Abstract
Ionizing radiation and many cancer drugs have the potential to produce germ-cell mutations that might lead to genetic disease in the next generation. In a population-based study, we identified, from records in the Danish Cancer Registry, 4,676 children treated for cancer. Their 6,441 siblings provided a comparison cohort. The results of a search of the Central Population Register identified 2,630 live-born offspring of the survivors and 5,504 live-born offspring of their siblings. The occurrence of abnormal karyotypes diagnosed in these offspring and also in any pregnancies terminated following prenatal diagnosis of a chromosome abnormality was determined from the Danish Cytogenetic Registry. After exclusion of hereditary cases and inclusion of the prenatal cases, after correction for expected viability, the adjusted proportion of live-born children in survivor families with abnormal karyotypes (5.5/2,631.5 [0.21%]) was the same as that among the comparison sibling families (11.8/5,505.8 [0.21%]). There were no significant differences in the occurrence of Down syndrome (relative risk [RR]=1.07; 95% CI 0.16–5.47) or Turner syndrome (RR=1.32; 95% CI 0.17–7.96) among the children of cancer survivors, compared with the children of their siblings. These reassuring results are of importance to the survivors, to their families, and to genetic counselors.
There are concerns about ill effects that cancer treatment may have on children born to cancer survivors. Although radiation and many cancer drugs produce somatic-cell mutations, there is little information in humans on the production of germ-cell mutations leading to genetic disease in the next generation. Adding to reassuring but scant information (Boice et al. 2003), we evaluated cancer treatment in Danish children and subsequent chromosomal aberrations in their offspring.
From the Danish Cancer Registry, 4,676 survivors were identified who were diagnosed with cancer at age <20 years between 1943 and 1996 and who survived until the onset of fertility (age 15 years). Patients had to be alive on or born after April 1, 1968, when the national Central Population Register (CPR) was established, with personal identification numbers for all citizens that permit linkage among registers. A search of the CPR resulted in identification of 2,630 live-born offspring (50.6% boys) of the survivors and 5,504 live-born offspring (52.3% boys) of their 6,441 siblings (comparison cohort). The proportion who were parents was lower among the survivors (30%; 1,381 parents) compared with their siblings (44%; 2,823 parents). The mean number of offspring per parent was the same (1.9) for both survivors and siblings. All subjects, including female partners of male survivors (Boice et al. 2003), were linked to the Danish Cytogenetic Registry, which includes all normal and abnormal karyotypes diagnosed pre- or postnatally in Denmark since 1960, excluding stillbirths. All children with an abnormal karyotype were linked to the National Hospital Register, which contains information for virtually every nonpsychiatric hospital admission in Denmark since 1977, to learn whether the child had been hospitalized for or with a malformation. Information on radiotherapy (yes/no) was obtained from the Danish Cancer Registry for the entire cohort and indicated that 37% of all cancer survivors received radiation treatment. Information on chemotherapy was abstracted from medical records only for survivors with affected offspring. For affected offspring and fetuses of survivors, radiation doses to parental gonads were estimated from details on medical records and from experimental simulations by use of tissue-equivalent and three-dimensional mathematical phantoms (Stovall et al., in press). Observed numbers of abnormal karyotypes in the 4,676 families of cancer survivors were compared with numbers in the comparison cohort.
Of 4,676 survivors of childhood cancer, 8 had at least one child or fetus with an abnormal karyotype (table 1 [individuals S1–S8]). In two affected families (of individuals S1 and S2), the abnormal karyotype is thought to be hereditary. The remaining six chromosomal abnormalities were found in four offspring with Down syndrome (two diagnosed prenatally) and two with Turner syndrome. It is noted that no cases of de novo structural chromosomal abnormalities were observed in the families with cancer survivors. Four of the eight survivors with affected children had received neither radiotherapy nor chemotherapy, and those who did undergo radiotherapy received relatively low gonadal doses. Of the 6,441 control siblings, 19 had affected children or fetuses (table 1 [individuals C1–C19). In six families, the abnormal karyotype was inferred to be hereditary. The offspring of these siblings were not registered in the Hospital Register as having a malformation. The three subjects with fragile-X syndrome who were born to a control sibling, who was herself a fragile-X carrier, were not included in the main analysis, which excluded hereditary cases. Among the 13 families with affected children or fetuses of nonhereditary origin, five subjects with Down syndrome (one prenatal), four of Turner syndrome (one prenatal and one a mosaic), and three subjects with Edward syndrome (two in one sibship, diagnosed prenatally) were recorded.
Table 1.
Abnormal Karyotypes among Fetuses and Offspring of Childhood-Cancer Survivors and of Their Healthy Siblings
|
Findings in Childhood-Cancer Survivor or Sibling Control Individuala |
Findings in Offspring |
|||||
| Individual(Sex) | Cancer Diagnosis (Age at Diagnosis) | Anatomical Region of Radiotherapy(Gonadal Dose)/Chemotherapy | Status at Time of Karyotype Test (Sex) | Abnormal Karyotype | Age atKaryotype Test | Commentary |
| S1 (M) | Cerebellum, cystic astrocytoma (16 years) | No radio- or chemotherapy | Child (M) | 4/8 translocation 46,XY,t(4;8)(p16.3; p23.1) | 8 years | Hereditary; father of child: 46,XY,t(4;8)(p16.3;p23.1) |
| Child (F) | 4/8 translocation 46,XX,t(4;8)(p13; p16)b | 23 years | Hereditary | |||
| Fetus (F) | 4/8 translocation 46,XX,t(4;8)(p16.3; p23.1) | Fetalc | Hereditary, singleton | |||
| Fetus (F) | 4/8 translocation 46,XX,t(4;8)(p16.3; p23.1) | Fetalc | Hereditary, singleton | |||
| S2 (M) | Right testis, malignant teratoma (16 years) | One side of pelvis and the abdomen (426 cGy to testes)/Methotrexate (total dose unknown) | Fetus (M) | Balanced Robertsonian translocation 45,XY, der(13;14) | Fetalc | Hereditary; mother of fetus: 45,XX, der(13;14) |
| S3 (M) | Nasopharynx, reticulosarcoma (14 years) | Head and neck (22 cGy to testes)/no chemotherapy | Fetus (F) | Down syndrome 47,XX,+21 | Fetal | Aborted |
| S4 (M) | Right testis, malignant Leydig cell tumor (18 years) | No radio- or chemotherapy | Fetus (F) | Down syndrome 47,XX,+21 | Fetal | Aborted |
| S5 (M) | Humerus, Ewing sarcoma (17 years) | Arm (2.4 cGy to testes)/no chemotherapy | Child (F) | Down syndrome 47,XX,+21 | At birth | Malformedd |
| S6 (F) | Unilateral retinoblastoma (10 mo) | No radio- or chemotherapy | Child (M) | Down syndrome 47,XY,+21 | At birth | Malformedd |
| S7 (F) | Skin of lower leg, malignant melanoma (19 years) | No radio- or chemotherapy | Child (F) | Turner syndrome 45,X | At birth | |
| S8 (F) | Lateral ventricle, tumor NOSe (16 years) | Brain (1.5 cGy to ovaries)/no chemotherapy | Child (F) | Turner syndrome 45,X | 17 years | |
| C1(F) | … | … | Child (F) | Fragile X 46,X,fra(X) | 11 years | Hereditary; mother of child: 46,X,fra(X)(q27.3) |
| Child (M) | Fragile X 46,Y,fra(X)(q27.3) | 7 years | ||||
| Fetus (F) | Fragile X 46,X,fra(X)(q27.3) | Fetalc | ||||
| C2 (M) | … | … | Child (M) | 4 pericentric inversion 46,XY,inv(4)(p11q11) | 3 years | Hereditary; father of child: 46,XY,inv(4)(p11q11) |
| C3 (F) | … | … | Child (F) | 2/8 translocation 46,XX,t(2;8)(p16;q22) | 6 years | Hereditary; mother of child: 46,XX,t(2;8) |
| C4 (M) | … | … | Fetus (F) | 6/18 translocation 46,XX,t(6;18)(p21.3;q22.1) | Fetalc | Hereditary; mother of fetus: 46,XX, t(6;18)(p21.3;q22.1) |
| C5 (F) | … | … | Fetus (M) | Balanced Robertsonian translocation 45,XY,der(13;14)(p11;q11) | Fetalc | Hereditary; mother of fetus: 45,XX,der(13;14)(p11;q11) |
| C6 (F) | … | … | Fetus (F) | 9/16 translocation 46,XX,t(9;16)(p11.2;p11.2) | Fetalc | Hereditary; father of fetus: 46,XY,t(9;16)(p11.2;p11.2) |
| C7 (M) | … | … | Child (F) | 14 q deletion 46,XX,del(14)(q?) | At birth | Malformedd |
| C8 (M) | … | … | Child (M) | Down syndrome 47,XY,+21 | At birth | Malformedd |
| C9 (M) | … | … | Child (F) | Down syndrome 47,XX,+21 | At birth | Malformedd |
| C10 (M) | … | … | Child (M) | Down syndrome 47,XY,+21 | At birth | Malformedd |
| C11 (F) | … | … | Child (F) | Down syndrome 47,XX,+21 | At birth | Malformedd |
| C12 (M) | … | … | Fetus (F) | Down syndrome 47,XX,+21 | Fetal | Aborted |
| C13 (F) | … | … | Child (F) | Turner syndrome 45,X | 2 years | Malformedd |
| C14 (F) | … | … | Child (F) | Turner syndrome 45,X | <1 year | Malformedd |
| C15 (F) | … | … | Fetus (F) | Turner syndrome 45,X | Fetal | Aborted |
| C16 (F) | … | … | Child (F) | Turner mosaicf 45,X/46,XX | <1 year | Malformedd |
| C17 (F) | … | … | Fetus (M) | Klinefelter mosaicf 47,XXY/46,XY (65%/35%)g | Fetal | Aborted |
| C18 (M) | … | … | Child (F) | Edward syndrome 47,XX,+18 | At birth | Malformedd |
| C19 (M) | … | … | Fetus (F) | Edward syndromeh 47,XX,+18 | Fetal | Aborted, singleton |
| Fetus (F) | Edward syndromeh 47,XX,+18 | Fetal | Aborted, singleton | |||
S = childhood-cancer survivor; C = control sibling.
Slightly different chromosome breakpoints to other members of the family; however, highly likely that it is the same balanced translocation, just interpreted differently.
Live born.
“Malformed” denotes that the child was born alive and subsequently registered as being malformed in the National Hospital Register. In all cases, multiple malformations typical for the respective syndromes were registered.
NOS = not otherwise specified.
Mosaicism might be a postzygotic mechanism.
The proportion of each type of cell.
Parental mosaicism might be a possible explanation for the unusual finding of two fetuses with Edward syndrome. Parental chromosomes, however, have not been examined in this family. The referral for prenatal test was because of advanced maternal age.
After exclusion of hereditary cases and adjustment of the prenatally diagnosed and terminated cases for viability (Hook et al. 1989; survival probabilities of 0.74 for Down, 0.35 for Turner, 0.36 for Edward), the adjusted proportion of live-born children with chromosome abnormalities in survivor families was similar—(4 liveborn+1.5 adjustedprenatalcases)/(2,630 liveborn+1.5 adjustedprenatalcases), or 0.21%—to the proportion among the comparison sibling families—(10 liveborn+1.8 adjustedprenatalcases)/(5,504 liveborn+1.8 adjustedprenatalcases), or 0.21%. Inclusion of the hereditary cases also resulted in similar proportions in the two cohorts; that is, 0.40% (10.5/2,631.5) and 0.36% (19.8/5,505.8) among survivors and siblings, respectively. These overall estimates were not adjusted for maternal age, but the median maternal age was similar in the two cohorts; that is, 26 years (range 15–51 years) among cancer survivors and 26 years (range 15–45 years) among siblings.
The adjusted live-born rates for Down syndrome were compared with expectations based on the maternal age–related rates of Cuckle et al. (1987). Both survivors and siblings had similar numbers of offspring with Down syndrome, as expected; that is, 3.5 versus a maternal age–adjusted expected number of 3.1 for the survivors and 4.7 versus 5.9 for the siblings. Both cohorts had similar adjusted live-born rates for Turner syndrome, although these were slightly higher than expected numbers, on the basis of a rate of 1 in 2,500 live-born females (Jensen 1998); that is, 2 versus 0.5 and 3.4 versus 1.1 expected among offspring of survivors and siblings, respectively. All but one of the live-born subjects were diagnosed some time after birth, which might explain the higher rates than normally quoted for birth prevalence.
A direct comparison, conducted by calculations of age-adjusted risk ratios, revealed an occurrence of Down syndrome among live-born offspring born to survivors comparable to that in offspring of siblings (relative risk 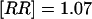 ; 95% CI 0.16–5.47), whereas a slightly increased risk for Turner syndrome was observed in offspring of survivors (RR=1.32; 95% CI 0.17–7.96).
; 95% CI 0.16–5.47), whereas a slightly increased risk for Turner syndrome was observed in offspring of survivors (RR=1.32; 95% CI 0.17–7.96).
In three of the four instances of Down syndrome reported in the offspring of cancer survivors, the parent affected with cancer was male, whereas it is evident that most cases of nondisjunction leading to this syndrome are maternal in origin (Jensen 1998). Similarly, the two instances of Turner syndrome in the childhood-cancer survivor group were born to female survivors, whereas this nondisjunction most often is paternal in origin. It is unfortunate that parent-of-origin studies for the nondisjunction resulting in the children with Down and Turner syndromes were not available, but it would seem unlikely that any disturbance of sex-related nondisjunction patterns had occurred in the survivors, since both Down syndrome and Turner prevalence rates were similar to those in the offspring of control siblings.
In this population-based study of 4,676 survivors of childhood cancer, there was no indication of increased risk of chromosomal abnormalities in their offspring. Although we were able to evaluate chromosomal abnormalities diagnosed pre- or postnatally in the entire population of Denmark since 1960, the relatively small number of affected children and fetuses and the fact that not all of the cancer survivors received mutagenic treatment precludes firm conclusions. However, our reassuring results accord with previous studies of survivors of childhood cancer (Byrne et al. 1998), including those using other endpoints to measure heritable genetic conditions such as congenital malformations (Boice et al. 2003), and with the study of the children of the Japanese atomic bomb survivors (UNSCEAR 2001). Because of the increasing number of cancer survivors who are capable of having children, the issue of genetic disease in offspring is of great importance to cancer survivors and their families and to clinicians who provide genetic counseling.
Acknowledgments
This study was supported by the Danish Cancer Society, the International Epidemiology Institute (IEI), and the Westlakes Research Institute (agreement 01/12/99 DC). None of the funding sources had any involvement in the conduction of this study, except for Dr. Boice, who is Scientific Director at IEI, and Dr. Tawn, who is head of Genetics at Westlakes Research Institute. We thank programmer Jan Hansen, Danish Cytogenetic Registry, for data extraction and introduction.
References
- Boice JD Jr, Tawn EJ, Winther JF, Donaldson SS, Green DM, Mertens AC, Mulvihill JJ, Olsen JH, Robison LL, Stovall M (2003) Genetic effects of radiotherapy for childhood cancer. Health Phys 85:65–80 10.1097/00004032-200307000-00013 [DOI] [PubMed] [Google Scholar]
- Byrne J, Rasmussen SA, Steinhorn SC, Connelly RR, Myers MH, Lynch CF, Flannery J, Austin DF, Holmes FF, Holmes GE, Strong LC, Mulvihill JJ (1998) Genetic disease in offspring of long-term survivors of childhood and adolescent cancer. Am J Hum Genet 62:45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuckle HS, Wald NJ, Thompson SG (1987) Estimating a woman’s risk of having a pregnancy associated with Down’s syndrome using her age and serum alpha-fetoprotein level. Br J Obstet Gynaecol 94:387–402 [DOI] [PubMed] [Google Scholar]
- Hook EB, Topol BB, Cross PK (1989) The natural history of cytogenetically abnormal fetuses detected at midtrimester amniocentesis which are not terminated electively: new data and estimates of the excess and relative risk of late fetal death associated with 47, +21 and some other abnormal karyotypes. Am J Hum Genet 45:855–861 [PMC free article] [PubMed] [Google Scholar]
- Jensen PKA (1998) Kromosomafvigelser hos mennesket (Chromosomal abberations in humans). Gads Forlag, Copenhagen (in Danish) [Google Scholar]
- Stovall M, Donaldson SS, Weathers RE, Robison LL, Mertens AC, Winther JF, Olsen JH, Boice JD Jr. Genetic effects of radiotherapy for childhood cancer: gonadal dose reconstruction. Int J Radiat Oncol Biol Phys (in press) [DOI] [PubMed] [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) (2001) Report to the General Assembly, with scientific annexes: hereditary effects of radiation. Report E.01.IX.2, United Nations, New York [Google Scholar]


