The finding of evidence for linkage to marker D17S784 was supported both by the TSAICG (1999) and by Zhang et al. (2002). The TSAICG found a weak peak, with a maximum-likelihood score (MLS) of 0.6, at this marker. However, as mentioned above, Zhang et al. (2002) found a high nonparametric LOD (NPL) score at marker D17S784 (P<.00002) in a subset of affected sib pairs positive for the OC symptom of hoarding. These results indicated that 17q25 deserved further evaluation as a possible GTS-susceptibility locus.
Here, we report results of an initial screen of chromosome 17 and the follow-up fine mapping of the candidate region. Furthermore, the linkage disequilibrium (LD) pattern of the region was studied in samples from eight populations of European ancestry to facilitate the interpretation of our association results and to provide background information for subsequent studies. Linkage analysis was performed initially on two large families with multiple members affected with GTS. Next, a map of increased density of STRPs was typed on a total of four large families, including the two original families, as well as two additional pedigrees. For one of the additional families, additional individuals were added to extend the pedigree structure. To further reduce the large candidate interval, we typed 25 SNPs, focusing on three loci that, according to their expression patterns and function, could constitute putative susceptibility genes for GTS—neuronal pentraxin 1 (NPTX1 [MIM 602367]), insulin receptor substrate p53 (IRSP53 [MIM 605475]), and tubulin specific chaperone D (TBCD [MIM 604649]). Tests of both single-marker and haplotype association were undertaken, and multiple positive results were obtained. The same SNPs were then typed in an independent sample of small nuclear families participating in the study at a second site (Toronto), and the initial findings were replicated to some extent.
Samples and Methods
Samples
The study was approved by the appropriate institutional review boards and ethics committees at both sites (Yale and Toronto), and informed consent was obtained from the participating individuals.
Yale family sample
At Yale, linkage and association analysis was performed on four large, multigenerational families with multiple members affected with GTS. The families investigated originated from Canada, Kansas (TSK), Michigan (TSM), and Oregon (TSO). The largest branch of the Canadian family has been analyzed separately and is designated hereafter as “TSC.” The pedigrees are extended to four generations and consist of 462 individuals, with 105 individuals affected with GTS (68 male and 37 female). None of the married-in spouses presented with any GTS symptoms, according to our data. These kindreds have been described in detail elsewhere (Kurlan et al. 1986; Pauls et al. 1990; Pakstis et al. 1991). In all families, each individual was assessed in a direct interview by use of a structured questionnaire (Pauls and Hurst 1987). Diagnoses were based on criteria from the revised third edition of the Diagnostic and Statistical Manual (DSM-IIIR) (American Psychiatric Association 1987) and were refined, as suggested elsewhere (Kurlan 1989), to indicate the quality of the information. This gives subjects a possible, probable, or definite diagnosis of GTS or chronic multiple tics. We decided to take a more conservative approach and considered as affected in our analysis only those individuals who presented with definite or probable GTS, according to the diagnostic scheme. DNA was extracted, using standard procedures, from permanent lymphoblastoid cell lines that were established for all the families at the Yale site.
Toronto family sample
In Toronto, 330 individuals from 96 small, nuclear families were available for analysis. DNA was extracted from whole blood according to standard procedures. The sample consisted of 41 families with one affected child and 55 families with two affected children. Of the 96 families studied, 90 are of European origin. Two families of southwestern Asian origin and one family of East Asian origin were also included in the sample. In addition, in two families, the father is of European origin, and the mother is East Asian; in one family, the mother is European and the father is African American. The diagnostic assessment of these families has been described elsewhere (TSAICG 1999). In brief, information about symptoms associated with GTS was collected, using a self- and family report, on the basis of the Yale Global Tic Severity Scale (Leckman et al. 1989). The information was checked by an experienced neuropsychiatrist or neurologist, who also performed a direct examination of each proband.
Yale population samples
Background LD in the genomic region studied was estimated in unrelated samples from seven populations originating from Europe (Adygei, Chuvash, Russians, Ashkenazi Jews, Finns, Danes, and Irish). A more diverse sample of European Americans was also studied. The mean sample size was 65 individuals. The vast majority of families used for linkage and association studies here (all of the Yale families and 90 of 96 Toronto families) are of European descent, and their ethnic origin cannot be easily defined any further. The populations used to obtain background information are representative of European ancestry, especially given the fact that genomic variation among European populations is quite homogeneous (Kidd et al., in press). Descriptive information and literature citations for these population samples can be found in the Allele Frequency Database (ALFRED) under the unique identification numbers (UIDs) shown in table 1. DNA was extracted from lymphoblastoid cell lines available at the Yale site.
Table 1.
Frequencies of Rarer Alleles[Note]
|
Allele Frequency ina |
|||||||||
| Known Gene and SNP | Physical Position(bp) | Adygei(UID SA000017I;2N=108) | Chuvash(UID SA000491O;2N=84) | Russians(UID SA000019K;2N=96) | Ashkenazim(UID SA000490N;2N=166) | Finns(UID SA000018J;2N=72) | Danes(UID SA000007H;2N=102) | Irish(UID SA000057M;2N=226) | European Americans(UID SA000020C;2N=184) |
| EVER2b: | |||||||||
| 1.C_11489838_10c | 76,572,635 | .396 | .500 | .573 | .380 | .410 | .314 | .405 | .511 |
| 2.C_3068804_10c | 76,595,312 | .031 | .025 | NA | .020 | .051 | .015 | .062 | .021 |
| 3.C_11488062_10c | 76,779,757 | .611 | .586 | .451 | .412 | .382 | .343 | .424 | .467 |
| NPTX1: | |||||||||
| 4.C_152603_10c | 78,956,093 | .422 | .488 | .478 | .411 | .353 | .490 | .395 | .375 |
| 5.C_465993_10c | 78,978,702 | .510 | .463 | .380 | .367 | .279 | .469 | .335 | .338 |
| 6.C_9176659_10c | 79,009,826 | .147 | .232 | .239 | .179 | .176 | .160 | .198 | .213 |
| 7.C_106441_10c | 79,091,403 | .250 | .317 | .283 | .272 | .309 | .280 | .293 | .283 |
| IRSP53: | |||||||||
| 8.C_216379_10c | 79,583,267 | .192 | .220 | .250 | .281 | .106 | .200 | .239 | .276 |
| 9.C_150018_10c | 79,589,081 | .231 | .341 | .375 | .453 | .348 | .382 | .365 | .365 |
| 10.C_7985808_10c | 79,598,546 | .173 | .171 | .156 | .270 | .088 | .216 | .159 | .192 |
| 11.C_213917_10c | 79,622,389 | .275 | .329 | .326 | .187 | .324 | .235 | .243 | .227 |
| 12.C_179850_10c | 79,641,502 | .327 | .263 | .344 | .393 | .265 | .324 | .252 | .318 |
| 13.C_209341_10c | 79,652,019 | .216 | .134 | .177 | .257 | .162 | .250 | .110 | .230 |
| 14.C_399266_10c | 79,664,539 | .067 | .063 | .052 | .101 | .030 | .088 | .041 | .076 |
| SECTM1: | |||||||||
| 15.C_11600340_10c | 80,819,047 | .481 | .519 | .571 | .569 | .450 | .386 | .495 | .490 |
| TBCD: | |||||||||
| 16. rs1056534d | 81,238,364 | .442 | .341 | .419 | .462 | .371 | .373 | .364 | .397 |
| 17. rs635996d | 81,284,335 | .450 | .313 | .430 | .468 | .424 | .430 | .399 | .410 |
| 18. rs662669d | 81,305,806 | .365 | .563 | .432 | .354 | .500 | .461 | .482 | .457 |
| 19. C_1674439_10d | 81,339,492 | .410 | .287 | .337 | .533 | .353 | .353 | .349 | .345 |
| 20. rs733342d | 81,351,843 | .202 | .171 | .239 | .348 | .286 | .265 | .234 | .224 |
| 21. rs3744161d | 81,357,820 | .423 | .305 | .375 | .538 | .357 | .410 | .361 | .393 |
| 22. C_1674406_10c | 81,373,505 | .202 | .171 | .227 | .348 | .271 | .270 | .231 | .238 |
| 23. C_1674398_10c | 81,380,863 | .390 | .333 | .386 | .538 | .357 | .412 | .360 | .400 |
| 24. rs3214032d | 81,417,007 | .202 | .154 | .239 | .329 | .271 | .265 | .216 | .212 |
| 25. C_1674327_10c | 81,437,491 | .442 | .488 | .512 | .558 | .586 | .598 | .472 | .506 |
Note.— Frequencies of alleles that are actually more frequent in that particular population are shown in bold italics.
2N = no. of studied chromosomes.
EVER2 = epidermodysplasia verruciformis gene 2.
Applied Biosystems assay SNP ID.
dbSNP ID.
Genotyping
STRPs
For the initial screen, the TSO and the TSC pedigrees were typed for 13 markers on chromosome 17 (ABI PRISM linkage mapping set, panels 24 and 25). The average intermarker distance was 10 cM. All of the large pedigrees at the Yale site were then typed for 17 additional STRPs, increasing the density of the map between marker D17S798 and 17qter (table 2). All STRPs were typed by use of fluorescently labeled primers for PCR amplification, and electrophoresis of the denatured products was performed on an acrylamide gel by use of the ABI 377 instrument. The fine-mapping markers were organized into two sets, so as not to overlap, according to their fluorescent label, and allele size and the PCR products of each set were pooled before electrophoresis. The primers used for PCR amplification can be found at the GenLink site for all markers, except for the 17qter STR. This is an STRP identified in our lab; the primers and the amplification conditions can be found on the ALFRED Web site. Size assignment and allele calling were performed using GeneScan and Genotyper software.
Table 2.
STRPs Used for Linkage Analysis in Large Families
|
Genetic Positionb (in cM),According to |
||||
| Marker | Physical Positiona(bp) | DeCode | Généthon | Marshfield |
| D17S849c | 386,285 | .63 | .6 | .63 |
| D17S938c | 6,194,052 | 17.06 | 14.8 | 14.69 |
| D17S945c | 9,766,279 | 27.99 | 22 | 21.01 |
| D17S799c | 13,114,012 | 37 | 32.8 | 31.96 |
| D17S925c | 27,158,736 | 52.17 | 49.5 | 49.67 |
| D17S798c | 31,138,469 | 55.6 | 53.9 | 53.41 |
| D17S933 | 33,045,783 | 62.09 | 58.3 | 57.71 |
| D17S1814 | 38,029,065 | 68.61 | 62.2 | 61.48 |
| D17S1299 | 38,903,071 | NA | NA | 62.01 |
| D17S791c | 45,098,507 | NA | 65 | 64.16 |
| D17S1795 | 48,267,058 | 75.53 | 69.4 | 68.44 |
| D17S787c | 53,523,963 | NA | 75.7 | 74.99 |
| D17S1799 | 53,782,650 | 81.61 | 75.7 | 74.99 |
| D17S957 | 55,815,576 | 87.36 | 81.7 | 80.38 |
| D17S808c | 61,012,911 | 91.86 | 84.2 | 82.56 |
| D17S1816 | 64,838,112 | 95.49 | 87.6 | 85.94 |
| D17S949c | 68,929,847 | 102.96 | 94.9 | 93.27 |
| D17S1826 | 70,999,722 | 106.23 | 97.2 | 95.99 |
| D17S1352 | 72,462,356 | 110.6 | 99.3 | 98.14 |
| D17S1807 | 72,824,696 | 113.1 | 100.4 | 99.21 |
| D17S785 | 74,895,412 | 115.34 | 104.7 | 103.53 |
| D17S802c | 76,695,601 | 120.84 | 108.2 | 106.8 |
| D17S1847 | 77,485,779 | 123.75 | 112.3 | 111.22 |
| D17S836 | 77,760,910 | 125.04 | 114 | 112.92 |
| D17S1806 | 77,906,603 | NA | 115.4 | 114.41 |
| D17S1822 | 78,344,934 | 129.24 | 117.7 | 116.86 |
| D17S1830 | 78,360,040 | NA | 117.7 | 116.86 |
| D17S784c | 78,366,192 | 129.62 | 117.7 | 116.86 |
| D17S928c | 80,782,601 | 135.67 | 128.7 | 126.46 |
| 17qter STR | 81,447,000 | NA | NA | NA |
Physical position refers to the beginning of each STRP. Positions are based on National Center for Biotechnology Information build 33. Both physical and genetic positions for the STRPs used can be found at the University of California–Santa Cruz Genome Bioinformatics Web site, except for 17qter STR (the position of which is based on local contig assembly).
Genetic positions are given relative to pter. NA = not applicable.
ABI PRISM linkage mapping set.
SNPs
Twenty-five SNPs were typed in all families available for this study. The SNPs were chosen from the Applied Biosystems “assays on demand” catalogue and were typed as 5′ TaqMan assays (Livak 1999). Most of the SNPs are intronic, with no apparent functional significance. SNPs rs3214032 and rs1056534 are exonic but code synonymous changes. The total region screened spans 4.8 Mb, with greater marker density in three regions of 135 kb (NPTX1 locus), 80 kb (IRSP53 locus), and 199 kb (TBCD locus) (table 1). We studied the whole of the IRSP53 and TBCD genes, with markers at an average spacing of ∼10 kb and ∼21 kb, respectively. In the NPTX1 locus, we studied three SNPs at the 3′ end as well as one SNP in intron 2–3 of the gene. The SNPs were chosen on the basis of their heterozygosity and also their chromosomal position. An attempt was made to investigate in more detail the region that produced high NPL scores but had low STRP coverage. At the same time, the genes studied could constitute candidate genes for GTS. In this article, for reasons of simplicity, we refer to each of the SNPs by its order number, as shown in table 1.
Statistical Analysis
The STRP genotyping data were used to perform both single- and multipoint nonparametric linkage analysis, as implemented by GENEHUNTER (Kruglyak et al. 1996). STRP allele frequencies were calculated in the unrelated individuals in the sample by use of locally written software. Haplotypes shared identical by descent among affected individuals were examined in detail in the families yielding high NPL scores (TSC and TSO). Haplotypes were constructed using GENEHUNTER and were then compared among affected and unaffected individuals in each pedigree.
The transmission/disequilibrium test (TDT) for individual markers (Spielman et al. 1993) was performed using the software package GAS. The program TRANSMIT (Clayton 1999) was used for the inference of haplotypes in the family samples and for the implementation of a haplotype-based TDT. The bootstrap option of the program was used, and, for each haplotype, the test was replicated 1,000 times, thus deriving exact P values. For some of the intervals, in which rare haplotypes or alleles existed, the −c5 or −agg3 flags were used to disregard rare haplotypes (frequency <0.05) or aggregate rare alleles (frequency <0.03), respectively, before haplotype construction.
Allele frequencies of individual SNP sites were calculated by gene counting. The assumption of Hardy-Weinberg ratios was tested by means of an auxiliary program, FENGEN (Kidd et al. 1998). The multisite haplotype frequencies were calculated with HAPLO (Hawley and Kidd 1995), which implements the expectation-maximization algorithm. By use of the haplotype frequency estimates, pairwise LD coefficients were computed as Δ2 (Pritchard and Przeworski 2001). The HAPLO/P program (Zhao et al. 1999) was used to perform permutation-based calculations of the pairwise LD values and to provide a test of statistical significance.
Results
Linkage and Association Studies
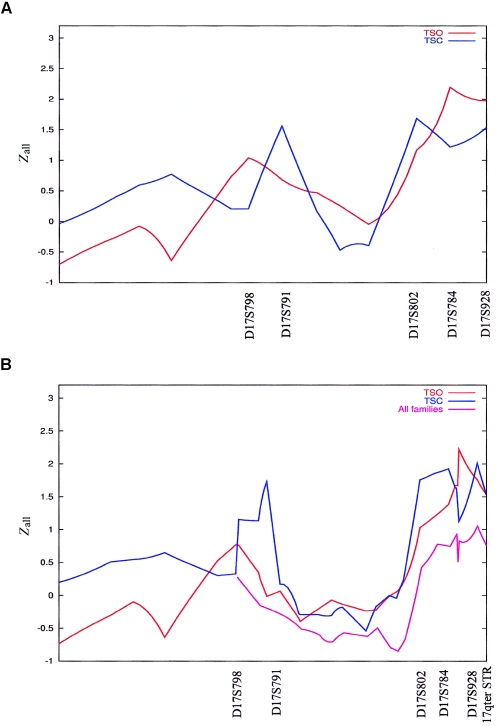
The results of the initial scan of chromosome 17, by use of only the ABI panel STRPs and a multipoint linkage analysis approach on each pedigree, are shown in figure 1A. Analysis of the TSO genotyping data gave a peak between marker D17S784 and 17qter (Zall=2.19) and a smaller peak at marker D17S798 (Zall=1.03). For TSC, three peaks were detected, at markers D17S791 (Zall=1.31), D17S802 (Zall=1.67), and D17S928 (Zall=1.51). With both pedigrees analyzed together, the multipoint NPL score reached 1.61 at marker D17S791, whereas, at 17q25, a broad peak between markers D17S802 and D17S928 was produced (highest Zall=2.46 at marker D17S928; P=.0037). Results of the single-point analysis are shown in table 3. For TSO, the highest NPL score was produced at marker D17S784 with an associated P value of .007, whereas TSC gave the highest NPL score at marker D17S928 (P=.033). Single-point nonparametric linkage analysis of the genotyping data of both pedigrees produced interesting NPL scores at markers D17S784 (P=.007) and D17S928 (P=.004).
Figure 1.
Multipoint linkage analysis (GENEHUNTER) of large pedigrees (Yale sample). A, Analysis of TSO and TSC with initial STRP panel. B, Analysis by use of a fine-mapping STRP panel.
Table 3.
Nonparametric Single-Point Linkage Analysis (GENEHUNTER)
| Pedigree and Marker | NPL Score | P |
| TSO: | ||
| D17S784 | 1.97 | .007 |
| D17S928 | 1.54 | .017 |
| TSC: | ||
| D17S802 | 1.70 | .041 |
| D17S928 | 1.82 | .033 |
| TSO and TSC: | ||
| D17S802 | 1.67 | .026 |
| D17S784 | 2.19 | .007 |
| D17S928 | 2.39 | .004 |
We decided to pursue these findings further and to increase the density of markers between marker D17S798 and 17qter. To this end, we typed 17 additional STRPs in both of these pedigrees as well as in additional individuals from TSC, TSK, and TSM. For TSO, the peak sharpened and shifted to marker D17S928, reaching a P value of .003, whereas the peak at marker D17S798 was reduced (fig. 1B). For TSC, the peak at marker D17S791 became more distinct, whereas, at 17q25, there was a broad peak between markers D17S802 and D17S1822 and a sharper peak at marker D17S928 (fig. 1B). With both pedigrees analyzed together, the peak NPL score at marker D17S928 reached 2.61, with a P value of .002. Multipoint analysis of the TSK pedigree produced an NPL score that peaked close to marker D17S802 (Zall=1.35; P=.07), whereas TSM analyzed alone did not provide positive results in this region (data not shown). When all of the families were analyzed together, the NPL score only reached 1 (P=.09) close to marker D17S928 (fig. 1B).
We examined in detail the haplotypes that were shared identical by descent in the two pedigrees (TSC and TSO) and that yielded the highest NPL scores. The pattern of haplotypes inherited by the affected individuals is quite complex, with no single haplotype always segregating with the affection status in either of the families analyzed. However, excessive haplotype sharing among affected individuals could be seen (table 4). In TSO, two haplotypes account for 43.3% of the chromosomes in the affected individuals and only 5.7% of the chromosomes in the unaffected individuals. In TSC, two different haplotypes in the same region are inherited identical by descent in 32.35% of the chromosomes in individuals with GTS and are found in only 8% of the chromosomes of the unaffected individuals.
Table 4.
Haplotype Analysis of TSO and TSC
|
Allele at Marker |
% (no./total) of Chromosomes |
|||||||||||
| Pedigree | D17S785 | D17S802 | D17S1847 | D17S836 | D17S1806 | D17S8122 | D17S1830 | D17S784 | D17S928 | 17qter STR | Affected Individuals | Unaffected Individuals |
| TSO: | ||||||||||||
| 6 | 7 | 1 | 2 | 2 | 2 | 7 | Allele 8 or 1 | 11 | 12 | 26.67 (8/30) | 2.86 (2/70) | |
| 6 | 7 | 3 | 2 | 11 | 1 | 5 | 3 | 2 | 1 | 16.67 (5/30) | 2.86 (2/70) | |
| TSC: | ||||||||||||
| 1 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 13.24 (9/68) | 1.61 (1/62) | |||
| 1 | 2 | 2 | 3 | 1 | 3 | Allele 2 or 3 | 6 | 19.12 (13/68) | 6.45 (4/62) | |||
We continued our fine-mapping efforts, focusingon 17q25 and, particularly, the regions producing thehighest NPL scores. We typed 3 SNPs around marker D17S802 and 22 SNPs between markers D17S784 and 17qter STR. Initially following a map-based approach, we chose to type SNPs in regions that gave strong indications of linkage but had low STRP coverage in our analysis. At the same time, 22 of the SNPs studied span three genes (NPTX1, IRSP53, and TBCD) that, according to their expression patterns and functions, could constitute susceptibility candidates for GTS (see the “Discussion” section). Finally, we decided to study TBCD, the last known gene on chromosome 17, as an anchoring point for our analysis.
The TDT revealed overtransmission of alleles and positive association with one SNP close to marker D17S802 (C_11488062_10) and two in the TBCD region (rs662669 and rs3744161) (table 5). To make the sample more informative, we continued our analysis, performing an association test with a three-site–haplotype moving window. Two-site–haplotype tests were also performed, but they did not add any information and pointed toward the same regions (data not shown). Multiple small haplotypes, spanning most of the region studied, were found to be overtransmitted or undertransmitted to affected offspring (table 6).
Table 5.
TDT for Single Markers (GAS)[Note]
|
No. of Alleles |
|||||
| Sample andMarker | Known Gene | Allele | Transmitted | Not Transmitted | P |
| Yale families:a | |||||
| C_11488062_10b | … | G | 27 | 9 | .002 |
| rs3744161c | TBCD | G | 29 | 17 | .052 |
| Canadian families:d | |||||
| C_11488062_10b | … | G | 18 | 8 | .038 |
| rs662669c | TBCD | T | 23 | 12 | .045 |
| rs3744161c | TBCD | G | 23 | 11 | .029 |
| Toronto families: | |||||
| C_11600340_10b | SECTM1 | G | 76 | 49 | .0098 |
| rs1056534c | TBCD | C | 65 | 43 | .021 |
| rs662669c | TBCD | T | 65 | 44 | .027 |
Note.— Transmission of all 25 SNPs genotyped was tested. Only statistically significant results are presented here.
Analysis performed on the entire sample of large families available at the Yale site.
Applied Biosystems assay SNP ID.
dbSNP ID.
Analysis performed on the large Canadian families only.
Table 6.
Results of TDT by Use of 3-Site–Haplotype Sliding Window in the Yale Families[Note]
|
Transmissions |
|||||
| SNPsa andHaplotype | Observed | Expected | Var(Observed−Expected) | χ2 (P) | Global χ2 (P)b |
| 2-3-4: | |||||
| A-G-G | 34.805 | 41.065 | 8.16 | 4.79 (.021c) | 6.68d (.271c) |
| 3-4-5: | |||||
| G-A-G | 34.275 | 28.078 | 5.76 | 6.65 (<.001) | |
| G-A-A | 16.313 | 21.648 | 3.73 | 7.62 (.01) | NC |
| 4-5-6: | |||||
| G-A-G | 12.771 | 9 | 1.93 | 7.31 (<.001) | |
| G-G-A | 21.98 | 27.37 | 8.78 | 3.31 (.031) | 18.07e (.022) |
| 5-6-7: | |||||
| G-G-C | 38.10 | 31.88 | 8.22 | 4.7 (.041) | |
| A-G-T | 4.44 | 3.18 | .89 | 1.79 (.02) | 17.08e (.019) |
| 6-7-8: | |||||
| A-C-G | 44.05 | 52.8 | 9.75 | 7.86 (.001) | |
| G-C-T | 43.33 | 35.96 | 10 | 5.40 (.005) | 16.03e (.01) |
| 7-8-9: | |||||
| C-G-A | 49.83 | 55.85 | 10.281 | 3.519 (.043) | 8.08e (.122) |
| 8-9-10: | |||||
| T-A-G | 8.53 | 5.58 | 2 | 4.28 (<.001) | |
| T-G-G | 11.014 | 16.18 | 2.79 | 9.54 (.001) | 36.4f (.041c) |
| 9-10-11: | |||||
| G-G-C | 17.26 | 25.75 | 7.07 | 10.186 (<.001) | 33.109g (.001) |
| 11-12-13: | |||||
| C-A-A | 63.915 | 59.26 | 5 | 4.31 (.032) | |
| T-A-A | 7.71 | 5.63 | 1.65 | 2.62 (.002) | 13.5f (.081) |
| 12-13-14: | |||||
| A-A-C | 71.414 | 65.465 | 5.24 | 6.74 (.016) | 37.3f (.023) |
| 13-14-15: | |||||
| G-C-A | 29.92 | 40.624 | 14 | 8.14 (.019) | 8.62d (.341c) |
| 17-18-19: | |||||
| T-T-G | 14.811 | 21.017 | 6.36 | 6.05 (<.001) | |
| C-T-G | 9.13 | 14 | 3.37 | 7.04 (.029) | 20.82e (.007) |
| 18-19-20: | |||||
| T-G-G | 15.693 | 24.356 | 6.66 | 11.268 (<.001) | |
| T-T-A | 12.823 | 9.91 | 2.79 | 3.025 (.002) | 21.72g (.009) |
| 19-20-21: | |||||
| T-A-G | 13.83 | 10.464 | 2.66 | 4.25 (<.001) | NC |
| 20-21-22: | |||||
| A-G-G | 16.792 | 12.966 | 3.1 | 4.72 (<.001) | NC |
| 21-22-23: | |||||
| G-G-T | 24.2 | 19.047 | 4.52 | 5.86 (<.001c) | NC |
| 23-24-25: | |||||
| C-T-C | 4.86 | 3.68 | 1.16 | 1.20 (.005) | 12.669e (.165) |
Note.— Results shown for joint analysis of all pedigrees.
SNP numbers correspond to SNP order shown in table 1.
NC = value could not be calculated.
Used −c5 flag.
4 df.
7 df.
5 df.
6 df.
This intriguing result required replication in an independent sample. Our collaborators at the Toronto Western Hospital agreed to type their sample of small nuclear families with one or two members affected with GTS for the 25 SNPs that we had typed in the large pedigrees. The TDT revealed positive association, with three of the markers (C_11600340_10, rs1056534, and rs662669) having an allele overtransmitted to the affected offspring (table 5). Markers rs1056534 and rs662669 are situated at the beginning of the TBCD gene, whereas marker C_11600340_10 resides 400 kb upstream of TBCD at an intronic region of secreted and transmembrane protein 1 (SECTM1 [MIM 602602]). The same allele of rs662669 that was overtransmitted in the Yale sample was also transmitted in excess in the Toronto sample. The three-site–haplotype TDT produced positive findings in six small haplotype regions (table 7).
Table 7.
Results of TDT by Use of 3-Site–Haplotype Sliding Window in the Toronto Families
|
Transmissions |
||||||
| SNPsa andHaplotype | Observed | Expected | Var(Observed−Expected) | χ2 | P | Global χ2 (P) |
| 2-3-4: | ||||||
| G-G-G | 2.37 | 5.95 | 2.16 | 5.91 | .017b | 17.294c (.027b) |
| 3-4-5: | ||||||
| G-A-G | 25.80 | 20.14 | 6.45 | 4.97 | <.001 | 9.54c (.076) |
| 4-5-6: | ||||||
| G-G-G | 13.04 | 17.27 | 5.58 | 3.19 | .02 | 6.25c (.308) |
| 7-8-9: | ||||||
| C-T-A | 14.90 | 11.44 | 3.10 | 3.85 | .032 | 6.56c (.447) |
| 11-12-13: | ||||||
| T-C-G | .68 | 4.03 | 1.52 | 7.36 | <.001 | 8.26d (.035) |
| 12-13-14: | ||||||
| C-G-C | .88 | 5.17 | 2.06 | 8.93 | <.001 | |
| C-A-T | 1.06 | 2.28 | .96 | 1.53 | <.001 | 11.173e (.007) |
| 14-15-16: | ||||||
| C-G-C | 95.10 | 81.59 | 26.52 | 6.88 | .017 | 10.762c (.064) |
| 15-16-17: | ||||||
| G-C-T | 101.10 | 85.40 | 27.16 | 8.97 | .004f | 10.557d,f (.037f) |
| 18-19-20: | ||||||
| C-G-A | 35.04 | 44.71 | 14.82 | 6.31 | .002 | 7.98d (.012) |
SNP numbers correspond to SNP order shown in table 1.
Used −agg3 flag.
7 df.
4 df.
5 df.
Used −c5 flag.
LD Patterns
We collected background information about the SNPs included in this study, to allow better interpretation of our association results and to provide a framework for subsequent studies. Since all the families analyzed at Yale and most of the families analyzed in Toronto are of European descent, we calculated the SNP frequencies and the pairwise LD values in unrelated samples from eight populations of European origin. The pairwise LD tests were performed between SNPs at the three loci (NPTX1, IRSP53, and TBCD) that were studied at a somewhat higher density.
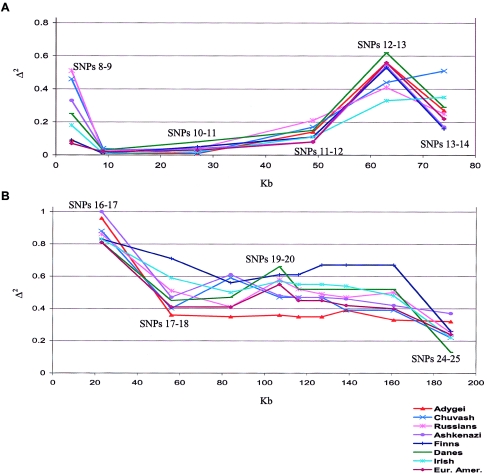
The frequencies of all SNPs that were genotyped are shown in table 1. The table shows only the results of pairwise LD tests between consecutive SNPs. At NPTX1, we studied four SNPs spanning 135 kb at an average spacing of 45 kb. Moderately significant LD was found only between SNPs 4 and 5 (C_152603_10 and C_465993_10), with an associated P value of <.006 for all the populations studied. At IRSP53, seven SNPs were studied spanning 80 kb at average intervals of 13 kb (fig. 2A). The highest LD was observed between SNPs 8 and 9 (C_216379_10–C_150018_10), SNPs 11 and 12 (C_213917_10–C_179850_10), and SNPs 12 and 13 (C_179850_10–C_209341_10). The LD test results are most interesting at the telomeric TBCD region (fig. 2B). One would expect little or no LD between markers, because of generally high recombination rates near telomeres. However, in a region spanning 199 kb with 10 SNPs at an average spacing of 22 kb, there is high LD between all the marker pairs studied. If selection has not operated, this finding indicates low recombination rates across this telomeric region. It should also be noted that, in all three regions, LD does not break down with distance in a simple manner (data not shown). The populations studied are historically close and genetically homogeneous, when compared with other regions of the world (Tishkoff et al. 1998; Kidd et al. 2000, in press). In the regions analyzed here, some variation among populations is seen, but it does not appear significant.
Figure 2.
Pairwise LD tests at IRSP53 and TBCD regions (X-axis corresponds to distance in kb; LD values are shown at the midpoint of each interval). A, Tests at IRSP53 are significant for all the populations at P<.006 for the intervals between SNPs 11 and 12, 12 and 13, and 13 and 14. At the interval between SNPs 8 and 9, the test is significant at P<.002 for Chuvash, Russians, Danes, Irish, and European Americans. B, Analysis of the TBCD region. Each point corresponds to adjacent SNP intervals (SNPs 16–17, 17–18, 18–19, 19–20, 20–21, 21–22, 22–23, 23–24, and 24–25). All Δ2 values are associated with P<.001, except for the test for the final interval (SNPs 24–25) for the Chuvash (P=.014).
Discussion
We have presented several lines of evidence implicating 17q25 in the etiopathogenesis of GTS. Our study was initiated as a fine-mapping effort following up on previous indicative findings. Nonparametric linkage analysis in the multigenerational pedigrees included in this study provided indications of linkage with a region between markers D17S802 and D17S784 as well as between marker D17S928 and 17qter STR. A previous linkage analysis study, performed on a large pedigree from Utah, yielded a parametric LOD score of 2.2 at marker D17S802 (Leppert et al. 1996). On the other hand, marker D17S784 produced one of the highest NPL scores in the genome screen performed by Zhang et al. (2002) on families with two sib pairs affected with GTS who were concordant for the OC phenotype of hoarding. In view of these previous results, our findings on independent samples become more significant.
In addition to the positive linkage suggested in the large pedigrees studied, we obtained multiple positive association results after analyzing the genotyping data from the 25 SNPs that we typed in our region of interest. TDT results with single markers point in the direction of the TBCD gene as well as to the region close to marker D17S802. A TDT that we performed using a three-site–haplotype sliding window seems also to be implicating the other two genes investigated (NPTX1 and IRSP53).
An independent sample of 96 small nuclear families with one or two children affected with GTS and their parents, was genotyped for the SNPs included in our initial study. The results make our case stronger by replicating a finding of positive association with two markers in the TBCD region as well as with over- or undertransmitted haplotypes in the entire region studied. The fact that the large-family sample produces many more positive results with the haplotype approach than do the Toronto small families can be explained by the nature of the sample. It is expected that fewer genetically independent haplotypes will exist in a sample of large families, thus reinforcing the positive results. It has also not escaped our attention that in each of the two family samples studied, different haplotypes are associated with GTS. This may be due to the LD pattern of the region. In genomic regions of low LD, the recombination rates are simply too high, increasing the number of observed haplotypes. Thus, mutations involved in the etiology of GTS may have occurred on multiple haplotype backgrounds. On the other hand, for intervals demonstrating strong LD (as is the case, e.g., in the TBCD region studied here), the existence of multiple haplotypes that are positively associated with the disorder implies the presence of more than one mutation allele. We have to note that, at this point, this is only speculation.
The two families that were used for the initial scan of chromosome 17 (TSO and TSC) have also been used in a whole-genome scan that did not produce any positive findings in the region investigated here (Barr et al. 1999). In that study, the diagnostic scheme used was much broader, including subjects who presented with chronic motor tics in the analysis of affected individuals. Furthermore, different statistics were estimated (the parametric LOD score and the nonparametric affected-pedigree method statistic).
We believe that, in the current study, the fact that not all large families show linkage to the markers suggested in the two largest pedigrees simply underlines the heterogeneity of the disorder. Bilineality of susceptibility transmission in large families is an issue that has long been proposed as the cause that hampers the linkage analysis studies on large pedigrees with GTS (Comings et al. 1989; Kurlan et al. 1994).
The comorbidity of GTS with other psychiatric disorders could be explained by a common neurological basis. OC symptoms among individual with GTS range from 11%–80% (King et al. 1998), whereas 30% of adults with GTS meet full criteria for OCD. Hanna et al. (2002), in a recent genomewide scan for OCD, obtained a suggestive NPL score of almost 1 at a marker close to 17qter. Mood disorders have also been associated with GTS, and some studies have suggested that bipolar disorder is overrepresented in patients with GTS (Kerbeshian et al. 1995; Berthier et al. 1998; Robertson 2000). A recent genomewide linkage analysis of bipolar disorder produced a LOD score of 2.4 at marker D17S928 (Dick et al. 2003). Such overlapping findings could be of special interest for disentangling the association of different psychiatric-disease phenotypes.
The genes studied in greater detail here could constitute candidate susceptibility genes for GTS. All three are related to neuronal plasticity, maintenance, and development. NPTX1 is a gene with a protein product that is expressed almost exclusively in the human brain and plays a role in excitatory synaptogenesis, most likely in the developing brain (Omeis et al. 1996; Xu et al. 2003). Insulin receptor substrate protein p53 (IRSP53) is also expressed primarily in the brain and functions as an insulin receptor tyrosine kinase substrate (Abbott et al. 1999). It is considered to play an important role in neurite outgrowth, influencing the shape and dynamic of cytoskeletal structures (Oda et al. 1999; Krugmann et al. 2001; Bockmann et al. 2002). It has also been identified as interacting with the dentatorubral-pallidoluysian atrophy (DRPLA) gene, which is associated with a neurodegenerative disease (Okamura-Oho et al. 1999). DRPLA symptoms are similar to those of Huntington disease and include chorea, ataxia, lack of coordination, and dementia (Ross et al. 1997). The third gene we chose to study encodes TBCD, a protein important for the correct folding of tubulin and the formation of the functional αβ-heterodimer (Fleming et al. 2000). When overexpressed in vitro, it acts as a microtubule-destabilizing protein (Martin et al. 2000). As part of the microtubule-assembly machinery, TBCD may play an important role in the establishment of neural networks as well as axonal transport and maintenance. It has been recently shown that mutations in the proteins responsible for the folding and assembly of tubulin subunits into functional heterodimers can cause neurological disease (Bommel et al. 2002; HRD/Autosomal Recessive Kenny-Caffey Syndrome Consortium 2002; Martin et al. 2002).
The etiopathogenesis of GTS seems to result from the interaction of genetic susceptibility, environmental factors, and neurobiological systems active in the developing brain. It is clear that the disorder is both genetically and phenotypically heterogeneous. Our study has identified three genes that could confer susceptibility or protection for GTS and that should be further investigated. The identification of genes that contribute to the genetic component of GTS will lead treatment of the disorder in new directions and will elucidate the complex brain procedures involved in habit formation and tics.
Acknowledgments
This work was supported by National Institute of Neurological Disorders and Stroke grant R01-NS40024. P.P. is supported by a Tourette Syndrome Association Research Grant Award. We thank Valeria Ruggeri, for her technical help, and Efstratios Kosmidis, for his help in generating figure 1 by use of GNUPLOT. We also acknowledge and thank the following individuals for their help over the years in assembling the samples from diverse populations: Kenneth Kendler, Leena Peltonen, Elena Grigorenko, Olga Zhukova, Batsheva Bonne-Tamir, and Joseph Parnas. Special thanks are due to the many hundreds of individuals who volunteered to give blood samples for studies such as this.
Electronic-Database Information
The URLs for data presented herein are as follows:
- ALFRED, http://alfred.med.yale.edu
- Applied Biosystems, http://www.appliedbiosystems.com/
- GenLink, http://www.genlink.wustl.edu/
- National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for GTS, OCD, DRD4, MAOA, CNTNAP2, NPTX1, IRSP53, TBCD, and SECTM1)
- University of California–Santa Cruz Genome Bioinformatics, http://www.genome.ucsc.edu/
References
- Abbott MA, Wells DG, Fallon JR (1999) The insulin receptor tyrosine kinase substrate p58/53 and the insulin receptor are components of CNS synapses. J Neurosci 19:7300–7308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (1987) Diagnostic and statistical manual of mental disorders (DSM-IIIR). 3rd rev ed. American Psychiatric Association, Washington, DC [Google Scholar]
- Barr CL, Wigg KG, Pakstis AJ, Kurlan R, Pauls D, Kidd KK, Tsui LC, Sandor P (1999) Genome scan for linkage to Gilles de la Tourette syndrome. Am J Med Genet 88:437–445 [DOI] [PubMed] [Google Scholar]
- Berthier ML, Kulisevsky J, Campos VM (1998) Bipolar disorder in adult patients with Tourette’s syndrome: a clinical study. Biol Psychiatry 43:364–370 10.1016/S0006-3223(97)00025-5 [DOI] [PubMed] [Google Scholar]
- Bockmann J, Kreutz MR, Gundelfinger ED, Bockers TM (2002) ProSAP/Shank postsynaptic density proteins interact with insulin receptor tyrosine kinase substrate IRSp53. J Neurochem 83:1013–1017 10.1046/j.1471-4159.2002.01204.x [DOI] [PubMed] [Google Scholar]
- Bommel H, Xie G, Rossoll W, Wiese S, Jablonka S, Boehm T, Sendtner M (2002) Missense mutation in the tubulin-specific chaperone E (Tbce) gene in the mouse mutant progressive motor neuronopathy, a model of human motoneuron disease. J Cell Biol 159:563–569 10.1083/jcb.200208001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett PM, Curtis D, Robertson MM, Dahlitz M, Gurling HM (1996) Linkage analysis and exclusion of regions of chromosomes 3 and 8 in Gilles de la Tourette syndrome following the identification of a balanced translocation 46,XY,t(3;8)(p21.3;q24.1) in a case of Tourette syndrome. Psychiatr Genet 6:99–105 [DOI] [PubMed] [Google Scholar]
- Brett PM, Curtis D, Robertson MM, Gurling HM (1995) Exclusion of the 5-HT1A serotonin neuroreceptor and tryptophan oxygenase genes in a large British kindred multiply affected with Tourette’s syndrome, chronic motor tics, and obsessive-compulsive behavior. Am J Psychiatry 152:437–440 [DOI] [PubMed] [Google Scholar]
- Carter AS, O’Donnell DA, Schultz RT, Scahill L, Leckman JF Pauls DL (2000) Social and emotional adjustment in children affected with Gilles de la Tourette’s syndrome: associations with ADHD and family functioning. J Child Psychol Psychiatry 41:215–223 10.1017/S0021963099005156 [DOI] [PubMed] [Google Scholar]
- Clayton D (1999) A generalization of the transmission/disequilibrium test for uncertain-haplotype transmission. Am J Hum Genet 65:1170–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comings DE, Comings BG, Knell E (1989) Hypothesis: homozygosity in Tourette syndrome. Am J Med Genet 34:413–421 [DOI] [PubMed] [Google Scholar]
- Comings DE, Gonzalez N, Wu S, Gade R, Muhleman D, Saucier G, Johnson P, Verde R, Rosenthal RJ, Lesieur HR, Rugle LJ, Miller WB, MacMurray JP (1999) Studies of the 48 bp repeat polymorphism of the DRD4 gene in impulsive, compulsive, addictive behaviors: Tourette syndrome, ADHD, pathological gambling, and substance abuse. Am J Med Genet 88:358–368 [DOI] [PubMed] [Google Scholar]
- Crawford FC, Ait-Ghezala G, Morris M, Sutcliffe MJ, Hauser RA, Silver AA, Mullan MJ (2003) Translocation breakpoint in two unrelated Tourette syndrome cases, within a region previously linked to the disorder. Hum Genet 113:154–161 [DOI] [PubMed] [Google Scholar]
- Cruz C, Camarena B, King N, Paez F, Sidenberg D, de la Fuente JR, Nicolini H (1997) Increased prevalence of the seven-repeat variant of the dopamine D4 receptor gene in patients with obsessive-compulsive disorder with tics. Neurosci Lett 231:1–4 10.1016/S0304-3940(97)00523-5 [DOI] [PubMed] [Google Scholar]
- Díaz-Anzaldúa A, Joober R, Rivière J-B, Dion Y, Lespérance P, Richer F, Chouinard S, Rouleau GA (2004) Tourette syndrome and dopaminergic genes: a family-based association study in the French Canadian founder population. Mol Psychiatry 9:272–277 10.1038/sj.mp.4001411 [DOI] [PubMed] [Google Scholar]
- Dick DM, Foroud T, Flury L, Bowman ES, Miller MJ, Rau NL, Moe PR, et al (2003) Genomewide linkage analyses of bipolar disorder: a new sample of 250 pedigrees from the National Institute of Mental Health Genetics Initiative. Am J Hum Genet 73:107–114 (erratum 73:979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eapen V, Pauls DL, Robertson MM (1993) Evidence for autosomal dominant transmission in Tourette’s syndrome: United Kingdom Cohort Study. Br J Psychiatry 162:593–596 [DOI] [PubMed] [Google Scholar]
- Elstner K, Selai CE, Trimble MR, Robertson MM (2001) Quality of life (QOL) of patients with Gilles de la Tourette’s syndrome. Acta Psychiatr Scand 103:52–59 10.1034/j.1600-0447.2001.00147.x [DOI] [PubMed] [Google Scholar]
- Fleming JA, Vega LR, Solomon F (2000) Function of tubulin binding proteins in vivo. Genetics 156:69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gade R, Muhleman D, Blake H, MacMurray J, Johnson P, Verde R, Saucier G, Comings DE (1998) Correlation of length of VNTR alleles at the X-linked MAOA gene and phenotypic effect in Tourette syndrome and drug abuse. Mol Psychiatry 3:50–60 10.1038/sj.mp.4000326 [DOI] [PubMed] [Google Scholar]
- Grice DE, Leckman JF, Pauls DL, Kurlan R, Kidd KK, Pakstis AJ, Chang FM, Buxbaum JD, Cohen DJ, Gelernter J (1996) Linkage disequilibrium between an allele at the dopamine D4 receptor locus and Tourette syndrome, by the transmission-disequilibrium test. Am J Hum Genet 59:644–652 [PMC free article] [PubMed] [Google Scholar]
- Hanna GL, Veenstra-VanderWeele J, Cox NJ, Boehnke M, Himle JA, Curtis GC, Leventhal BL, Cook EH Jr (2002) Genome-wide linkage analysis of families with obsessive-compulsive disorder ascertained through pediatric probands. Am J Med Genet 114:541–552 10.1002/ajmg.10519 [DOI] [PubMed] [Google Scholar]
- Hasstedt SJ, Leppert M, Filloux F, van de Wetering BJM, McMahon WM (1995) Intermediate inheritance of Tourette syndrome, assuming assortative mating. Am J Hum Genet 57:682–689 [PMC free article] [PubMed] [Google Scholar]
- Hawley ME, Kidd KK (1995) HAPLO: a program using the EM algorithm to estimate the frequencies of multi-site haplotypes. J Hered 86:409–411 [DOI] [PubMed] [Google Scholar]
- Hebebrand J, Nothen MM, Ziegler A, Klug B, Neidt H, Eggermann K, Lehmkuhl G, Poustka F, Schmidt MH, Propping P, Remschmidt H (1997) Nonreplication of linkage disequilibrium between the dopamine D4 receptor locus and Tourette syndrome. Am J Hum Genet 61:238–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- HRD/Autosomal Recessive Kenny-Caffey Syndrome Consortium (2002) Mutation of TBCE causes hypoparathyroidism-retardation-dysmorphism and autosomal recessive Kenny-Caffey syndrome. Nat Genet 32:448–452 10.1038/ng1012 [DOI] [PubMed] [Google Scholar]
- Hyde TM, Aaronson BA, Randolph C, Rickler KC, Weinberger DR (1992) Relationship of birth weight to the phenotypic expression of Gilles de la Tourette’s syndrome in monozygotic twins. Neurology 42:652–658 [DOI] [PubMed] [Google Scholar]
- Kerbeshian JB, Burd L, Klug M (1995) Comorbid Tourette’s disorder and bipolar disorder: an etiologic perspective. Am J Psychiatry 152:1646–1651 [DOI] [PubMed] [Google Scholar]
- Kidd JR, Pakstis AJ, Zhao H, Lu R-B, Okonofua FE, Odunsi A, Grigorenko E, Bonne-Tamir B, Friedlaender J, Schulz LO, Parnas J, Kidd KK (2000) Haplotypes and linkage disequilibrium at the phenylalanine hydroxylase locus, PAH, in a global representation of populations. Am J Hum Genet 66:1882–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd KK, Morar B, Castiglione CM, Zhao H, Pakstis AJ, Speed WC, Bonne-Tamir B, Lu RB, Goldman D, Lee C, Nam YS, Grandy DK, Jenkins T, Kidd JR (1998) A global survey of haplotype frequencies and linkage disequilibrium at the DRD2 locus. Hum Genet 103:211–227 10.1007/s004390050809 [DOI] [PubMed] [Google Scholar]
- Kidd KK, Pakstis AJ, Speed WC, Kidd JR. Understanding human DNA sequence variation. J Hered (in press) [DOI] [PubMed] [Google Scholar]
- King RA, Leckman JF, Scahill LD, Cohen DJ (1998) Obsessive-compulsive disorder, anxiety, and depression. In: Leckman JF, Cohen DJ (eds) Tourette’s syndrome tics, obsessions, compulsions: developmental psychopathology and clinical care. John Wiley and Sons, New York, pp 43–62 [Google Scholar]
- Kroisel PM, Petek E, Emberger W, Windpassinger C, Wladika W, Wagner K (2001) Candidate region for Gilles de la Tourette syndrome at 7q31. Am J Med Genet 101:259–261 [DOI] [PubMed] [Google Scholar]
- Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES (1996) Parametric and nonparametric linkage analysis: a unified multipoint approach. Am J Hum Genet 58:1347–1363 [PMC free article] [PubMed] [Google Scholar]
- Krugmann S, Jordens I, Gevaert K, Driessens M, Vandekerckhove J, Hall A (2001) Cdc42 induces filopodia by promoting the formation of an IRSp53:Mena complex. Curr Biol 11:1645–1655 10.1016/S0960-9822(01)00506-1 [DOI] [PubMed] [Google Scholar]
- Kurlan R (1989) Tourette’s syndrome: current concepts. Neurology 39:1625–1630 [DOI] [PubMed] [Google Scholar]
- Kurlan R, Behr J, Medved L, Shoulson I, Pauls D, Kidd JR, Kidd KK (1986) Familial Tourette’s syndrome: report of a large pedigree and potential for linkage analysis. Neurology 36:772–776 [DOI] [PubMed] [Google Scholar]
- Kurlan R, Como PG, Miller B, Palumbo D, Deeley C, Andresen EM, Eapen S, McDermott MP (2002) The behavioral spectrum of tic disorders: a community-based study. Neurology 59:414–420 [DOI] [PubMed] [Google Scholar]
- Kurlan R, Eapen V, Stern J, McDermott MP, Robertson MM (1994) Bilineal transmission in Tourette’s syndrome families. Neurology 44:2336–2342 [DOI] [PubMed] [Google Scholar]
- Leckman JF (2002) Tourette’s syndrome. Lancet 360:1577–1586 10.1016/S0140-6736(02)11526-1 [DOI] [PubMed] [Google Scholar]
- Leckman JF, Riddle MA (2000) Tourette’s syndrome: when habit-forming systems form habits of their own? Neuron 28:349–354 10.1016/S0896-6273(00)00114-8 [DOI] [PubMed] [Google Scholar]
- Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, Cohen DJ (1989) The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry 28:566–573 [DOI] [PubMed] [Google Scholar]
- Leckman JF, Zhang H, Vitale A, Lahnin F, Lynch K, Bondi C, Kim YS, Peterson BS (1998) Course of tic severity in Tourette syndrome: the first two decades. Pediatrics 102:14–19 10.1542/peds.102.1.14 [DOI] [PubMed] [Google Scholar]
- Leppert M, Peiffer A, Snyder B, van de Wetering BJM, Filloux F, Coon H, McMahon WM (1996) Two loci of interest in a family with Tourette syndrome. Am J Hum Genet Suppl 59:A225 [Google Scholar]
- Livak KJ (1999) Allelic discrimination using fluorogenic probes and the 5′ nuclease assay. Genet Anal 14:143–149 [DOI] [PubMed] [Google Scholar]
- Martin L, Fanarraga ML, Aloria K, Zabala JC (2000) Tubulin folding cofactor D is a microtubule destabilizing protein. FEBS Lett 470:93–95 10.1016/S0014-5793(00)01293-X [DOI] [PubMed] [Google Scholar]
- Martin N, Jaubert J, Gounon P, Salido E, Haase G, Szatanik M, Guenet JL (2002) A missense mutation in Tbce causes progressive motor neuronopathy in mice. Nat Genet 32:443–447 10.1038/ng1016 [DOI] [PubMed] [Google Scholar]
- Mérette C, Brassard A, Potvin A, Bouvier H, Rousseau F, Émond C, Bissonnette L, Roy M-A, Maziade M, Ott J, Caron C (2000) Significant linkage for Tourette syndrome in a large French Canadian family. Am J Hum Genet 67:1008–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink JW (2001) Basal ganglia dysfunction in Tourette’s syndrome: a new hypothesis. Pediatr Neurol 25:190–198 10.1016/S0887-8994(01)00262-4 [DOI] [PubMed] [Google Scholar]
- Oda K, Shiratsuchi T, Nishimori H, Inazawa J, Yoshikawa H, Taketani Y, Nakamura Y, Tokino T (1999) Identification of BAIAP2 (BAI-associated protein 2), a novel human homologue of hamster IRSp53, whose SH3 domain interacts with the cytoplasmic domain of BAI1. Cytogenet Cell Genet 84:75–82 [DOI] [PubMed] [Google Scholar]
- Okamura-Oho Y, Miyashita T, Ohmi K, Yamada M (1999) Dentatorubral-pallidoluysian atrophy protein interacts through a proline-rich region near polyglutamine with the SH3 domain of an insulin receptor tyrosine kinase substrate. Hum Mol Genet 8:947–957 10.1093/hmg/8.6.947 [DOI] [PubMed] [Google Scholar]
- Omeis IA, Hsu YC, Perin MS (1996) Mouse and human neuronal pentraxin 1 (NPTX1): conservation, genomic structure, and chromosomal localization. Genomics 36:543–545 10.1006/geno.1996.0503 [DOI] [PubMed] [Google Scholar]
- Pakstis AJ, Heutink P, Pauls DL, Kurlan R, van de Wetering BJ, Leckman JF, Sandkuyl LA, Kidd JR, Breedveld GJ, Castiglione CM, Weber J, Sparkes RS, Cohen DJ, Kidd KK, Oostra B (1991) Progress in the search for genetic linkage with Tourette syndrome: an exclusion map covering more than 50% of the autosomal genome. Am J Hum Genet 48:281–294 [PMC free article] [PubMed] [Google Scholar]
- Pauls DL (2003) An update on the genetics of Gilles de la Tourette syndrome. J Psychosom Res 55:7–12 10.1016/S0022-3999(02)00586-X [DOI] [PubMed] [Google Scholar]
- Pauls DL, Hurst CR (1987) Schedule for Tourette and other behavioral syndromes, version IV. Yale University Press, New Haven, CT [Google Scholar]
- Pauls DL, Leckman JF (1986) The inheritance of Gilles de la Tourette’s syndrome and associated behaviors: evidence for autosomal dominant transmission. N Engl J Med 315:993–997 [DOI] [PubMed] [Google Scholar]
- Pauls DL, Pakstis AJ, Kurlan R, Kidd KK, Leckman JF, Cohen DJ, Kidd JR, Como P, Sparkes R (1990) Segregation and linkage analyses of Tourette’s syndrome and related disorders. J Am Acad Child Adolesc Psychiatry 29:195–203 [DOI] [PubMed] [Google Scholar]
- Pauls DL, Raymond CL, Stevenson JM, Leckman JF (1991) A family study of Gilles de la Tourette syndrome. Am J Hum Genet 48:154–163 [PMC free article] [PubMed] [Google Scholar]
- Petek E, Windpassinger C, Vincent JB, Cheung J, Boright AP, Scherer SW, Kroisel PM, Wagner K (2001) Disruption of a novel gene (IMMP2L) by a breakpoint in 7q31 associated with Tourette syndrome. Am J Hum Genet 68:848–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Pine DS, Cohen P Brook JS (2001) Prospective, longitudinal study of tic, obsessive-compulsive, and attention-deficit/hyperactivity disorders in an epidemiological sample. J Am Acad Child Adolesc Psychiatry 40:685–695 10.1097/00004583-200106000-00014 [DOI] [PubMed] [Google Scholar]
- Price RA, Kidd KK, Cohen DJ, Pauls DL, Leckman JF (1985) A twin study of Tourette syndrome. Arch Gen Psychiatry 42:815–820 [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Przeworski M (2001) Linkage disequilibrium in humans: models and data. Am J Hum Genet 69:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson MM (2000) Tourette syndrome, associated conditions and the complexities of treatment. Brain 123:425–462 10.1093/brain/123.3.425 [DOI] [PubMed] [Google Scholar]
- ——— (2003) Diagnosing Tourette syndrome: is it a common disorder? J Psychosom Res 55:3–6 10.1016/S0022-3999(02)00580-9 [DOI] [PubMed] [Google Scholar]
- Ross CA, Becher MW, Colomer V, Engelender S, Wood JD, Sharp AH (1997) Huntington’s disease and dentatorubral-pallidoluysian atrophy: proteins, pathogenesis and pathology. Brain Pathol 7:1003–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seuchter SA, Hebebrand J, Klug B, Knapp M, Lehmkuhl G, Poustka F, Schmidt M, Remschmidt H, Baur MP (2000) Complex segregation analysis of families ascertained through Gilles de la Tourette syndrome. Genet Epidemiol 18:33–47 [DOI] [PubMed] [Google Scholar]
- Simonic I, Gericke GS, Ott J, Weber JL (1998) Identification of genetic markers associated with Gilles de la Tourette syndrome in an Afrikaner population. Am J Hum Genet 63:839–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonic I, Nyholt DR, Gericke GS, Gordon D, Matsumoto N, Ledbetter DH, Ott J, Weber JL (2001) Further evidence for linkage of Gilles de la Tourette syndrome (GTS) susceptibility loci on chromosomes 2p11, 8q22 and 11q23-24 in South African Afrikaners. Am J Med Genet 105:163–167 10.1002/ajmg.1192 [DOI] [PubMed] [Google Scholar]
- Singer HS (2000) Current issues in Tourette syndrome. Mov Disord 15:1051–1063 [DOI] [PubMed] [Google Scholar]
- Singer HS, Minzer K (2003) Neurobiology of Tourette’s syndrome: concepts of neuroanatomic localization and neurochemical abnormalities. Brain Dev Suppl 25:S70–S84 14980376 [DOI] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Harding M, O’Donnell D, Wilens T, Faraone S, Coffey B, Geller D (1998) Disentangling the overlap between Tourette’s disorder and ADHD. J Child Psychol Psychiatry 39:1037–1044 10.1017/S0021963098002984 [DOI] [PubMed] [Google Scholar]
- Spielman RS, McGinnis RE, Ewens WJ (1993) Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus (IDDM). Am J Hum Genet 52:506–516 [PMC free article] [PubMed] [Google Scholar]
- State MW, Greally JM, Cuker A, Bowers PN, Henegariu O, Morgan TM, Gunel M, DiLuna M, King RA, Nelson C, Donovan A, Anderson GM, Leckman JF, Hawkins T, Pauls DL, Lifton RP, Ward DC (2003) Epigenetic abnormalities associated with a chromosome 18(q21-q22) inversion and a Gilles de la Tourette syndrome phenotype. Proc Natl Acad Sci USA 100:4684–4689 10.1073/pnas.0730775100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishkoff SA, Goldman A, Calafell F, Speed WC, Deinard AS, Bonne-Tamir B, Kidd JR, Pakstis AJ, Jenkins T, Kidd KK (1998) A global haplotype analysis of the myotonic dystrophy locus: implications for the evolution of modern humans and for the origin of myotonic dystrophy mutations. Am J Hum Genet 62:1389–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourette Syndrome Association International Consortium for Genetics (TSAICG) (1999) A complete genome screen in sib pairs affected by Gilles de la Tourette syndrome. Am J Hum Genet 65:1428–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkerk AJ, Mathews CA, Joosse M, Eussen BH, Heutink P, Oostra BA, Tourette Syndrome Association International Consortium for Genetics (2003) CNTNAP2 is disrupted in a family with Gilles de la Tourette syndrome and obsessive compulsive disorder. Genomics 82:1–9 10.1016/S0888-7543(03)00097-1 [DOI] [PubMed] [Google Scholar]
- Walkup JT, LaBuda MC, Singer HS, Brown J, Riddle MA, Hurko O (1996) Family study and segregation analysis of Tourette syndrome: evidence for a mixed model of inheritance. Am J Hum Genet 59:684–693 [PMC free article] [PubMed] [Google Scholar]
- Xu D, Hopf C, Reddy R, Cho RW, Guo L, Lanahan A, Petralia RS, Wenthold RJ, O’Brien RJ, Worley P (2003) Narp and NP1 form heterocomplexes that function in developmental and activity-dependent synaptic plasticity. Neuron 39:513–528 10.1016/S0896-6273(03)00463-X [DOI] [PubMed] [Google Scholar]
- Zhang H, Leckman JF, Pauls DL, Tsai C-P, Kidd KK, Campos MR, Tourette Syndrome Association International Consortium for Genetics (2002) Genomewide scan of hoarding in sib pairs in which both sibs have Gilles de la Tourette syndrome. Am J Hum Genet 70:896–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Pakstis AJ, Kidd JR, Kidd KK (1999) Assessing linkage disequilibrium in a complex genetic system. I. Overall deviation from random association. Ann Hum Genet 63:167–179 10.1046/j.1469-1809.1999.6320167.x [DOI] [PubMed] [Google Scholar]