Abstract
In a female infant with dysmorphic features, severe neurological defects, and congenital blindness, a positive urinary Bratton-Marshall test led to identification of a massive excretion of 5-amino-4-imidazolecarboxamide (AICA)–riboside, the dephosphorylated counterpart of AICAR (also termed “ZMP”), an intermediate of de novo purine biosynthesis. ZMP and its di- and triphosphate accumulated in the patient’s erythrocytes. Incubation of her fibroblasts with AICA-riboside led to accumulation of AICAR, not observed in control cells, suggesting impairment of the final steps of purine biosynthesis, catalyzed by the bifunctional enzyme AICAR transformylase/IMP cyclohydrolase (ATIC). AICAR transformylase was profoundly deficient, whereas the IMP cyclohydrolase level was 40% of normal. Sequencing of ATIC showed a K426R change in the transformylase region in one allele and a frameshift in the other. Recombinant protein carrying mutation K426R completely lacks AICAR transformylase activity.
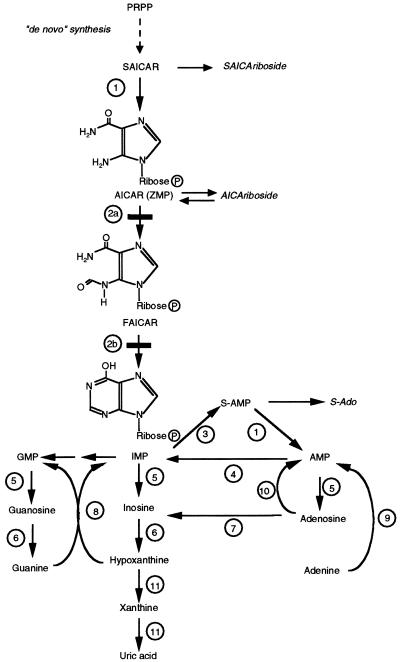
The de novo purine biosynthesis pathway involves 10 steps which lead from 5-phosphoribosylpyrophosphate (PRPP) to inosine monophosphate (IMP), from which the adenine and guanine nucleotides are formed (fig. 1). Hitherto, only a single inborn deficiency of purine synthesis had been identified in humans, namely the deficiency of adenylosuccinate lyase (ADSL [MIM 103050]) (Jaeken and Van den Berghe 1984; Van den Berghe and Jaeken 2001). This disorder is characterized by the presence in urine and cerebrospinal fluid (CSF) of succinyl-5-amino-4-imidazolecarboxamide riboside (SAICA-riboside) and succinyladenosine (S-Ado), the nucleosides corresponding to SAICA-ribotide (SAICAR) and adenylosuccinate (S-AMP), respectively, the two substrates of ADSL. The presence of SAICA-riboside results in a positive Bratton-Marshall test (Laikind et al. 1986). Since false-positive results are recorded in patients who receive certain medications, a positive Bratton-Marshall test should be followed by identification by high-performance liquid chromatography (HPLC) coupled with UV spectral analysis (Jaeken and Van den Berghe 1984; Van de Berghe and Jaeken 2001) of the succinylpurines SAICA-riboside and S-Ado.
Figure 1.
Pathway of purine metabolism. 1, ADSL. 2a, AICAR-TF. 2b, IMP-CH. 3, Adenylosuccinate synthetase. 4, AMP deaminase. 5, 5′-nucleotidase. 6, Purine nucleoside phosphorylase. 7, Adenosine deaminase. 8, HGPRT. 9, APRT. 10, Adenosine kinase. 11, Xanthine oxidase.
We have observed a 4-year-old girl who presented with a devastating neurological picture, involving profound mental retardation, epilepsy, dysmorphic features (prominent forehead and metopic suture, brachycephaly, wide mouth with thin upper lip, low-set ears, and prominent clitoris due to fused labia minora), and congenital blindness. She is the second child of healthy, unrelated French parents whose first child is a healthy son. She was born at term after an uneventful pregnancy. Birth weight was 3,030 g, length 49 cm, and head circumference 34 cm. The baby was hypotonic and displayed hypoglycemia (1.5 mmol/liter) and hyponatremia, which resolved after dietary treatment. Fundoscopy showed normal results. A heart murmur was shown to be due to an ostium secundum–type atrial septal defect. At age 5 mo, psychomotor delay was manifest, and partial occipital seizures appeared. Dysmorphic features were more prominent and included high bridge of the nose, anteverted nostrils, and cutaneous dimples on the extensor side of knees, elbows, and shoulders. At age 6 mo, congenital blindness was noticed. At age 12 mo, bilateral atrophic pigmented chorioretinal macular lesions had developed with optic atrophy, abnormal electroretinograms, and visual evoked potentials. Right-side esotropia was also present. At follow-up at age 4 years, psychomotor delay and visual handicap remained the major concerns.
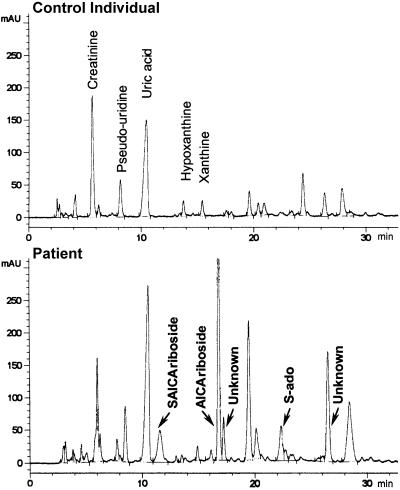
The results of routine biochemical analyses, including serum (0.18 mM) and urinary uric acid (table 1), were normal. Cholesterol was 4.2 mM (normal 4.4–6.4 mM), and free fatty acids 0.17 mM (normal 0.2–0.6 mM). Results of laboratory investigations for organic acidurias, aminoacidopathies, sterol, lysosomal, peroxysomal, biotin, and protein glycosylation enzymes were negative. High-resolution karyotype, pantelomeric screening, and mDNA were normal. Infectious embryopathy and chorioretinitis were also ruled out because of the normality of brain imaging (CT scan and magnetic resonance imaging) and brain auditory-evoked response. A positive urinary Bratton-Marshall test (Laikind et al. 1986) suggested accumulation of SAICA-riboside. We performed HPLC analysis of urine and CSF, and both revealed the presence of SAICA-riboside and S-Ado (fig. 2; table 1), although at a lower concentration than that found in ADSL-deficient patients (table 1). The chromatograms also revealed three additional peaks (fig. 2) that are seen neither in control individuals' nor in ADSL-deficient patients' urine. The major peak was identified as 5-amino-4-imidazolecarboxamide riboside (AICA-riboside), on the basis of a positive Bratton-Marshall test, spectral analysis, and spiking with the authentic compound. The two other compounds displayed spectral characteristics similar to those of AICA-riboside. They were not further characterized, although they were not due to the medications taken by the patient.
Table 1.
Analysis of Urine and CSF by HPLC, Compared with Reference Values[Note]
|
Findings ina |
|||
| Sample and Content | Control Individuals(n=5) | Patient | ADSL-Deficient Patients(n=8) |
| Urine (mmol/mole creatinine): | |||
| S-Ado | ND | 45 | 132–439 |
| SAICA-riboside | ND | 80 | 79–802 |
| AICA-riboside | ND | 280 | ND |
| Uric acid | 185–1,134b | 829 | 400–695 |
| Cerebrospinal fluid (μM): | |||
| S-Ado | 0–5.4 | 16 | 158–443 |
| SAICA-riboside | ND | 15 | 132–783 |
| AICA-riboside | ND | 12 | ND |
| Uric acid | 2–40 | ND | 0–8 |
Note.— Urine and CSF samples were analyzed by use of HPLC with diode-array UV detection on an Alltima C18 5u (250×246 mm) reversed-phase column (Alltech), as described elsewhere (Marie et al. 2000).
ND = not detectable.
Normal values for 2–5-year-old children.
Figure 2.
HPLC of urine. Urine samples were analyzed by HPLC, with diode-array UV detection, on an Alltima C18 5u (250×246 mm) reversed-phase column (Alltech), as described elsewhere (Marie et al. 2000).
AICA-riboside is the nucleoside corresponding to AICAR (AICA-ribotide, also termed “ZMP”), an intermediate of the de novo purine biosynthetic pathway. AICA-riboside is formed by dephosphorylation of AICAR (ZMP), most likely by IMP-GMP 5′-nucleotidase (Vincent et al. 1996a). Its presence in trace amounts in control human urine has been demonstrated (Sweetman and Nyhan 1971). Increased urinary excretion of AICA-riboside is associated with vitamin B12 and folic acid deficiencies, which impair AICAR transformylase (TF) activity (Middleton et al. 1964). It has also been detected in children with acute leukemia (Lulenski et al. 1970) and in patients with hypoxanthine-guanine phosphoribosyltransferase (HGPRT [MIM 308000]) deficiency (Newcombe 1970; Sweetman and Nyhan 1971). In the latter two disorders, increased excretion of AICA-riboside can be explained by enhancement of de novo purine synthesis, which renders AICAR-TF rate limiting. However, in all the conditions listed, the excretion of AICA-riboside is orders of magnitude lower than in the present patient.
Nucleotide analysis of the patient’s red blood cells (table 2) revealed a huge accumulation of AICAR (ZMP) and also of its di- and triphosphate derivatives, ZDP and ZTP. The concentration of ZTP markedly surpassed that of ATP. In turn, ATP concentration was depressed by 60% in the patient’s erythrocytes, as compared with those of the control individuals and the parents. Accumulation of ZTP also is associated with HGPRT deficiency and other disorders of purine overproduction and has been attributed to the increased rate of de novo purine synthesis in these conditions (Sidi and Mitchell 1985). However, in those cases, ZTP accumulation is much less prominent than in our patient.
Table 2.
Concentrations of Nucleotides in Red Blood Cells[Note]
|
Concentrations of Nucleotidesin Red Blood Cells ofa |
||||
| Nucleotide | Control Individualsb(n=3) | Father | Mother | Patient |
| ATP | 599±95 | 750 | 631 | 222 |
| ADP | 54±6 | 56 | 61 | 67 |
| AMP | 4.5±3 | 6 | 6 | 61 |
| IMP | ND | 12 | 18 | 23 |
| GTP | 29±12 | 21 | 17 | 43 |
| ZMP | ND | ND | ND | 243 |
| ZDP | ND | ND | ND | 224 |
| ZTP | ND | ND | ND | 716 |
Note.— Concentrations are given in mmol/ml of blood. Red blood cells were packed by centrifugation for 15 min at 500 g and resuspended in 3 volumes of Krebs buffer. Suspensions of packed erythrocytes were analyzed by HPLC, after extraction with 1 volume of 10% HClO4 and neutralization, on a Partisphere SAX (4.6×125 mm) ion-exchange column (Whatman) (Vincent et al. 1991).
ND = not detectable.
Mean±SD.
Cultured skin fibroblasts obtained from the patient were incubated with AICA-riboside, which can be used by the de novo synthesis pathway, following phosphorylation into AICAR through the action of adenosine kinase (fig. 1). Fibroblasts were trypsinized and resuspended in Krebs buffer at a density of 106 cells per 0.5 ml. Cells were incubated at 37°C for 60 min in a shaking incubator with intermittent oxygenation, with or without AICA-riboside 0.5 mM. The cell pellet was extracted with 0.1 ml HClO4 2.5% and was analyzed, as described for red blood cells in the note of table 2. This revealed marked accumulation of AICAR (ZMP) in the patient’s cells (to 6.21 μmol per g of protein, after 60 min), not seen in control cells, confirming impairment of AICAR metabolism.
The presence of massive amounts of AICA-riboside in the patient’s urine and the accumulation of AICAR and its derivatives in her erythrocytes and fibroblasts are a clear indication of a deficiency in the enzyme that utilizes this intermediate of de novo purine biosynthesis, the bifunctional enzyme AICAR-TF/IMP cyclohydrolase (ATIC [MIM 601731], also known as purH) (Greasley et al. 2001) (fig. 1). AICAR-TF catalyzes the transformylation of AICAR and 10-formyl-tetrahydrofolate to produce formyl-AICAR (FAICAR) and tetrahydrofolate, whereas the IMP cyclohydrolase (IMP-CH) moiety cyclizes FAICAR to IMP. The C-terminal domain of the protein is responsible for the AICAR-TF activity, whereas the IMP-CH activity resides in the N-terminal domain. We assayed the presence of both catalytic activities in the patient’s cultured skin fibroblasts (table 3). This revealed a profound deficiency of AICAR-TF activity. By contrast, IMP-CH activity was 40% of normal (table 3).
Table 3.
Assay of AICAR-TF, IMP-CH, and ADSL Activities in Fibroblasts[Note]
|
Assay of |
||||
| ADSL with |
||||
| Subjects | AICAR-TF | IMP-CH | S-AMP | SAICAR |
| Control Individuals | 1.52±.44 | 2.3±.24 | 2.75±.37 | 2.15±.15 |
| Patient | NDa | .9±.36 | 2.9, 2.75 | 2.3, 2.1 |
Note.— Results are given in nmol/min/mg of soluble proteins. Mean±SD of 3–4 determinations or individual values. Activities were measured on extracts prepared from cultured skin fibroblasts. AICAR-TF and IMP-CH were assayed as described by Rayl et al. (1996), except that 10-formyltetrahydrofolate was prepared from 5,10-methenyltetrahydrofolate purchased from Shrick. ADSL activity was assayed, as described elsewhere, by use of both SAICAR and S-AMP as substrates and measurement of the products of the reaction by HPLC (Marie et al. 2000).
ND = not detectable.
Sequencing of the gene (GenBank accession number NT_005403) showed a frameshift in exon 2 in one allele, caused by a duplication/deletion event (125–129dupGGGAT; 130–132delGCT). This results in instability of mRNA (GenBank accession number BC008879), as assessed by sequencing of the product from RT-PCR, in which this allele was absent. This mutation was also found in the mother’s DNA. In the other allele, a K426R (c.1277 A→G) change was found in exon 13, which is part of the AICAR-TF region. This mutation was also found in the father’s DNA. This second mutation is located within a conserved region that has been shown to be implicated in the binding of a potassium ion in the avian protein (Greasley et al. 2001). This potassium ion has been proposed as playing a key role in stabilization of the tertiary structure of the protein. In expression studies, recombinant protein carrying mutation K426R completely lacks AICAR-TF activity but still shows IMP-CH activity.
The accumulation of AICAR in the patient’s erythrocytes probably results from phosphorylation of circulating AICA-riboside by adenosine kinase. Utilization of ATP in this process, in combination with the phosphorylation of ZMP into ZDP by nucleoside monophosphate kinase and of ZDP into ZTP by nucleoside diphosphate kinase, probably explains the lower ATP concentrations in the patient’s erythrocytes.
The observation that deficiency of ATIC does not influence the patient’s production of uric acid, as apparent from normal serum and urinary concentrations, is in accordance with a normally minor contribution of the de novo pathway to the synthesis of purine nucleotides. As in ADSL deficiency, a depletion of purine nucleotides is not found in ATIC deficiency, most probably because the enzyme defect on the de novo pathway can be circumvented by supply of purines by the salvage pathway encompassing the enzymes HGPRT, adenine phosphoribosyltransferase (APRT), and adenosine kinase (fig. 1). Nevertheless, ATIC deficiency could result in depletion of purine nucleotides in some hitherto-unidentified cell types or during embryonic development and organogenesis, when the contribution of the de novo pathway to the synthesis of purine nucleotides could be more important.
As in ADSL deficiency, the succinylpurines S-Ado and SAICA-riboside accumulate in ATIC deficiency, although to a lesser extent. This accumulation is probably the result of inhibition of ADSL by accumulating AICAR (Sabina et al. 1982) and could exert toxicity by still-unexplained mechanisms. Moreover, AICA-riboside, the major accumulating compound in ATIC deficiency, might have several potentially deleterious effects. Indeed, in recent years, AICA-riboside has been shown to possess a diversity of metabolic properties (reviewed by Vincent [1997]), probably as a consequence of its structural similarity with adenosine. A cardioprotective action—involving a broad variety of effects on cardiac tissue, neutrophils, and platelets, possibly mediated by the release of adenosine (Gruber et al. 1989)—has been extensively investigated. On the other hand, we have shown that AICA-riboside has marked inhibitory effects on carbohydrate and lipid metabolism in the liver. These include inhibition of gluconeogenesis (Vincent et al. 1991), capable of inducing hypoglycemia (Vincent et al. 1996b), of glycolysis (Vincent et al. 1992), and of fatty-acid and cholesterol synthesis (Henin et al. 1995). These inhibitory effects can be explained by the rapid phosphorylation of AICA-riboside by adenosine kinase, resulting in accumulation of AICAR. AICAR, because of its structural similarity with AMP (Sabina et al. 1984), influences the activity of a number of AMP-sensitive enzymes, among which are fructose-1,6-bisphosphatase, glucokinase, and AMP-activated protein kinase (Henin et al. 1996). AICA-riboside has also been shown to stimulate glucose uptake by muscle (Holmes et al. 1999) and to induce apoptosis in human neuroblastoma cells (Garcia-Gil et al. 2003). It remains to be determined whether the pathology of the present patient—including transient neonatal hypoglycemia, low cholesterol and free fatty acids—is due to the accumulation of AICA-riboside and its phosphorylated counterpart, AICAR, and the precise mechanisms of the potentially deleterious effects of these compounds. Moreover, possible toxic effects of the two unidentified compounds, which might be derivatives of upstream components of the de novo pathway, should also be investigated.
Systematic screening for ADSL deficiency with the Bratton-Marshall test has been regularly advocated in the numerous patients with unexplained psychomotor retardation, convulsions, autistic features, or neurological disease without clear etiology. The present observation—which led to the discovery of the first patient affected with a second inborn defect of purine biosynthesis, AICA-ribosiduria caused by the deficiency of ATIC—strongly reinforces this point.
Acknowledgments
This work was supported by grants from the Fund for Medical Scientific Research (Belgium), the Concerted Research Action Programme of the Communauté Française de Belgique, the Interuniversity Attraction Poles Programme-Belgian Science Policy, and by European Community concerted action BMH4-CT98–3079. G.V.d.B. is director of research of the Belgian National Fund for Scientific Research.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for ATIC mRNA [accession number BC008879]; nucleotides numbered from initiation codon, A1TG; the entire sequence of the ATIC gene found in the human chromosome 2 [genomic contig NT_005403]; and primers designed according to these sequences)
- Online Mendelian Inheritance in Man (OMIM) http://www.ncbi.nlm.nih.gov/Omim/ (for ADSL, HGPRT, and ATIC)
References
- Garcia-Gil M, Pesi R, Perna S, Allegrini S, Giannecchini M, Camici M, Tozzi (2003) 5-Aminoimidazole-4-carboxamide riboside induces apoptosis in human neuroblastoma cells. Neuroscience 117:811–820 10.1016/S0306-4522(02)00836-9 [DOI] [PubMed] [Google Scholar]
- Greasley SE, Horton P, Ramcharan J, Beardsley GP, Benkovic SJ, Wilson IA (2001) Crystal structure of a bifunctional transformylase and cyclohydrolase enzyme in purine biosynthesis. Nat Struct Biol 8:402–406 10.1038/87555 [DOI] [PubMed] [Google Scholar]
- Gruber HE, Hoffer ME, McAllister DR, Laikind PK, Lane TA, Schmid-Schoenbein GW, Engler RL (1989) Increased adenosine concentration in blood from ischemic myocardium by AICA riboside: effects on flow, granulocytes and injury. Circulation 80:1400–1411 [DOI] [PubMed] [Google Scholar]
- Henin N, Vincent MF, Gruber HE, Van den Berghe G (1995) Inhibition of fatty acid and cholesterol synthesis by stimulation of AMP-activated protein kinase. FASEB J 9:541–546 [DOI] [PubMed] [Google Scholar]
- Henin N, Vincent MF, Van den Berghe G (1996) Stimulation of rat liver AMP-activated protein kinase by AMP analogues. Biochim Biophys Acta 1290:197–203 [DOI] [PubMed] [Google Scholar]
- Holmes BF, Kurth-Kraczek EJ, Winder WW (1999) Chronic activation of 5′-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J Appl Physiol 87:1990–1995 [DOI] [PubMed] [Google Scholar]
- Jaeken J, Van den Berghe G (1984) An infantile autistic syndrome characterised by the presence of succinylpurines in body fluids. Lancet 2:1058–1061 [PubMed] [Google Scholar]
- Laikind PK, Seegmiller JE, Gruber HE (1986) Detection of 5′-phosphoribosyl-4-(N-succinylcarboxamide)-5-aminoimidazole in urine by use of the Bratton-Marshall reaction: identification of patients deficient in adenylosuccinate lyase activity. Anal Biochem 156:81–90 [DOI] [PubMed] [Google Scholar]
- Lulenski G, Donaldson M, Newcombe D (1970) Urinary aminoimidazolecarboxamide levels in children with acute leukemia. Pediatrics 45:983–995 [PubMed] [Google Scholar]
- Marie S, Flipsen JW, Duran M, Poll-The BT, Beemer FA, Bosschaart AN, Vincent MF, Van den Berghe G (2000) Prenatal diagnosis in adenylosuccinate lyase deficiency. Prenat Diagn 20:33–36 [DOI] [PubMed] [Google Scholar]
- Middleton JE, Coward RF, Smith P (1964) Urinary excretion of A.I.C. in vitamin-B12 and folic-acid deficiencies. Lancet 284:258–259 10.1016/S0140-6736(64)90216-8 [DOI] [Google Scholar]
- Newcombe DS (1970) The urinary excretion of aminoimidazolecarboxamide in the Lesch-Nyhan syndrome. Pediatrics 46:508–512 [PubMed] [Google Scholar]
- Rayl EA, Moroson BA, Beardsley GP (1996) The human purH gene product, 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase/IMP cyclohydrolase: cloning, sequencing, expression, purification, kinetic analysis, and domain mapping. J Biol Chem 271:2225–2233 10.1074/jbc.271.4.2225 [DOI] [PubMed] [Google Scholar]
- Sabina RL, Holmes EW, Becker MA (1984) The enzymatic synthesis of 5-amino-4-imidazolecarboxamide riboside triphosphate (ZTP). Science 223:1193–1195 [DOI] [PubMed] [Google Scholar]
- Sabina RL, Kernstine KH, Boyd RL, Holmes EW, Swain JL (1982) Metabolism of 5-amino-4-imidazolecarboxamide riboside in cardiac and skeletal muscle: effects on purine nucleotide synthesis. J Biol Chem 257:10178–10183 [PubMed] [Google Scholar]
- Sidi Y, Mitchell BS (1985) Z-nucleotide accumulation in erythrocytes from Lesch-Nyhan patients. J Clin Invest 76:2416–2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetman L, Nyhan WL (1971) Detailed comparison of the urinary excretion of purines in a patient with the Lesch-Nyhan syndrome and a control subject. Biochem Med 4:121–134 5134918 [DOI] [PubMed] [Google Scholar]
- Van den Berghe G, Jaeken J (2001) Adenylosuccinate lyase deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease, 8th ed. McGraw-Hill, New York, pp 2653–2662 [Google Scholar]
- Vincent MF (1997) AICA riboside: a nucleoside with interesting metabolic properties. PhD thesis, Université Catholique de Louvain, Brussels [Google Scholar]
- Vincent MF, Bontemps F, Van den Berghe G (1992) Inhibition of glycolysis by 5-amino-4-imidazolecarboxamide riboside in isolated rat hepatocytes. Biochem J 281:267–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ——— (1996a) Substrate cycling between 5-amino-4-imidazolecarboxamide riboside and its monophosphate in isolated rat hepatocytes. Biochem Pharmacol 52:999–1006 10.1016/0006-2952(96)00413-3 [DOI] [PubMed] [Google Scholar]
- Vincent MF, Erion MD, Gruber HE, Van den Berghe G (1996b) Hypoglycaemic effect of AICAriboside in mice. Diabetologia 39:1148–1155 [DOI] [PubMed] [Google Scholar]
- Vincent MF, Marangos PJ, Gruber HE, Van den Berghe G (1991) Inhibition by AICA riboside of gluconeogenesis in isolated rat hepatocytes. Diabetes 40:1259–1266 [DOI] [PubMed] [Google Scholar]