Abstract
Humans vary >100-fold in their sensitivity to the harmful effects of ultraviolet radiation. The main determinants of sensitivity are melanin pigmentation and less-well-characterized differences in skin inflammation and repair processes. Pigmentation has a high heritability, but susceptibility to cancers of the skin, a key marker of sun sensitivity, is less heritable. Despite a large number of murine coat-color mutations, only one gene in humans, the melanocortin 1 receptor (MC1R), is known to account for substantial variation in skin and hair color and in skin cancer incidence. MC1R encodes a 317–amino acid G-coupled receptor that controls the relative amounts of the two major melanin classes, eumelanin and pheomelanin. Most persons with red hair are homozygous for alleles of the MC1R gene that show varying degrees of diminished function. More than 65 human MC1R alleles with nonsynonymous changes have been identified, and current evidence suggests that many of them vary in their physiological activity, such that a graded series of responses can be achieved on the basis of (i) dosage effects (of one or two alleles) and (ii) individual differences in the pharmacological profile in response to ligand. Thus, a single locus, identified within a Mendelian framework, can contribute significantly to human pigmentary variation.
Introduction
Understanding the variation in human skin color and cutaneous sensitivity to sunshine (UV radiation) is of interest to at least two groups of researchers. First, skin-color variation is one of the most striking polymorphic traits of humans, and understanding its genesis is one of the classic evolutionary problems of human genetics (Bodmer and Cavalli-Sforza 1976; Cavalli-Sforza et al. 1996). Many aspects of this are still not fully understood, including how skin color is generated, what genes underpin it, how color relates to other genetic traits, and the extent to which natural selection accounts for the world diversity in skin colors of people with different genetic and geographical ancestries. This is therefore an area of great interest, not only to human geneticists but also to physical anthropologists and social scientists. Second, skin color, as a determinant of sensitivity to UV radiation (UVR), is of major medical importance. Sunshine exerts both beneficial and harmful effects on human health. In the presence of substantial ambient UVR, those with pale skin are more at risk of most major skin cancers (including melanoma, basal cell carcinoma, and squamous cell carcinoma) (Rees 2002b, 2002c) and are more likely to suffer from one of a range of photodermatoses (i.e., a pathological sensitivity to sunshine like that seen in the cutaneous forms of porphyria [MIM 176100]). Conversely, those with dark skin—and therefore with a greater degree of protection against UVR—are more at risk of vitamin D deficiency (Holick 2001) and are less amenable to the therapeutic uses of UVR for the treatment of skin diseases such as psoriasis or atopic dermatitis.
In this review, I seek to summarize our knowledge of the genetics of sensitivity to sunshine, with an emphasis on the understanding of physiological variation in sensitivity; rare Mendelian disorders will be mentioned only when they provide particular insights into the more common variation. The emphasis will be the integration of genetic advances with physiology, so that the spectrum of human pigmentation can be understood.
Skin Structure
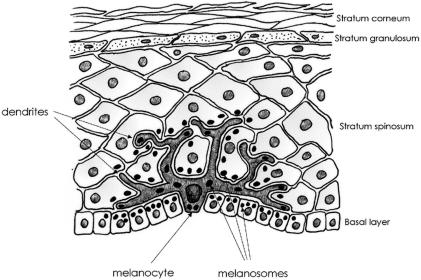
Skin comprises two compartments: a stratified epithelium composed predominantly of keratinocytes, which measures 50–100 μm in thickness in many body regions; and a relatively acellular dermis of ∼1,000 μm thickness, which contains a complex extracellular matrix comprising many types of collagen, fibroblasts that secrete collagen, and a range of supporting structures, including blood vessels, inflammatory cells, nerves, and ground substance (fig. 1). In addition to the keratinocytes, an estimated 10% of the cellular component of the epidermis is composed of neural crest–derived melanocytes and Langerhans cells, the latter of which are bone marrow–derived professional macrophages (fig. 1). Within the epidermis, as keratinocytes proliferate, they move upward from the basal layer and express a range of different differentiation markers, before undergoing a form of programmed cell death to produce the dead but not inert stratum corneum (fig. 1).
Figure 1.
A schematic of human epidermis. Keratinocytes within the basal layer proliferate and differentiate as they move through the strata spinosum and granulosum to form the dead but chemically noninert stratum corneum. Melanocytes are dendritic cells, located close to the basal layer, that synthesize melanin in melanosome before passing the melanosomes to surrounding keratinocytes. Melanosomes can be seen collecting above the nuclei of keratinocytes, which provide protection against UVR (note that, for clarity of presentation, they are shown only in some cells). Not shown are Langerhans cells (antigen-presenting cells within the epidermis) or the underlying dermis, which contains collagen fibers, fibroblasts, nerves, vessels, and other immune and inflammatory cells.
Exposure of Skin to UVR (Sunshine)
Exposure of skin to a significant dose of UVR leads to an inflammatory reaction characterized by erythema (redness), edema, and possibly pain and blistering (“sunburn”). The erythema is due to superficial vasodilatation and peaks 8–24 h after exposure (Farr and Diffey 1985); the edema is the result of an inflammatory cell infiltrate and extravastation of fluid from capillaries, and the pain is the result of sensitization of peripheral nocireceptors by a range of inflammatory mediators released from keratinocytes and inflammatory cell infiltrate. In most persons, a UVR-dose–dependent increase in melanin pigmentation will be seen several days later (Oh et al., in press).
Histological examination reveals a sequence of changes after UVR exposure: an initial inhibition of S phase within the keratinocytes, followed by the appearance of keratinocyte apoptotic cells (historically called “sunburn cells”), followed 48–72 h later by a wave of keratinocyte hyperproliferation that leads to an increase in epidermal thickness and an increase in the thickness of the stratum corneum (Gilchrest et al. 1981; Stierner et al. 1989; Soter 1990). These acute changes usually subside completely, and the skin returns to normal, although erythema and pigmentation may persist for several weeks or longer (Ha et al. 2003b, 2003c; Oh et al., in press).
Repeated exposure of skin to UVR over the course of days and weeks leads to a number of changes that appear to be adaptive, adaptive in the sense that subsequent exposures are associated with a diminished inflammatory response—that is, the skin is undergoing photoadaptation (Oh et al., in press). This can often be noticed by the way that a pale-skinned person burns less easily at the end of a summer holiday than at the start of the holiday. There are several processes that appear to underpin this adaptive response. First, the amount of melanin, as alluded to above, increases in response to sunshine, and this melanin, as discussed below, affords some protection against further UVR exposure. Second, even in the absence of any melanin, the epidermis—especially the stratum corneum—thickens, to protect underlying structures from further UVR (Everett 1961). Both these processes can be viewed as “sun-blocking” activities, but it is important to note that the stimulus that leads to adaptation is already associated with initial cellular damage.
Repeated chronic exposure of skin (over decades), whether or not visible erythema is induced, is associated with a number of features distinct from the acute reaction (Rees 2002a). These include an increased risk of keratinocyte and melanocyte carcinomas and a number of changes in both epidermis and dermis, commonly referred to as “skin aging.” These features include changes in the epidermis, such as the presence of light brown spots (“age spots”), and in the dermis, such as a reduction in the volume and quality of the dermis that accompanies wrinkle formation.
The UV spectrum of 300–400 nm is responsible for most of the effects of sunshine on skin (Diffey 1999). Rare disorders, such as solar urticaria, may sometimes be due to light in the visible spectrum (>400 nm), but most biological effects arise from exposure to the UVR component of sunshine (Diffey 1999). Different biological effects of UVR all have their own action spectrum, a measure of the degree to which any particular wavelength acts on a particular biological endpoint (Urbach 1999). For instance, the most studied action spectrum is that of the induction of erythema. This spectrum is identical to that of DNA absorption (and damage) and, at least in mice, to the spectrum that induces squamous skin cancer (Urbach 1999). This observation strongly suggests that one of the major targets harmed by UVR is DNA. By contrast, the melanoma action spectrum is unknown, although some believe longer wavelengths (UVA) are relatively more important than they are for squamous cell carcinoma (Wang et al. 2001; Ortonne 2002).
Three factors contribute to the optical properties of UVR transmission through the epidermis (Young 1997): melanin pigmentation, the presence of amino acids, and light scattered by melanin and amino acids. The optical characteristics of all these factors are wavelength specific (just as different wavelengths of white light are scattered differently, which leads to the sky's blue appearance). Thus, an increase in melanin or an increase in the thickness of the stratum corneum (or total epidermis), as seen in the thickening that follows UVR irradiation, leads to penetration of fewer photons to the basal layer of the epidermis—the dermis. Fewer photons implies less damage to DNA or other macromolecules and a less inflammatory response.
Variation in the Response to UVR
Although formal studies that compare world populations with standardized insults have not been performed, sensitivity to the effects of UVR on skin is highly polymorphic within “normal” nondiseased populations. Within Northern European populations, there is a 4–5-fold variation in response, both between persons at the same body site and between different body sites on the same person (Ha et al. 2003a; Waterston et al., in press). To produce the same change in blood flux as is seen in a pale-skinned European, a >100-fold increase in UVR dose is required for a person with black skin from the African subcontinent (J.L.R., unpublished data). If sensitivity is viewed in terms of skin cancer rates, then up to a 500-fold difference in sensitivity is evident (Urbach 1999; Rees, in press).
In seeking to understand this variation, two rare genodermatoses—albinism (MIM 203100) and xeroderma pigmentosum (XP [MIM 278730])—have offered important insights into the molecular mechanisms underpinning sun sensitivity. When these insights are coupled with the morphological changes seen when skin is exposed to UVR (described above), a simple model to explain variation in sensitivity, both to acute inflammation and to skin cancer, can be suggested.
The first rare Mendelian disorder is albinism (for a recent review, see King et al. [2001]). Albinos are unable to make adequate amounts of melanin, and their phenotype provides a clear example of the role of melanin in protection against the harmful effects of UVR. Albinos are intensely sensitive to the effects of UVR: they develop acute erythema, swelling, blistering, and pain in response to what would be trivial amounts of UVR to others; in the long term, albinism leads to a greatly increased risk of some forms of skin cancer (Kromberg et al. 1989). In equatorial regions, skin cancers—chiefly squamous cell carcinoma rather than melanoma—may develop in albinos during the teenage years, which leads to a significant mortality—as well as morbidity—rate in the 3rd decade of life (Lookingbill et al. 1995).
The second Mendelian disorder is the DNA nuclear excision repair defect syndrome, XP (for a recent review, see Bootsma et al. [2001]). With XP, as with albinism, most subjects are highly sensitive to the harmful effects of UVR, which leads to acute complications similar to those seen with albinism, and—at an even earlier age than for albinism—to the development of malignancies, including melanoma and squamous cell carcinoma (Bootsma et al. 2001).
The heuristic importance of XP is that it has provided links between several originally disparate pathological features—namely, DNA defects, skin cancer, acute erythema, and the DNA action spectrum of UVR. Patients with XP have abnormally increased and prolonged erythemal responses, have increased rates of skin cancer, and are deficient in the particular types of DNA repair that are maximal at those wavelengths that are most effective in the induction of erythema in normal subjects (and of most cancers in experimental animals). Furthermore, within the XP spectrum of disorders, it is possible to associate different repair subtypes (such as transcription-coupled repair and global-damage repair) with particular phenotypes, such as enhanced erythemal response or skin cancer (see Kolgen et al. [2003] and Berg et al. [1998] for further discussion).
These Mendelian disorders, coupled with what we know of the physiology of skin when irradiated, suggest pathways that may be important for explanation of variation in response to UVR within the normal population. First, resting pigmentation (pigmentation prior to sun exposure) can be viewed as a protective factor that acts by preventing harmful photons from reaching the viable part of the skin and causing damage to DNA and other macromolecules. Second, once DNA damage has occurred, DNA repair pathways are activated. Third, in ways that are poorly understood, UVR-induced inflammation and DNA damage are associated with a series of changes that limit further insults from UVR, including an increase in pigmentation (tanning) and an increase in the thickness of the epidermis. Finally, the degree of variation in inflammation (to a uniform insult) is also likely to vary among persons, and, since inflammation may well be related causally to the ability to photoadapt, this variation feeds back into the variation attributable to the other factors.
In practice, the genetics of pigmentation and, to a lesser degree, the genetics of DNA repair are better understood than the genetic factors that determine the degree of epidermal thickening or variation in the inflammatory response between persons (Kulms and Schwarz 2000; Murphy et al. 2001).
Human Pigmentation
Skin color, with the exception of some very rare disorders, is due to the presence of blood in superficial plexuses and to melanin. All but transient differences in human skin color are due to differences in the amount and type of melanin within skin. Differences in skin color account for much of the variation seen in erythemal sensitivity to UVR, both between different persons at the same body site and within persons at different body sites (Wagner et al. 2002b; Ha et al. 2003a; Waterston et al., in press). Similarly, skin color is an excellent predictor of cancer risk, with rates of skin cancer varying >100-fold between subjects with different skin color (Urbach 1999).
Variation in skin color between people is due to the amount, type, and arrangement of melanin produced. It has traditionally been said that melanocyte density, although varying by body site, is similar in different continental population groups, although a recent study questions this and suggests that this issue should be revisited (Alaluf et al. 2003). Melanocytes are neural crest–derived cells that migrate into skin during the first trimester of gestation and produce melanin. Melanin is a mixture of polymers with different physicochemical properties that is enzymatically derived from tyrosine via a complex series of intermediate steps (Ito 2003). Melanin is biosynthesized within melanosomes that, when mature, are passed to the surrounding keratinocytes. Visible skin color is therefore the result of the melanin in keratinocytes but is synthesized in melanocytes. Within the keratinocyte layers of skin, melanin is arranged in particular ways, most notably in the basal layer, which contains the keratinocyte-proliferative compartment, where little crescents of melanin overlie the nuclei and provide protection against UVR photons (Tadokoro et al. 2003). Some in vitro reconstitution experiments studying persons with different skin colors suggest that keratinocytes may exert a significant role in determining skin color, contrary to the conventional view that keratinocytes are passive recipients of melanosomes (Bessou-Touya et al. 1998; Minwalla et al. 2001).
Chemical characterization of melanin has proved difficult because of its insolubility and because it is not one substance but a range of polymers (Ito 2003). Genetic studies in mice have provided considerable insight into melanogenesis, and a number of gene products important in alteration of the balance of the various polymer types—and therefore the nature of melanin—have been identified (Barsh 1996; Jackson 1997; Rees 2003). A useful, albeit simplistic, classification characterizes melanin content in terms of the type of melanin and the amount produced. There are two broad classes: eumelanin, which is brown or black, and pheomelanin, which is rich in cysteine and is red or yellow. The relative amounts of the types of melanin are often expressed as the ratio of eumelanin to pheomelanin (“pigment switch”). Red hair has a low ratio of eumelanin to pheomelanin, and black hair has a high ratio of eumelanin to pheomelanin, whereas blond hair contains little of either class of melanin (Rees 2003). Studies of epidermal melanins (rather than hair melanins) are few—because they require skin biopsies—but suggest that those with black skin have 2–3-fold as much melanin as Northern Europeans but that the ratio of eumelanin to pheomelanin may differ in skin and hair (Thody et al. 1991; Alaluf et al. 2001, 2002a; Tadokoro et al. 2003). Note that these relatively small differences in total melanin content apparently account for up to a 100-fold difference in UVR sensitivity, as judged by erythemal responses and skin cancer rates (Urbach 1999), which suggests there may be important differences in the quality of the melanin not recognized by current methods of characterization (Alaluf et al. 2001, 2002a). It is known, for instance, that the shape, size, and grouping of melanosomes differs between people of different skin colors (Alaluf et al. 2001, 2002a).
The Genetics of Human Skin Color
Skin pigmentation is a highly heritable trait. Twin studies of a sample of 134 Australian twins showed a heritability of 0.83 for skin color, measured at a wavelength of 685 nm at the upper inner arm (Clark et al. 1981). It is not surprising that heritability estimates were lower at other sites that receive more ambient sun exposure. Anthropologists frequently measure skin color on the upper inner arm, although this site is also dependent on sun exposure and shows a seasonal trend (Lock-Andersen and Wulf 1997). In general, the greater the level of constitutive pigmentation, the greater the ability to develop facultative pigment in response to UVR. Although this topic has received little systematic study, it appears that the increase in pigmentation is proportional to the inflammatory damage following UVR, when basal skin color is taken into account (Wagner et al. 2002b).
A large number of anthropological studies have been performed measuring skin color in diverse populations, although differences in instrumentation—and failure to account for differences in sun exposure—mean that these studies are not always directly comparable. Relethford (2002) has performed a useful review and reanalysis of some of the studies. Unlike many traits, and as is now well known, most variance in skin color is between continents rather than within continents, which is compatible with the view that there has been strong and recent selection on skin pigmentation. Variability (within a region) is greater in Africa than in other regions (Relethford 2000).
Although estimates of the number of genes involved in determination of human skin color have been made (e.g., see Harrison and Owen [1964], who have suggested at least four genes), they hardly seem credible because of the limited sampling of human populations, inappropriate assumptions about gene action in the humans studied, and the inability to distinguish between tanning and constitutive skin color. In mice, a large number of alleles (>700) at a large number of loci (>125)—almost half of which have been cloned and sequenced—have been identified that influence coat color and a range of other phenotypes (Bennett and Lamoreux 2003). Some of the human homologues of these genes are known to cause rare Mendelian disorders that involve pigmentation, with or without extracutaneous effects (e.g., Waardenburg-Shah syndrome [MIM 277580] and piebaldism [MIM 172800] [Jackson 1997; Rees 2003]), but, to date, only one gene, the melanocortin 1 receptor gene (MC1R), has been implicated in normal variation in human skin and hair color (Rees 2003). MC1R therefore has been discussed in depth, although it seems likely that other candidates from mice will be important in determination of variation in normal human skin color (Barsh 2003).
MC1R
The elucidation of the role of human MC1R in pigmentation followed the identification of the mc1r as the locus underpinning a series of mutations in mice at the classical coat-color locus extension (Silvers 1979; Robbins et al. 1993). In recessive yellow mice (e−/−), mc1r loss-of-function alleles lead to a yellow, pheomelanic coat (low eumelanin:pheomelanic ratio), whereas dominant gain-of-function alleles lead to a dark, eumelanic coat (high eumelanin:pheomelanin ratio) (Cone et al. 1996). Subsequently, MC1R mutations have been found to underpin a range of analogous coat-color mutants in dogs, sheep, foxes, horses, and birds (Klungland et al. 1995; Marklund et al. 1996; Våge et al. 1997, 2003; Kijas et al. 1998; Newton et al. 2000; Theron et al. 2001; Ling et al. 2003).
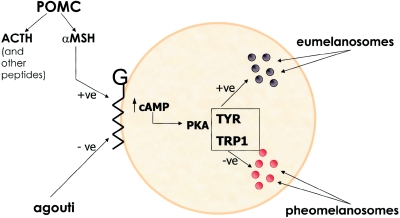
The human MC1R gene encodes a 317–amino acid member of the subfamily of melanocortin receptors of G-coupled receptors. Other receptors in this family include MC2R—the receptor for ACTH—and MC4R, which plays a central role in body-weight regulation. Mutations in either MC2R or MC4R lead to disease states (glucocorticoid deficiency 1 [MIM 202200] and melanocortin 4 receptor [MIM 155541], respectively). α-Melanocyte–stimulating hormone (αMSH), a cleavage product of pro-opiomelanocortin (POMC), is a ligand at the MC1R gene, and activation leads to an increase in cellular cAMP. ACTH, at least in vitro and in pharmacological doses in humans, also has activity at the receptor MC1R (Hunt et al. 1994; Cone et al. 1996). Agouti-signaling protein (ASP) antagonizes the effects of αMSH at MC1R, with overexpression of agouti in mice leading to yellow coat color (fig. 2). The role of agouti in humans is as yet unclear (see below). Finally, it would seem that the MC1R receptor has some degree of constitutive (ligand-independent) activity (Sanchez-Mas et al. 2004).
Figure 2.
A schematic melanocyte that shows the membrane-bound G-coupled receptor MC1R (indicated by “G”) and intracellular and extracellular signaling pathways. “+ve” and “−ve” indicate positive and negative influences, respectively, on the ratio of eumelanin to pheomelanin. TYR = tyrosinase; TRP1 = tyrosinase related protein 1.
The exact pathways by which elevation in intracellular cAMP leads to changes in melanin production are imperfectly understood. Elevation in cAMP leads to activation of protein kinase A (PKA), which in turn leads to increased transcription of microphthalmia transcription factor (MITF). MITF appears to be a key player in the control of melanogenesis that leads to increased transcription of a range of genes (including genes encoding tyrosinase and tyrosinase-related protein 1 and 2) involved in the control of the relative and absolute amounts of eumelanin and pheomelanin (reviewed by Bertolotto [2002]) (fig. 2).
In humans, early, small association studies in Northern Europeans showed that nonsynonymous changes at the MC1R gene were common and that such variants were seen more commonly in persons with red hair and pale skin (Valverde et al. 1995). Red hair has been shown, by the majority (but not all) of previous studies (e.g., Neel 1943), to approximate to an autosomal recessive trait. The early human MC1R association studies were therefore difficult to interpret, with respect to the mode of inheritance, because some persons with red hair had one variant, whereas others had two or even three changes (in comparison with the consensus sequence), and it was unclear which of the many changes were functionally significant. A range of studies, including family studies, have been performed subsequently that have largely resolved these issues: heterologous MC1R transfection assays with use of cAMP as an endpoint in response to αMSH, BAC rescue of mc1r-null mice (e−/e−) with use of human MC1R variants, and several large allelic association studies between MC1R variation and phenotype and between MC1R and cancer (Box et al. 1997; Smith et al. 1998; Schioth et al. 1999; Flanagan et al. 2000; Healy et al. 2000, 2001; Duffy et al. 2004; Ringholm et al., in press). The results are further discussed below.
Family studies show that most persons with red hair (>80%) harbor functionally significant changes on both MC1R alleles (Flanagan et al. 2000). These alleles—D84E, R142H, R151C, R160H, and D294H—are highly penetrant (∼0.8) (Flanagan et al. 2000). A number of low-penetrance but common alleles have also been described, including V60L (penetrance of ∼0.15), V92M, and, arguably, R163Q (Flanagan et al. 2000; Duffy et al. 2004). More than 65 nonsynonymous changes have been described in the human MC1R gene (Flanagan et al. 2000; T. H. Wong and J. L. Rees, unpublished data). Large association studies have shown that the highly penetrant alleles have odds ratios (ORs) for red hair of ∼50, whereas the lower penetrant alleles show ORs of ∼5. These figures are obviously population dependent (Duffy et al. 2004), because the prevalence of variant MC1R alleles varies between populations.
These MC1R alleles are also associated with skin (rather than just hair) color phenotypes. Most persons with red hair in Northern European populations have pale, heavily freckled skin that tends to burn easily and to tan very poorly in response to UVR. Studies in Northern European populations have shown that MC1R status underpins tanning ability, skin color, and freckling (Flanagan et al. 2000; Healy et al. 2000; Bastiaens et al. 2001a; Duffy et al. 2004).
Some of these characteristics can be assessed quantitatively or semiquantitatively; when this is done, a clear heterozygote or dosage effect is apparent. For instance, the number of body sites affected by freckling is intermediate in heterozygotes, as compared with consensus sequence or those with homozygous-diminished function alleles (Flanagan et al. 2000; Bastiaens et al. 2001a). Similarly, if hair color is measured using objective spectrophotometry or by high-performance liquid-chromatography assessment of melanins, a clear heterozygous effect is seen (Naysmith et al. 2004). Skin color can also be assessed spectrophotometrically, although it would seem the magnitude of the heterozygote effect (and absolute effect of MC1R) on skin color is less than that for hair color (Duffy et al. 2004).
In populations such as those of the British Isles, over half the population will be heterozygotes, with ∼8% being homozygous for the highly penetrant diminished-function alleles (Smith et al. 1998; Flanagan et al. 2000).
Transfection studies in heterologous cell types through use of cAMP as a readout, have confirmed the markedly diminished function of the R142H, R151C, R160H, and D294H alleles (Frändberg et al. 1998; Schioth et al. 1999; Ringholm et al., in press). Attempts to rescue the pigmentation defect of mice null at the extension (e−/e−) locus by use of BACs containing human alleles have also confirmed that these human alleles have diminished—but not complete loss—of function (Healy et al. 2001). The mouse studies also suggest that the various human alleles should not be considered functionally equivalent in terms of the degree of loss of function (Healy et al 2001). Recent heterologous transfection studies are in keeping with these findings and suggest that many of the range of mutations have different dose-response kinetics (Ringholm et al., in press). When viewed together with the range of ORs for red hair with different MC1R alleles, these studies suggest that variation in the degree and type of pigment can be achieved by (i) dosage effect of one or two alleles and (ii) a range of mutations with quantitatively different effects on binding and signaling through the MC1R gene. One locus can therefore produce a graded range of phenotypes, albeit for just one aspect of the total pigmentary phenotype.
The previous discussion of MC1R covered loss-of-function or diminished-function alleles. In mice and other animals, gain-of-function mutations at the MC1R gene can also be found. These produce a dark (eumelanic) coat color (Cone et al. 1996; Kijas et al. 1998; Våge et al. 1999), but no such mutations have been found in humans. Similarly, in mice, there is a competitive antagonist to the mc1r—agouti—which can be switched on in a body-site-specific manner to produce a yellow hair color (Miller et al. 1993; Vrieling et al. 1994). In humans, the physiological role of the homologue of agouti—ASP—is uncertain, although, of course, body site differences in hair color are known. For instance, beard color is often redder than scalp hair, and there is evidence of an MC1R effect on beard color (Flanagan et al. 2000). Two association studies of agouti allelic variants and human pigmentary phenotype have been published, one showing a weak (OR <2) association between a SNP in the 3′ UTR of agouti-related peptide and dark hair (and brown eyes) and the other failing to find such an association (Kanetsky et al. 2002; Zeigler-Johnson et al. 2004). This issue will be resolved only with a combination of more-precise phenotyping and a better understanding of the functional consequences of the various SNPs.
Finally, the importance of the MC1R-signaling pathway in humans was confirmed, and the need for ligand at the receptor (rather then just constitutive activity by the receptor) was shown by the identification of individuals with loss-of-function mutations of the αMSH precursor molecule—POMC—with a complex endocrine phenotype that included red hair (Krude et al. 1998) (fig. 2).
Population Variation at MC1R
Several systematic or case-based studies of MC1R diversity have been published. In African populations, low genetic diversity at MC1R suggests it is under functional constraint, whereas diversity is increased in European populations and, to a lesser degree, in Asian populations (Rana et al. 1999; Harding et al. 2000; Makova et al. 2001). Two interpretations of these findings have been considered. One is that there is selection for functionally significant variants in non-African populations (Rana et al. 1999; Makova et al. 2001). Others show that the diversity in the non-African populations is not beyond what might be expected under neutral theory (Harding et al. 2000). In terms of the latter interpretation, the limited power of the testing, given available data sets, needs to be borne in mind. Dating of some of the mutations under a coalescent model that assumes neutrality suggests ages of ∼25,000–50,000 years (Harding et al. 2000).
Several case reports have also suggest that MC1R may exert phenotypic effects on a number of different genetic backgrounds and may not be important just in Northern European populations. King et al. (2003) reported that albinos with P gene mutations, who would be expected to have yellow hair, have red hair if (in addition to mutation at P) MC1R mutations are present. McKenzie and colleagues reported individuals from Jamaica who are “black-skinned” but have red hair and are homozygous for diminished-function MC1R alleles (McKenzie et al. 2003). The hair melanin levels in these persons were within the range that is seen in persons with red hair in northern U.K. populations (J.L.R., unpublished data). Although precise measurements of skin color were not available, these persons were still “dark skinned,” again compatible with the idea that the magnitude of MC1R effects on hair and skin are quantitatively different and that it is more meaningful to think in term of a quantitative trait rather than penetrance of “red/not red.”
An elegant demonstration of interaction between MC1R and other loci was provided by studies of MC1R status in families with the melanoma-predisposition gene p16 (Box et al. 2001; van der Velden et al. 2001). In these families, as one might expect, those who carried MC1R variants in addition to a p16 mutation presented with melanomas at an earlier age than did those who carried a p16 mutation but showed consensus MC1R sequence.
Other Skin Pigmentation Genes in Humans
With the possible exceptions of ASP (Kanetsky et al. 2002) and a single publication reporting epistasis between P and MC1R in Tibetans (Akey et al. 2001), MC1R is the only gene identified that underpins normal physiological variation in humans. It does, however, seem likely that some of the other genes implicated in mouse fancy mutations and in highly penetrant human disorders may be important (Barsh 2003). There are several reasons why such putative effects have not come to light so far: (i) some alleles (as with MC1R) may have a modest effect, (ii) human phenotyping is often poor and confounded by sun exposure, and (iii) large-scale sequencing of genes and their control regions on a large number of subjects has not been performed.
More than Just Pigment
A simplistic model views pigment as a single homogeneous substance that is distributed as a continuous variable in the population and achieves its biological effects by blocking UVR photons that reach the viable layers of the epidermis and dermis. Genes that influence the degree of pigmentation would uniformly influence skin cancer rates and other endpoints for UVR sensitivity, such as erythema. Such influence could be quantified by measures of skin color. This caricature is, of course, misleading and why it is so is illustrative.
First, pigmentation measured as skin color explains much of the variation in skin cancer rates and erythemal sensitivity, but not all (Wagner et al. 2002b; Ha et al. 2003a; Waterston et al., in press). Within even relatively homogeneous Europeans, there is considerable variation in skin cancer rates and erythemal responsiveness that is unaccounted for, even when pigmentation is taken into account (Ha et al. 2003a; Waterston et al., in press). Other factors, such as DNA repair or other modulators of repair, may also be important, as are differences in environmental exposure. Second, the use of skin color as a proxy for melanin is a simplification. Melanin is a mixture of poorly characterized biopolymers, and their physical properties in the visible region in vivo may not reflect adequately their characteristics in the UV spectrum in vivo, nor do most current assays capture differences in structure and packaging of melanin within the epidermis (Alaluf et al. 2001, 2002a; Tadokoro et al. 2003). Third, genes involved in pigmentation may play other roles in skin physiology.
MC1R Effects on Skin Cancer, Independent of Pigmentary Status
MC1R is expressed in melanocytes, and the historically described phenotype in mice null for mc1r is a sole defect in the pigmentary system, although recent studies indicate that mc1r status may also determine aspects of nociception (Mogil et al. 2003). Recent studies in humans support the assertion that those with red hair may respond differently to anaesthetic agents and or analgesics (Liem et al. 2004). A variety of cell types—including keratinocytes, inflammatory cells, and dermal cells—express MC1R, and some work suggests that this receptor is functionally important in these cells (Bohm et al. 1999, 2002, 2004; Luger et al. 2003). Allelic association studies of MC1R status and cancer have consistently shown that, even when adjustments for skin phenotype are made, an influence of MC1R is still evident (Valverde et al. 1996; Palmer et al. 2000; Bastiaens et al. 2001b; Kennedy et al. 2001). These findings have suggested that MC1R may play a role beyond its influence on the pigmentary phenotype. It is important that the association with MC1R genotype, after pigmentary phenotype is accounted for, is present for both melanoma and nonmelanoma skin cancers (NMSCs), which suggests that the association is not due to an autonomous effect on melanocyte growth (Palmer et al. 2000; Bastiaens et al. 2001b; Kennedy et al. 2001). Two explanations for this association have been suggested. First, the MC1R-signaling pathway is playing an undescribed role in cutaneous biology, a theme for which there is considerable supportive literature, but which, at the level of physiological relevance, is hard to interpret. Second, current measures of skin phenotype, such as color or self-reported burning, may miss some aspect of the phenotype determined by MC1R status. For instance, if skin color alone is used as a phenotypic measure, then it is possible to imagine that information about the different amounts of the different types of melanin, influenced by MC1R, will not be captured (Alaluf et al. 2001, 2002a, 2002b). Dwyer and colleagues have recently studied in greater detail the relationship among MC1R status, skin, and various types of skin cancer (Dwyer et al. 2004). They assessed skin color spectrophotometrically, which is preferable to subjective judgment. This type of measurement allowed Dwyer et al. (2004) to show (through use of receiver operator curves, a statistical method that, in this context, examines the ability of explanatory or screening tests to predict outcomes) that the influence of MC1R status beyond skin color is small and unlikely to be clinically useful. However, even the measure of skin color they use may capture only some of the variation in skin pigmentation, when assessed using histological methods (Dwyer et al. 1998). Nonetheless, their study highlights our inadequate understanding of the physiology of the relationship among MC1R-sequence change, epidermal melanin, skin color, and cancer risk.
Nonpigmentary Influences on Skin Sensitivity to UVR
The study of the effects of pigmentation genes on sun sensitivity has been greatly facilitated by the prior study of coat-color mutations that allow gene identification in mice and subsequent candidate studies in humans. It is worth emphasizing how much more difficult it would have been—without the intermediate phenotype of red hair (with an OR for some alleles of ∼50), mouse models, and simple assay systems in which to test variants—to identify the relationship between MC1R SNPs (>75 in the coding region of a gene <1 Kb) and the complex disease of cutaneous cancer. The study of other genetic influences on sun sensitivity has not shared these advantages, and the resultant literature is far less decisive. In what follows, I illustrate what we know of the genetics of sun sensitivity with two simple endpoints: first, erythemal response, as the short-term intermediate phenotype, and, second, the various forms of skin cancer that are the result of chronic exposure.
UVR Sensitivity and Skin Cancer
A limited number of studies have assessed the heritability of skin cancer. No studies have been performed that have attempted to partition variance between pigmentary effects and nonpigmentary effects.
Large and well-conducted twin studies have failed to show a need for heritable factors in explanation of the risk of NMSC in Finland (Milan et al. 1998). Similarly, large studies in Scandinavia have shown only a modest (18%) heritable contribution to melanoma risk (Hemminki et al. 2001). The specificity of these findings needs to be remembered, in that the heritable component is, by definition, dependent on the degree of genetic diversity of the population studied. A very different result would have been obtained in a more genetically admixed population, such as in parts of Africa (Ha and Rees 2000; Naysmith et al. 2004). The finding is, however, in keeping with the observation that the heritability of most human cancers is small (Hoover 2000). They also suggest that influences beyond the known pigmentary factors (which are subsumed within these figures) may be difficult to detect. Twin or family studies on erythemal sensitivity have not been conducted. Given the possibility of similarities in sun-seeking behavior within families, twin studies would be particularly helpful. Environmental influences are relatively unexplored. For instance, dietary effects, albeit with large supplements, are known to influence acute UVR sensitivity (Rhodes et al. 1994).
Despite the relatively low heritability of skin cancer, a number of allelic associations between various candidate genes and skin cancer have been reported. Most plausible are reports of associations between genes involved in DNA repair and skin cancer. As discussed above, patients with XP have a grossly elevated risk of both melanoma and NMSC (Bootsma et al. 2001). Various studies in normal populations have recently reported associations between some of the genes implicated in XP and skin cancer (Dybdahl et al. 1999; Winsey et al. 2000; Tomescu et al. 2001). Associations between skin cancer and genes involved in control of UVR-induced oxidative damage to skin—such as glutathione transferases—and those involved in the immune response in skin—such as TNF-α—have also been reported (Lear et al. 1997; Hajeer et al. 2000; Ramsay et al. 2001). The reported ORs for all these candidate-gene studies have tended to be modest (<2), and some results have not been confirmed in other populations, so it is possible that they are due to chance.
A few allelic association studies between candidate genes and experimentally induced UVR erythema have also been reported. After reports of associations between the codon 72 P53 polymorphism and various types of cancer (including skin cancer), associations were reported between these same polymorphisms and the erythemal response (McGregor et al. 2002). A subsequent study has failed to confirm these findings, which suggests that the association of particular P53 alleles with groups that have different genetic ancestries (and hence skin color) might have confounded the detection of erythema (Manson et al. 2004). A single study has suggested that allelic variants in some of the glutathione S transferase (GST) family of genes might be related to acute erythemal responses, although the reported effects are modest (Kerb et al. 2002).
Conclusions
Given its interest for a wide range of scientific disciplines, it is worth consideration why more progress has not already been made in determination of the genetic determinants of human UVR sensitivity. This is particularly so given the resource of the mouse fancy strains and the power of modern mouse genetics. There are a number of factors that might account for this relative lack of progress, some of which are of historical interest only. First, the availability of cheap large-scale sequencing now means that hunting for differences in noncoding regions has become much easier. Second, distinguishing causal changes from neutral changes in allelic association studies between populations remains challenging. Third, we remain ignorant of the heritability of some basic aspects of the UVR response. Fourth, studies that try to explain variation in erythemal response have suffered from a lack of a useful animal or physiological model, from ignorance of the magnitude of the likely heritable influence, and, consequently, from inadequate attention to statistical design. Finally, human phenotyping has not always been performed in a way that allows studies to be repeated in other populations with equivalent methods, nor has it always been sufficiently sensitive—and, at any one time, there are several methods being used by different researchers to measure what is assumed to be the same characteristic (Dwyer et al. 1998; Rees 2002a; Wagner et al. 2002a; Tadokoro et al. 2003; Oh et al., in press; Waterston et al., in press). If we are to understand the evolution of skin pigmentation and sensitivity to UVR, standardized quantitative methods that can be applied in diverse world populations are needed.
Acknowledgments
This work was supported by The Wellcome Trust. I thank Karen Waterston for artwork.
Electronic-Database Information
The URL for data presented herein is as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for porphyria, albinism, XP, Waardenburg-Shah syndrome, piebaldism, glucocorticoid deficiency 1, and melanocortin 4 receptor)
References
- Akey JM, Wang H, Xiong M, Wu H, Liu W, Shriver MD, Jin L (2001) Interaction between the melanocortin-1 receptor and P genes contributes to inter-individual variation in skin pigmentation phenotypes in a Tibetan population. Hum Genet 108:516–520 [DOI] [PubMed] [Google Scholar]
- Alaluf S, Atkins D, Barrett K, Blount M, Carter N, Heath A (2002a) Ethnic variation in melanin content and composition in photoexposed and photoprotected human skin. Pigment Cell Res 15:112–118 [DOI] [PubMed] [Google Scholar]
- ——— (2002b) The impact of epidermal melanin on objective measurements of human skin colour. Pigment Cell Res 15:119–126 [DOI] [PubMed] [Google Scholar]
- Alaluf S, Barrett K, Blount M, Carter N (2003) Ethnic variation in tyrosinase and TYRP1 expression in photoexposed and photoprotected human skin. Pigment Cell Res 16:35–42 [DOI] [PubMed] [Google Scholar]
- Alaluf S, Heath A, Carter N, Atkins D, Mahalingam H, Barrett K, Kolb R, Smit N (2001) Variation in melanin content and composition in type V and VI photoexposed and photoprotected human skin: the dominant role of DHI. Pigment Cell Res 14:337–347 [DOI] [PubMed] [Google Scholar]
- Barsh GS (1996) The genetics of pigmentation: from fancy genes to complex traits. Trends Genet 12:299–305 [DOI] [PubMed] [Google Scholar]
- ——— (2003) What controls variation in human skin color? PLoS Biol 1:E27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaens M, ter Huurne J, Gruis N, Bergman W, Westendorp R, Vermeer BJ, Bouwes Bavinck JN (2001a) The melanocortin-1-receptor gene is the major freckle gene. Hum Mol Genet 10:1701–1708 [DOI] [PubMed] [Google Scholar]
- Bastiaens MT, ter Huurne JAC, Kielich C, Gruis NA, Westendorp RGJ, Vermeer BJ, Bavinck JN, for the Leiden Skin Cancer Study Team (2001b) Melanocortin-1 receptor gene variants determine the risk of nonmelanoma skin cancer independently of fair skin and red hair. Am J Hum Genet 68:884–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DC, Lamoreux ML (2003) The color loci of mice: a genetic century. Pigment Cell Res 16:333–344 [DOI] [PubMed] [Google Scholar]
- Berg RJ, Ruven HJ, Sands AT, de Gruijl FR, Mullenders LH (1998) Defective global genome repair in XPC mice is associated with skin cancer susceptibility but not with sensitivity to UVB induced erythema and edema. J Invest Dermatol 110:405–409 [DOI] [PubMed] [Google Scholar]
- Bertolotto C (2002) The molecular mechanism of cAMP induced melanogenesis. In: Ortonne J-P, Ballotti R (eds) Mechanisms of suntanning. Martin Dunitz, London, pp 99–108 [Google Scholar]
- Bessou-Touya S, Picardo M, Maresca V, Surlève-Bazeille JE, Pain C, Taïeb A (1998) Chimeric human epidermal reconstructs to study the role of melanocytes and keratinocytes in pigmentation and photoprotection. J Invest Dermatol 111:1103–1108 [DOI] [PubMed] [Google Scholar]
- Bodmer WF, Cavalli-Sforza LL (1976) Genetics, evolution, and man. W H Freeman, San Francisco [Google Scholar]
- Bohm M, Metze D, Schulte U, Becher E, Luger TA, Brzoska T (1999) Detection of melanocortin-1 receptor antigenicity on human skin cells in culture and in situ. Exp Dermatol 8:453–461 [DOI] [PubMed] [Google Scholar]
- Bohm M, Raghunath M, Sunderkotter C, Schiller M, Stander S, Brzoska T, Cauvet T, Schioth HB, Schwarz T, Luger TA (2004) Collagen metabolism is a novel target of the neuropeptide α-melanocyte-stimulating hormone. J Biol Chem 279:6959–6966 [DOI] [PubMed] [Google Scholar]
- Bohm M, Schiller M, Stander S, Seltmann H, Li Z, Brzoska T, Metze D, Schioth HB, Skottner A, Seiffert K, Zouboulis CC, Luger TA (2002) Evidence for expression of melanocortin-1 receptor in human sebocytes in vitro and in situ. J Invest Dermatol 118:533–539 [DOI] [PubMed] [Google Scholar]
- Bootsma D, Kraemer KH, Cleaver JE, Hoeijmakers JHJ (2001) Nucleotide excision repair syndromes: xeroderma pigmentosum, Cockayne syndrome, and trichothiodystrophy. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B (eds) The metabolic and molecular bases of inherited disease, 8th ed. McGraw Hill, New York, pp 677–704 [Google Scholar]
- Box NF, Duffy DL, Chen W, Stark M, Martin NG, Sturm RA, Hayward NK (2001) MC1R genotype modifies risk of melanoma in families segregating CDKN2A mutations. Am J Hum Genet 69:765–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box NF, Wyeth JR, O’Gorman LE, Martin NG, Sturm RA (1997) Characterization of melanocyte stimulating hormone receptor variant alleles in twins with red hair. Hum Mol Genet 6:1891–1897 [DOI] [PubMed] [Google Scholar]
- Cavalli-Sforza LL, Menozzi P, Piazza A (1996) The history and geography of human genes. Princeton University Press, Princeton, NJ [Google Scholar]
- Clark P, Stark AE, Walsh RJ, Jardine R, Martin NG (1981) A twin study of skin reflectance. Ann Hum Biol 8:529–541 [DOI] [PubMed] [Google Scholar]
- Cone RD, Lu D, Koppula S, Vage DI, Klungland H, Boston B, Chen W, Orth DN, Pouton C, Kesterson RA (1996) The melanocortin receptors: agonists, antagonists, and the hormonal control of pigmentation. Rec Prog Horm Res 51:287–317 [PubMed] [Google Scholar]
- Diffey BL (1999) Human exposure to ultraviolet radiation. In: Hawk JLM (ed) Photodermatology. Arnold, London pp 5–24 [Google Scholar]
- Duffy DL, Box NF, Chen W, Palmer JS, Montgomery GW, James MR, Hayward NK, Martin NG, Sturm RA (2004) Interactive effects of MC1R and OCA2 on melanoma risk phenotypes. Hum Mol Genet 13:447–461 [DOI] [PubMed] [Google Scholar]
- Dwyer T, Muller HK, Blizzard L, Ashbolt R, Phillips G (1998) The use of spectrophotometry to estimate melanin density in Caucasians. Cancer Epidemiol Biomarkers Prev 7:203–206 [PubMed] [Google Scholar]
- Dwyer T, Stankovich JM, Blizzard L, FitzGerald LM, Dickinson JL, Reilly A, Williamson J, Ashbolt R, Berwick M, Sale MM (2004) Does the addition of information on genotype improve prediction of the risk of melanoma and nonmelanoma skin cancer beyond that obtained from skin phenotype? Am J Epidemiol 159:826–833 [DOI] [PubMed] [Google Scholar]
- Dybdahl M, Vogel U, Frentz G, Wallin H, Nexo BA (1999) Polymorphisms in the DNA repair gene XPD: correlations with risk and age at onset of basal cell carcinoma. Cancer Epidemiol Biomarkers Prev 8:77–81 [PubMed] [Google Scholar]
- Everett MA (1961) Protection from sunlight in vitiligo. Arch Dermatol 84:997–998 [DOI] [PubMed] [Google Scholar]
- Farr PM, Diffey BL (1985) The erythemal response of human skin to ultraviolet radiation. Br J Dermatol 113:65–76 [DOI] [PubMed] [Google Scholar]
- Flanagan N, Healy E, Ray A, Philips S, Todd C, Jackson IJ, Birch-Machin MA, Rees JL (2000) Pleiotropic effects of the melanocortin 1 receptor (MC1R) gene on human pigmentation. Hum Mol Genet 9:2531–2537 [DOI] [PubMed] [Google Scholar]
- Frändberg PA, Doufexis M, Kapas S, Chhajlani V (1998) Human pigmentation phenotype: a point mutation generates nonfunctional MSH receptor. Biochem Biophys Res Commun 245:490–492 [DOI] [PubMed] [Google Scholar]
- Gilchrest BA, Soter NA, Stoff JS, Mihm MC (1981) The human sunburn reaction: histologic and biochemical studies. J Am Acad Dermatol 5:411–422 [DOI] [PubMed] [Google Scholar]
- Ha T, Javedan H, Waterston K, Naysmith L, Rees JL (2003a) The relation between constitutive pigmentation and sensitivity to ultraviolet radiation induced erythema is dose dependant. Pigment Cell Res 16:477–479 [DOI] [PubMed] [Google Scholar]
- Ha T, Naysmith L, Waterston K, Oh C, Weller R, Rees JL (2003b) Defining the quantitative contribution of the melanocortin 1 receptor (MC1R) to variation in pigmentary phenotype. Ann NY Acad Sci 994:339–347 [DOI] [PubMed] [Google Scholar]
- Ha T, Rees JL (2000) Keeping genetics simple. Acta Derm Venereol 80:401–403 [DOI] [PubMed] [Google Scholar]
- Ha T, Waterston K, Bisset Y, Ray A, Rees JL (2003c) The time course of ultraviolet B induced erythema in people with red hair harbouring homozygous melanocortin 1 receptor (MC1R) mutations. Exp Dermatol 12:514–517 [DOI] [PubMed] [Google Scholar]
- Hajeer AH, Lear JT, Ollier WE, Naves M, Worthington J, Bell DA, Smith AG, Bowers WP, Jones PW, Strange RC, Fryer AA (2000) Preliminary evidence of an association of tumour necrosis factor microsatellites with increased risk of multiple basal cell carcinomas. Br J Dermatol 142:441–445 [DOI] [PubMed] [Google Scholar]
- Harding RM, Healy E, Ray AJ, Ellis NS, Flanagan N, Todd C, Dixon C, Sajantila A, Jackson IJ, Birch-Machin MA, Rees JL (2000) Evidence for variable selective pressures at the human pigmentation locus, MC1R. Am J Hum Genet 66:1351–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison GA, Owen JJT (1964) Studies on the inheritance of human skin colour. Ann Hum Genet 28:27–37 [DOI] [PubMed] [Google Scholar]
- Healy E, Flannagan N, Ray A, Todd C, Jackson IJ, Matthews JNS, Birch-Machin MA, Rees JL (2000) Melanocortin-1-receptor gene and sun sensitivity in individuals without red hair. Lancet 355:1072–1073 [DOI] [PubMed] [Google Scholar]
- Healy E, Jordan SA, Budd PS, Suffolk R, Rees JL, Jackson IJ (2001) Functional variation of MC1R alleles from red-haired individuals. Hum Mol Genet 10:2397–2402 [DOI] [PubMed] [Google Scholar]
- Hemminki K, Lonnstedt I, Vaittinen P, Lichtenstein P (2001) Estimation of genetic and environmental components in colorectal and lung cancer and melanoma. Genet Epidemiol 20:107–116 [DOI] [PubMed] [Google Scholar]
- Holick MF (2001) Sunlight “D”ilemma: risk of skin cancer or bone disease and muscle weakness. Lancet 357:4–6 [DOI] [PubMed] [Google Scholar]
- Hoover RN (2000) Cancer—nature, nurture, or both. N Engl J Med 343:135–136 [DOI] [PubMed] [Google Scholar]
- Hunt G, Todd C, Kyne S, Thody AJ (1994) ACTH stimulates melanogenesis in cultured human melanocytes. J Endocrinol 140:R1–R3 [DOI] [PubMed] [Google Scholar]
- Ito S (2003) The IFPCS presidential lecture: a chemist’s view of melanogenesis. Pigment Cell Res 16:230–236 [DOI] [PubMed] [Google Scholar]
- Jackson IJ (1997) Homologous pigmentation mutations in human, mouse and other model organisms. Hum Mol Genet 6:1613–1624 [DOI] [PubMed] [Google Scholar]
- Kanetsky PA, Swoyer J, Panossian S, Holmes R, Guerry D, Rebbeck TR (2002) A polymorphism in the agouti signaling protein gene is associated with human pigmentation. Am J Hum Genet 70:770–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C, ter Huurne J, Berkhout M, Gruis N, Bastiaens M, Bergman W, Willemze R, Bouwes Bavinck JN (2001) Melanocortin 1 receptor (MC1R) gene variants are associated with an increased risk for cutaneous melanoma which is largely independent of skin type and hair color. J Invest Dermatol 117:294–300 [DOI] [PubMed] [Google Scholar]
- Kerb R, Brockmoller J, Schlagenhaufer R, Sprenger R, Roots I, Brinkmann U (2002) Influence of GSTT1 and GSTM1 genotypes on sunburn sensitivity. Am J Pharmacogenomics 2:147–154 [DOI] [PubMed] [Google Scholar]
- Kijas JMH, Wales R, Törnsten A, Chardon P, Moller M, Andersson L (1998) Melanocortin receptor 1 (MC1R) mutations and coat color in pigs. Genetics 150:1177–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RA, Hearing VJ, Creel DJ, Oetting WS (2001) Albinism. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B (eds) The metabolic and molecular bases of inherited disease, 8th ed. McGraw Hill, New York, pp 5587–5628 [Google Scholar]
- King RA, Willaert RK, Schmidt RM, Pietsch J, Savage S, Brott MJ, Fryer JP, Summers CG, Oetting WS (2003) MC1R mutations modify the classic phenotype of oculocutaneous albinism type 2 (OCA2). Am J Hum Genet 73:638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klungland H, Vage DI, Gomez-Raya L, Adalsteinsson S, Lien S (1995) The role of melanocyte-stimulating hormone (MSH) receptor in bovine coat color determination. Mamm Genome 6:636–639 [DOI] [PubMed] [Google Scholar]
- Kolgen W, Van Steeg H, van der Horst GT, Hoeijmakers JH, Van Vloten WA, de Gruijl FR, Garssen J (2003) Association of transcription-coupled repair but not global genome repair with ultraviolet-B-induced Langerhans cell depletion and local immunosuppression. J Invest Dermatol 121:751–756 [DOI] [PubMed] [Google Scholar]
- Kromberg JG, Castle D, Zwane EM, Jenkins T (1989) Albinism and skin cancer in Southern Africa. Clin Genet 36:43–52 [DOI] [PubMed] [Google Scholar]
- Krude H, Biebermann H, Luck W, Horn R, Brabant G, Grüters A (1998) Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nature Genet 19:155–157 [DOI] [PubMed] [Google Scholar]
- Kulms D, Schwarz T (2000) Molecular mechanisms of UV-induced apoptosis. Photodermatol Photoimmunol Photomed 16:195–201 [DOI] [PubMed] [Google Scholar]
- Lear JT, Smith AG, Bowers B, Heagearty AH, Jones PW, Gilford J, Alldersea J, Strange RC, Fryer AA (1997) Truncal tumor site is associated with high risk of multiple basal cell carcinoma and is influenced by glutathione S-transferase, GSTT1, and cytochrome P450, CYP1A1 genotypes, and their interaction. J Invest Dermatol 108:519–522 [DOI] [PubMed] [Google Scholar]
- Liem EB, Lin CM, Suleman MI, Doufas AG, Gregg RG, Veauthier JM, Loyd G, Sessler DI (2004) Anesthetic requirement is increased in redheads. Anesthesiology 101:279–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling MK, Lagerstrom MC, Fredriksson R, Okimoto R, Mundy NI, Takeuchi S, Schioth HB (2003) Association of feather colour with constitutively active melanocortin 1 receptors in chicken. Eur J Biochem 270:1441–1449 [DOI] [PubMed] [Google Scholar]
- Lock-Andersen J, Wulf HC (1997) Seasonal variation of skin pigmentation. Acta Derm Venereol 77:219–221 [DOI] [PubMed] [Google Scholar]
- Lookingbill DP, Lookingbill GL, Leppard B (1995) Actinic damage and skin cancer in albinos in northern Tanzania: findings in 164 patients enrolled in an outreach skin care program. J Am Acad Dermatol 32:653–658 [DOI] [PubMed] [Google Scholar]
- Luger TA, Scholzen TE, Brzoska T, Bohm M (2003) New insights into the functions of α-MSH and related peptides in the immune system. Ann N Y Acad Sci 994:133–140 [DOI] [PubMed] [Google Scholar]
- Makova KD, Ramsay M, Jenkins T, Li WH (2001) Human DNA sequence variation in a 6.6-kb region containing the melanocortin 1 receptor promoter. Genetics 158:1253–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson C, Naysmith L, Waterston K, Melton DW, Rees JL (2004) No association between p53 codon 72 polymorphisms and erythemal response. J Invest Dermatol 122:1334–1335 [DOI] [PubMed] [Google Scholar]
- Marklund L, Moller MJ, Sandberg K, Andersson L (1996) A missense mutation in the gene for melanocyte-stimulating hormone receptor (MC1R) is associated with the chestnut coat color in horses. Mamm Genome 7:895–899 [DOI] [PubMed] [Google Scholar]
- McGregor JM, Harwood CA, Brooks L, Fisher SA, Kelly DA, O’nions J, Young AR, Surentheran T, Breuer J, Millard TP, Lewis CM, Leigh IM, Storey A, Crook T (2002) Relationship between p53 codon 72 polymorphism and susceptibility to sunburn and skin cancer. J Invest Dermatol 119:84–90 [DOI] [PubMed] [Google Scholar]
- McKenzie CA, Harding RM, Tomlinson JB, Ray AJ, Wakamatsu K, Rees JL (2003) Phenotypic expression of melanocortin-1 receptor (MC1R) mutations in black Jamaicans. J Invest Dermatol 121:207 [DOI] [PubMed] [Google Scholar]
- Milan T, Kaprio J, Verkasalo PK, Jansen CT, Teppo L, Koskenvuo M (1998) Hereditary factors in basal cell carcinoma of the skin: a population-based cohort study in twins. Br J Cancer 78:1516–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Duhl DM, Vrieling H, Cordes SP, Ollmann MM, Winkes BM, Barsh GS (1993) Cloning of the mouse agouti gene predicts a secreted protein ubiquitously expressed in mice carrying the lethal yellow mutation. Genes Dev 7:454–467 [DOI] [PubMed] [Google Scholar]
- Minwalla L, Zhao Y, Le Poole IC, Wickett RR, Boissy RE (2001) Keratinocytes play a role in regulating distribution patterns of recipient melanosomes in vitro. J Invest Dermatol 117:341–347 [DOI] [PubMed] [Google Scholar]
- Mogil JS, Wilson SG, Chesler EJ, Rankin AL, Nemmani KVS, Lariviere WR, Groce MK, Wallace MR, Kaplan L, Staud R, Ness TJ, Glover TL, Stankova M, Mayorov A, Hruby VJ, Grisel JE, Fillingim RB (2003) The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc Natl Acad Sci USA 100:4867–4872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G, Young AR, Wulf HC, Kulms D, Schwarz T (2001) The molecular determinants of sunburn cell formation. Exp Dermatol 10:155–160 [DOI] [PubMed] [Google Scholar]
- Naysmith L, Waterston K, Ha T, Flanagan N, Bisset Y, Ray A, Wakamatsu K, Ito S, Rees JL (2004) Quantitative measures of the effect of the melanocortin 1 receptor on human pigmentary status. J Invest Dermatol 122:423–428 [DOI] [PubMed] [Google Scholar]
- Neel JV (1943) Concerning the inheritance of red hair. J Hered 34:93–96 [Google Scholar]
- Newton JM, Wilkie AL, He L, Jordan SA, Metallinos DL, Holmes NG, Jackson IJ, Barsh GS (2000) Melanocortin 1 receptor variation in the domestic dog. Mamm Genome 11:24–30 [DOI] [PubMed] [Google Scholar]
- Oh C, Hennessy A, Ha T, Bisset Y, Diffey B, Rees JL. The time course of photoadaptation and pigmentation studied using a novel methods to distinguish pigmentation from erythema. J Invest Dermatol (in press) [DOI] [PubMed] [Google Scholar]
- Ortonne JP (2002) Photobiology and genetics of malignant melanoma. Br J Dermatol Suppl 146:11–16 [DOI] [PubMed] [Google Scholar]
- Palmer JS, Duffy DL, Box NF, Aitken JF, O’Gorman LE, Green AC, Hayward NK, Martin NG, Sturm RA (2000) Melanocortin-1 receptor polymorphisms and risk of melanoma: is the association explained solely by pigmentation phenotype? Am J Hum Genet 66:176–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay HM, Harden PN, Reece S, Smith AG, Jones PW, Strange RC, Fryer AA (2001) Polymorphisms in glutathione S-transferases are associated with altered risk of non-melanoma skin cancer in renal transplant patients. J Invest Dermatol 117:251–255 [DOI] [PubMed] [Google Scholar]
- Rana BK, Hewett-Emmett D, Jin L, Chang BHJ, Sambuughin N, Lin M, Watkins S, Bamshad M, Jorde LB, Ramsay M, Jenkins T, Li WH (1999) High polymorphism at the human melanocortin 1 receptor locus. Genetics 151:1547–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees JL (2002a) Molecular phototypes. In: Ortonne J-P, Ballotti R (eds) Mechanisms of suntanning. Martin Dunitz, London, pp 333–339 [Google Scholar]
- ——— (2002b) Skin cancer (including nevoid basal cell carcinoma syndrome). In: Vogelstein B, Kinzler K (eds) The genetic basis of human cancer, 2nd ed. McGraw-Hill, New York, pp 529–548 [Google Scholar]
- ——— (2002c) Why are redheads so susceptible to melanoma? In: Newton-Bishop J, Gore M (eds) Melanoma: critical debates. Blackwell Science, Oxford, pp 49–60 [Google Scholar]
- ——— (2003) Genetics of hair and skin colour. Annu Rev Genet 37:67–90 [DOI] [PubMed] [Google Scholar]
- ——— Melanogenesis, skin colour, and sun exposure as cancer risk factors in Africa. In: Ny A (ed) World Health Organization, Geneva (in press) [Google Scholar]
- Relethford JH (2000) Human skin color diversity is highest in sub-Saharan African populations. Hum Biol 72:773–780 [PubMed] [Google Scholar]
- ——— (2002) Apportionment of global human genetic diversity based on craniometrics and skin color. Am J Phys Anthropol 118:393–398 [DOI] [PubMed] [Google Scholar]
- Rhodes LE, O’Farrell S, Jackson MJ, Friedmann PS (1994) Dietary fish-oil supplementation in humans reduces UVB-erythemal sensitivity but increases epidermal lipid peroxidation. J Invest Dermatol 103:151–154 [DOI] [PubMed] [Google Scholar]
- Ringholm A, Klovins J, Rudzish R, Phillips S, Rees JL, Schioth HB. Pharmacological characterization of loss of function mutations of the human melanocortin 1 receptor that are associated with red hair. J Invest Dermatol (in press) [DOI] [PubMed] [Google Scholar]
- Robbins LS, Nadeau JH, Johnson KR, Kelly MA, Roselli-Rehfuss L, Baack E, Mountjoy KG, Cone RD (1993) Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell 72:827–834 [DOI] [PubMed] [Google Scholar]
- Sanchez-Mas J, Hahmann C, Gerritsen I, Garcia-Borron JC, Jimenez-Cervantes C (2004) Agonist-independent, high constitutive activity of the human melanocortin 1 receptor. Pigment Cell Res 17:386–395 [DOI] [PubMed] [Google Scholar]
- Schioth HB, Phillips S, Rudzish R, Birch-Machin M, Wikberg J, Rees JL (1999) Loss of function mutations of the human melanocortin 1 receptor are common and associated with red hair. Biochem Biophys Res Commun 260:488–491 [DOI] [PubMed] [Google Scholar]
- Silvers WK (1979) Coat colors of mice. Springer-Verlag, New York [Google Scholar]
- Smith R, Healy E, Siddiqui S, Flanagan N, Steijlen PM, Rosdahl I, Jacques JP, Rogers S, Turner R, Jackson IJ, Birch-Machin MA, Rees JL (1998) Melanocortin 1 receptor variants in an Irish population. J Invest Dermatol 111:119–122 [DOI] [PubMed] [Google Scholar]
- Soter NA (1990) Acute effects of ultraviolet radiation on the skin. Semin Dermatol 9:11–15 [PubMed] [Google Scholar]
- Stierner U, Rosdahl I, Augustsson A, Kagedal B (1989) UVB irradiation induces melanocyte increase in both exposed and shielded human skin. J Invest Dermatol 92:561–564 [DOI] [PubMed] [Google Scholar]
- Tadokoro T, Kobayashi N, Zmudzka BZ, Ito S, Wakamatsu K, Yamaguchi Y, Korossy KS, Miller SA, Beer JZ, Hearing VJ (2003) UV-induced DNA damage and melanin content in human skin differing in racial/ethnic origin. FASEB J 17:1177–1179 [DOI] [PubMed] [Google Scholar]
- Theron E, Hawkins K, Bermingham E, Ricklefs RE, Mundy NI (2001) The molecular basis of an avian plumage polymorphism in the wild: a melanocortin-1-receptor point mutation is perfectly associated with the melanic plumage morph of the bananaquit, Coereba flaveola. Curr Biol 11:550–557 [DOI] [PubMed] [Google Scholar]
- Thody AJ, Higgins EM, Wakamatsu K, Ito S, Burchill SA, Marks JM (1991) Pheomelanin as well as eumelanin is present in human epidermis. J Invest Dermatol 97:340–344 [DOI] [PubMed] [Google Scholar]
- Tomescu D, Kavanagh G, Ha T, Campbell H, Melton DW (2001) Nucleotide excision repair gene XPD polymorphisms and genetic predisposition to melanoma. Carcinogenesis 22:403–408 [DOI] [PubMed] [Google Scholar]
- Urbach F (1999) The cumulative effects of ultraviolet radiation on the skin: photocarcinogenesis. In: Hawk JLM (ed) Photodermatology. Arnold, London, pp 89–111 [Google Scholar]
- Våge DI, Fleet MR, Ponz R, Olsen RT, Monteagudo LV, Tejedor MT, Arruga MV, Gagliardi R, Postiglioni A, Nattrass GS (2003) Mapping and characterization of the dominant black colour locus in sheep. Pigment Cell Res 16:693–697 [DOI] [PubMed] [Google Scholar]
- Våge DI, Klungland H, Lu D, Cone RD (1999) Molecular and pharmacological characterization of dominant black coat color in sheep. Mamm Genome 10:39–43 [DOI] [PubMed] [Google Scholar]
- Våge DI, Lu DS, Klungland H, Lien S, Adalsteinsson S, Cone RD (1997) A non-epistatic interaction of agouti and extension in the fox, Vulpes vulpes. Nat Genet 15:311–315 [DOI] [PubMed] [Google Scholar]
- Valverde P, Healy E, Jackson I, Rees JL, Thody AJ (1995) Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat Genet 11:328–330 [DOI] [PubMed] [Google Scholar]
- Valverde P, Healy E, Sikkink S, Haldane F, Thody AJ, Carothers A, Jackson IJ, Rees JL (1996) The Asp84Glu variant of the melanocortin 1 receptor (MC1R) is associated with melanoma. Hum Mol Genet 5:1663–1666 [DOI] [PubMed] [Google Scholar]
- van der Velden PA, Sandkuijl LA, Bergman W, Pavel S, van Mourik L, Frants RR, Gruis NA (2001) Melanocortin-1 receptor variant R151C modifies melanoma risk in Dutch families with melanoma. Am J Hum Genet 69:774–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieling H, Duhl DM, Millar SE, Miller KA, Barsh GS (1994) Differences in dorsal and ventral pigmentation result from regional expression of the mouse agouti gene. Proc Natl Acad Sci USA 91:5667–5671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JK, Jovel C, Norton HL, Parra EJ, Shriver MD (2002a) Comparing quantitative measures of erythema, pigmentation and skin response using reflectometry. Pigment Cell Res 15:379–384 [DOI] [PubMed] [Google Scholar]
- Wagner JK, Parra EJ, Norton L, Jovel C, Shriver MD (2002b) Skin responses to ultraviolet radiation: effects of constitutive pigmentation, sex, and ancestry. Pigment Cell Res 15:385–390 [DOI] [PubMed] [Google Scholar]
- Wang SQ, Setlow R, Berwick M, Polsky D, Marghoob AA, Kopf AW, Bart RS (2001) Ultraviolet A and melanoma: a review. J Am Acad Dermatol 44:837–846 [DOI] [PubMed] [Google Scholar]
- Waterston K, Naysmith L, Rees JL. Physiological variation in the erythemal response to ultraviolet radiation and photoadaptation. J Invest Dermatol (in press) [DOI] [PubMed] [Google Scholar]
- Winsey SL, Haldar NA, Marsh HP, Bunce M, Marshall SE, Harris AL, Wojnarowska F, Welsh KI (2000) A variant within the DNA repair gene XRCC3 is associated with the development of melanoma skin cancer. Cancer Res 60:5612–5616 [PubMed] [Google Scholar]
- Young AR (1997) Chromophores in human skin. Phys Med Biol 42:789–802 [DOI] [PubMed] [Google Scholar]
- Zeigler-Johnson C, Panossian S, Gueye SM, Jalloh M, Ofori-Adjei D, Kanetsky PA (2004) Population differences in the frequency of the agouti signaling protein g.8818A>G polymorphism. Pigment Cell Res 17:185–187 [DOI] [PubMed] [Google Scholar]