Abstract
Deficiency of 3-methylcrotonyl-CoA carboxylase (MCC) results in elevated excretion of 3-methylcrotonylglycine (3-MCG) and 3-hydroxyisovaleric acid (3-HIVA). MCC is a heteromeric mitochondrial enzyme comprising biotin-containing α subunits and smaller β subunits, encoded by MCCA and MCCB, respectively. Mutations in these genes cause isolated MCC deficiency, an autosomal recessive disorder with a variable phenotype that ranges from severe neonatal to asymptomatic adult forms. No reported patients have responded to biotin therapy. Here, we describe two patients with a biochemical and, in one case, clinical phenotype of MCC deficiency, both of whom were responsive to biotin. The first patient presented at 3 months with seizures and progressive psychomotor retardation. Metabolic investigation at 2 years revealed elevated excretion of 3-MCG and 3-HIVA, suggesting MCC deficiency. High-dose biotin therapy was associated with a dramatic reduction in seizures, normalization of the electroencephalogram, and correction of the organic aciduria, within 4 weeks. MCC activity in fibroblasts was 25% of normal levels. The second patient, a newborn detected by tandem-mass-spectrometry newborn screening, displayed the same biochemical phenotype and remained asymptomatic with biotin up to the age of 18 months. In both patients, sequence analysis of the complete open reading frames of MCCA and MCCB revealed heterozygosity for MCCA-R385S and for the known polymorphic variant MCCA-P464H but revealed no other coding alterations. MCCA-R385S is unusual, in that it has a normal amount of MCCα protein but confers no MCC activity. We show that MCCA-R385S, but not other MCCA missense alleles, reduces the MCC activity of cotransfected MCCA–wild-type allele. Our results suggest that MCCA-R385S is a dominant negative allele and is biotin responsive in vivo.
Introduction
3-methylcrotonyl-CoA carboxylase (MCC [Enzyme Commission number 6.4.1.4]) is a biotin-dependent enzyme that catalyzes the fourth step in the leucine catabolic pathway. It carboxylates 3-methylcrotonyl-CoA to 3-methylglutaconyl-CoA in an ATP-requiring reaction that uses bicarbonate as the source of the carboxyl group (Sweetman and Williams 2001).
MCC is a member of the family of biotin-dependent carboxylases, a group of enzymes with diverse metabolic functions but common structural features (Samols et al. 1988; Wolf 2001). In addition to MCC, there are three other biotin-dependent carboxylases in humans: propionyl-CoA carboxylase (PCC), pyruvate carboxylase, and acetyl-CoA carboxylase (Samols et al. 1988; Wolf 2001). Biotin is covalently bound to the apocarboxylases by holocarboxylase synthetase (HCS [GenBank accession number BC060787]) and, after proteolytic degradation of the active holocarboxylase into short biotinyl peptides or biocytin, it is released by biotinidase, enabling the recycling of biotin (Wolf 2001).
MCC deficiency is caused either by defects of the MCC enzyme itself or by deficient activity of the enzymes involved in the metabolism of its cofactor biotin—that is, the enzymes HCS or biotinidase—deficiences of which cause multiple carboxylase deficiency.
Isolated biotin-resistant MCC deficiency (also known as “methylcrotonylglycinuria” [MIM 210200 and 210210]) is inherited as an autosomal recessive trait (Sweetman and Williams 2001). The clinical phenotype is highly variable. Some patients present in the neonatal period with seizures and hypotonia (Bannwart et al. 1992; Lehnert et al. 1996); others are asymptomatic adult women discovered only by detection of abnormal metabolites in the neonatal-screening samples from their healthy babies (Gibson et al. 1998). Most patients are asymptomatic until an episode of acute metabolic decompensation after intercurrent illness in early childhood, which leads to their diagnosis. These patients usually respond to intravenous fluids and the cessation of protein feeding and are asymptomatic between episodes. Some children have been placed on a leucine-restricted diet supplemented with oral L-carnitine, but the efficacy of this approach is unproven. In contrast to patients with multiple carboxylase deficiency due to HCS or biotinidase deficiency, no reported patient has been biotin-responsive, defined as showing a significant clinical and/or biochemical improvement in response to oral administration of biotin at doses of 1–2 mg/kg/d or higher.
Patients with MCC deficiency have a characteristic organic aciduria with greatly increased excretion of 3-hydroxyisovaleric acid (3-HIVA) and 3-methylcrotonylglycine (3-MCG), usually in combination with severe secondary carnitine deficiency. In addition, acyl-CoA derivatives accumulate and are trans-esterified to acylcarnitine esters; the major abnormal metabolite, 3-hydroxyisovalerylcarnitine, is found in blood and urine (Sweetman and Williams 2001). It is surprising that MCC deficiency appears to be the most frequent organic aciduria detected in tandem mass spectrometry (TMS) newborn-screening programs in North America (Naylor and Chace 1999; Koeberl et al. 2003), Europe (Roscher et al. 2000; Schulze et al. 2003), and Australia (Wilcken et al. 2003).
Isolated MCC deficiency can be confirmed by demonstration of deficient MCC activity in the presence of normal activity of another biotin-dependent carboxylase in cultured skin fibroblasts or in isolated lymphocytes. MCC activity in cultured fibroblasts of patients is usually <2% of the mean control value. Two patients with higher residual activity, varying from 4% to 12%, in cultured fibroblasts have been reported elsewhere (Tuchman et al. 1993; Wiesmann et al. 1998). No correlation between the level of residual enzyme activity and clinical presentation has been observed.
Bovine MCC has a size of ∼835 kD and appears to comprise six heterodimers: (αβ)6 (Lau et al. 1980). MCC is predominantly localized to the inner mitochondrial membrane. Like PCC, MCC is composed of larger α-subunits, which covalently bind biotin, and smaller β-subunits, encoded by the MCCA (MCCC1 [GenBank accession number BC004214]) and MCCB (MCCC2 [GenBank accession number BC065027]) genes, respectively, which have been cloned and characterized (Baumgartner et al. 2001; Gallardo et al. 2001; Holzinger et al. 2001). To date, 1 functionally neutral polymorphic variant, 9 functionally significant mutant MCCA alleles, and 13 functionally significant mutant MCCB alleles have been reported in probands with severe MCC deficiency (Baumgartner et al. 2001; Gallardo et al. 2001; Holzinger et al. 2001; Obata et al. 2001; Desviat et al. 2003). Several affected sibs of symptomatic patients have been clinically normal (Beemer et al. 1982; Jurecki and Packman 1992; Mourmans et al. 1995), suggesting that the genotypes at the MCCA and MCCB loci are not the sole determinants of the clinical phenotype.
One of the identified missense alleles, MCCA-R385S, is unusual in that it is the only one of seven missense alleles detected in six MCCα-deficient patients for whom protein-blot analysis of fibroblast homogenates shows the presence of MCCα protein (i.e., shows cross-reactive material [CRM]) (Baumgartner et al. 2001; Gallardo et al. 2001). Indeed, these studies suggest that the amount of MCCα protein in fibroblasts from a patient homozygous for the MCCA-R385S allele is normal or greater than normal, whereas six other MCCA missense alleles had little or no detectable CRM (Baumgartner et al. 2001). MCCA-R385S appears to be the most frequent allele found in MCC-deficient patients of German origin. Of seven such patients, one is homozygous and six are compound heterozygous for MCCA-R385S (M.R.B. and M.F.D, unpublished data).
Here, we describe two patients with elevated excretion of 3-MCG and 3-HIVA and with partial deficiency of MCC, one of whom has severe neurological symptoms. Both patients showed evidence of biotin responsiveness and were heterozygous for MCCA-R385S and the polymorphic variant MCCA-P464H. We provide evidence that MCCA-R385S is a dominant negative allele leading to biochemical abnormalities and clinical symptoms in heterozygous individuals and that it is responsive to pharmacologic doses of biotin in vivo.
Subjects and Methods
Case Reports
Patient 1 (MCC047)
This boy was the second child of healthy, nonconsanguineous German parents. At the age of 3 wk, he developed infantile spasms. Electroencephalogram (EEG) results showed hypsarrhythmia at age 3 mo. Routine cerebrospinal-fluid and brain magnetic resonance imaging (MRI) investigations were normal. Therapeutic trials with vitamin B6 and vigabatrin did not lead to clinical improvement. At 2 years of age, he presented with severe psychomotor retardation, persistent infantile spasms, and hypsarrhythmia. MRI of the brain showed significant frontal and parietal cerebral atrophy mainly of the white matter. Metabolic investigations revealed elevated excretion of 3-HIVA and 3-MCG without other abnormalities, suggesting MCC deficiency (table 1). Biotin treatment with 10 mg/d had no clinical or biochemical effect. An increase in the dose to 40 mg/d (2.7 mg/kg) was associated with dramatic reduction of seizure frequency. Within 4 wk, the EEG markedly improved, showing the disappearance of hypsarrhythmia, and the organic aciduria became virtually normal (table 1). On a biotin regimen (50 mg/d) and a protein-restricted diet (0.8 g/kg/d, introduced at age 2.5 years), the patient showed no further progression of symptoms, and excretion of 3-HIVA and 3-MCG completely normalized. Biotinidase activity in plasma was normal. At 7 years of age, he was seizure free on a protein-restricted diet (0.8 g/kg/d) and a regimen of vigabatrin and biotin (50 mg/d), and his EEG was unremarkable. Formal psychometrics were not obtained.
Table 1.
Effect of Biotin Substitution on 3-HIVA and 3-MCG Concentration in Urine
|
Concentration in Urine ofb (mmol/mol creatinine) |
|||
| Subject andBiotin Substitution | Time after Leucine Loada(h) | 3-HIVA | 3-MCG |
| MCC047: | |||
| No biotin | … | 276 | 653 |
| 10 mg/d for 7 d | … | 358 | 300 |
| 40 mg/d for 7 d | … | 135 | 44 |
| 40 mg/d for 3 more wk | … | 17 | 9 |
| Father of MCC047: | |||
| No biotin | … | 26 | ND |
| Mother of MCC047: | |||
| No biotin | … | 24 | ND |
| MCC048: | |||
| No biotin | Before load | 105 | 2.2 |
| No biotin | 0–2 | 183 | 12 |
| No biotin | 2–4 | 956 | 33 |
| No biotin | 4–6 | 794 | 35 |
| No biotin | Before load | 100 | ND |
| 5 mg/kg with leucine at 0 h | 0–2 | 368 | 6.2 |
| No biotin | 2–4 | 471 | 25 |
| No biotin | 4–6 | 335 | 12 |
| Controls: | |||
| No biotin | … | <18 | ND |
Patient MCC048 was given a leucine load of 150 mg/kg with and without a simultaneous biotin load, and urine was collected in three portions after the load.
ND = not detectable.
Patient 2 (MCC048)
This boy was the second child of healthy, nonconsanguineous Greek parents. His pre- and postpartum history was unremarkable except for a hyperbilirubinemia requiring phototherapy. He was referred at age 5 wk because of elevated 3-hydroxyisovalerylcarnitine detected by TMS newborn screening.
Urinary organic acid analysis displayed elevated 3-HIVA and trace amounts of 3-MCG with no other abnormalities (table 1). To test for a suspected defect in leucine catabolism, the patient received an oral leucine load (150 mg/kg). This challenge resulted in transient hyperammonemia (up to 134 μmol/L; normal < 80) and in a significant increase in urinary excretion of 3-HIVA and 3-MCG (table 1) that was partially suppressed by simultaneous administration of biotin (5 mg/kg) (table 1), suggesting in vivo biotin responsiveness similar to that of patient MCC047. Ten other infants who were referred because of elevated isovalerylcarnitine detected by TMS were similarly tested but showed normal organic acid excretion before and after leucine loading (data not shown). At 18 mo of age, patient MCC048 received biotin (5 mg/kg) and carnitine (50 mg/kg) and was developing normally. His urinary excretion of 3-HIVA and 3-MCG remained elevated (334 and 11.7 mmol/mol creatinine, respectively). Samples from the parents of patient MCC048 were not available. Informed consent for this study was obtained from the parents of both patients.
Cell Cultures and Carboxylase Assays
Skin fibroblasts were cultured in a standard medium containing 10% fetal calf serum (FCS). The biotin concentration of this medium, supplied by the natural biotin content of FCS, was 9 nmol/L, or 5-fold the mean normal plasma biotin concentration in humans (1.80 ± 0.84 nmol/L, range 0.65–4.83 nmol/L; n=126) (Suormala et al. 1997). We prepared a biotin-depleted medium by replacing FCS with newborn calf serum (NBCS), which resulted in a final biotin concentration of 0.1 nmol/L. To obtain physiological biotin concentration, we supplemented the low-biotin medium with 2.0 nmol/L biotin and, to obtain a high-biotin medium, with 10 μmol/L biotin (i.e., 5,000 times higher than normal plasma levels).
The activities of PCC and MCC were assayed in fibroblast homogenates by measurement of the incorporation of 14C-bicarbonate into acid-nonvolatile products, by methods established elsewhere (Suormala et al. 1985).
Mutation Analysis by RT-PCR and Genomic PCR
We extracted RNA and genomic DNA from skin fibroblast cultures between 5–15 passages, using RNA and DNA isolation kits from Qiagen. We performed RT-PCR using 2–5 μg of total cellular fibroblast RNA and a cDNA cycle kit (Invitrogen) in accordance with the manufacturer's recommendations. To search for mutations, we used primer pairs in the 5′- and 3′-UTR to amplify by RT-PCR the complete MCCA or MCCB ORF, as described elsewhere (Baumgartner et al. 2001). We used the same approach to amplify the complete ORF of the HCS gene. The PCR products were gel purified and were sequenced directly.
To confirm mutations identified in RT-PCR products, we amplified a genomic fragment containing the corresponding exon, using flanking intronic primers, and sequenced the PCR product directly (Baumgartner et al. 2001). All PCR reactions (50 μl) contained primers (100 ng of each), standard PCR buffer (Gibco-BRL), dNTPs (200 μM), and Taq polymerase (2.5 U) (Gibco-BRL). The sequences of all primers are available on request.
Construction of Wild-Type and Mutant MCCA Expression Vectors
We cloned the full-length human MCCA (−51 to +2275, where +1 is the A nucleotide of the initiation methionine codon) cDNA into a mammalian expression vector (pTracer-CMV2 [Invitrogen]) at the EcoRI sites, as described elsewhere (Baumgartner et al. 2001). To introduce R385S, A289V, L437P, and P464H into MCCA, we harvested an 896-bp ACCI fragment from RT-PCR–amplified cDNA from individuals with these variants and subcloned the fragment into the wild-type MCCA construct, as described elsewhere (Baumgartner et al. 2001). The pTracer-CMV2 vector contains a green fluorescent protein (GFP) gene fused to the Zeocin-resistance gene. We sequenced all constructs in both directions to validate the sequences.
Transfections
For expression studies, we electroporated the indicated constructs into SV40T-transformed skin fibroblasts from a patient homozygous for MCCA Q421fs(+1), as described elsewhere (Braverman et al. 1997; Baumgartner et al. 2001). These cells have no detectable MCC activity. The cells were harvested after 72 h and were assayed for MCC and PCC activities. All transfections were performed in duplicate. Transfection efficiency was assessed by scoring a subset of cells in each transfection for the presence of GFP and ranged from 10%–20% in these experiments. Transformed fibroblasts from an unaffected individual were used as control.
Allelic Variation in MCCA Expression
We used the MCCA SNP c.1391C→A, which results in the P464H amino acid change, to quantitate allele-specific transcripts by means of single-nucleotide primer extension (SnuPE) and laser-induced fluorescence capillary electrophoresis, as described elsewhere (Mátyás et al. 2002). This method takes advantage of distinct mobilities of SnuPE products with different nucleotides incorporated at their 3′ ends and has been independently shown to yield highly quantitative results (Mátyás et al. 2002; Yan and Zhou 2003).
Results
Carboxylase Activities
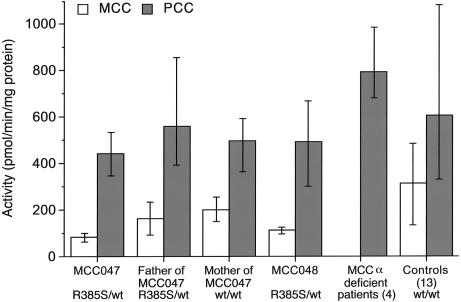
MCC activity in crude homogenates of fibroblasts cultured in standard medium was reduced below the normal range in patients MCC047 and MCC048, with an average of 26% and 36% of the mean control value, respectively (fig. 1). This residual activity is greater than that in fibroblasts from four typical CRM− MCC-deficient patients (0%–1% of mean control value) (fig. 1) but is less than that in fibroblasts from six heterozygotes for CRM− MCCA or MCCB alleles (72%–118% of control). The activity of another biotin-dependent carboxylase, PCC, was within the normal range in patients MCC047 and MCC048, indicating that the deficiency of MCC is specific. Moreover, the ratio of MCC to PCC activity was consistently below the control range inboth patients (MCC047: mean 0.19, range 0.18–0.19; MCC048: mean 0.24, range 0.19–0.32; 13 controls: mean 0.52, range 0.41–0.75). Fibroblast MCC activity levels from the father and the mother of patient MCC047 were both at the low end of the normal range (52% and 63% of the mean control value, respectively). However, the ratio of MCC to PCC activity was consistently below the control range in the father, who was also heterozygous for MCCA-R385S, whereas the ratio was normal in three of four experiments in the mother (father of MCC047: mean 0.29, range 0.24–0.36; mother of MCC047: mean 0.4, range 0.34–0.42).
Figure 1.
MCC and PCC activities in fibroblasts from patient MCC047 and his parents, from patient MCC048, from four typical CRM− MCC-deficient patients (probands 009, 010, 012, and 013 in Baumgartner et al. 2001), and from 13 control cell lines grown in standard FCS-based medium with a biotin concentration of 9 nmol/L. The indicated activities are the mean and range (vertical lines) of four individual experiments each with duplicate determinations. Values in the CRM− MCC-deficient patients are the mean and range of activities obtained in four different patients. Control values represent the mean and range of activities obtained in 13 different cell lines.
To determine whether the extent of the MCC deficiency in our patients’ fibroblasts was influenced by the biotin concentration of the medium, we cultured the cells for one passage (17–27 d) in either biotin-depleted (0.1 nmol/L) or high-biotin (10,000 nmol/L) medium (table 2). After one passage in the biotin-depleted medium, MCC activities in fibroblasts from patients MCC047 and MCC048 and from the parents of MCC047 were decreased to 24%–35% of activity in the high-biotin medium. A similar decrease occurred in control cells. Even after four passages in the low-biotin medium, MCC activity in the cells from patient MCC047 was decreased to only 27% (data not shown). Cells from patients with HCS deficiency exhibit a dramatic decrease in MCC activity when cultured in biotin-depleted medium (Suormala et al. 1997), which makes it unlikely that an abnormality of this enzyme contributes to our patients' biochemical phenotype. Furthermore, MCC and PCC activities in cells cultured in medium with a physiological concentration of biotin (2.1 nmol/L) were similar to those in cells cultured in the high-biotin medium. Increasing the biotin concentration to 100 μmol/L in the medium failed to increase the activities of MCC further (data not shown). Thus, we found no evidence for an increased biotin requirement as the cause of the low MCC activity in the patients’ fibroblasts.
Table 2.
Activities of 3-MCC and PCC in Fibroblasts Grown in NBCS-Based Low-Biotin Medium (0.1 nmol/L) and in the Same Medium Supplemented with Physiological (2.1 nmol/L) and High (10 μmol/L) Biotin Concentrations
|
Carboxylase Activitiesa(pmol/min/mg protein) |
|||
| Subject andBiotin in Medium(nmol/L) | MCC | PCC | MCC/PCC Ratio |
| MCC047: | |||
| .1 | 31.5 | 375 | .08 |
| 2.1 | 102 | 628 | .16 |
| 10,000 | 100 | 638 | .16 |
| Father of MCC047: | |||
| .1 | 42.0 | 280 | .15 |
| 2.1 | 121 | 446 | .27 |
| 10,000 | 130 | 498 | .26 |
| Mother of MCC047: | |||
| .1 | 61.2 | 315 | .20 |
| 2.1 | 176 | 500 | .35 |
| 10,000 | 177 | 472 | .38 |
| MCC048: | |||
| .1 | 30.4 | 283 | .11 |
| 2.1 | 120 | 603 | .20 |
| 10,000 | 128 | 603 | .21 |
| 21 controls, mean ± SD (range): | |||
| .1 | 145 ± 57 (70.4–292) | 494 ± 172 (300–889) | .3 ± .08 (.17–.43) |
| 10,000 | 340 ± 130 (140–606) | 694 ± 246 (347–1,246) | .5 ± .13 (.29–.85) |
Values are the mean of duplicate determinations within one experiment.
Normal kinetics of MCC activity were obtained in crude homogenates of fibroblasts from MCC047 when they were assayed with varying concentrations of bicarbonate, 3-methylcrotonyl-CoA, ATP, magnesium, and chloride in the assay mixture (results not shown). These results indicate that a decreased affinity of MCC for these assay components is highly unlikely to be the cause of the reduced activity in this patient. Despite these results, the ratio of MCC to PCC activity in fibroblasts from patient MCC047, his father, and patient MCC048 was reproducibly below the control range, whereas the ratio in fibroblasts from the mother of patient MCC047 was within the range of controls (table 2).
Mutation Analysis
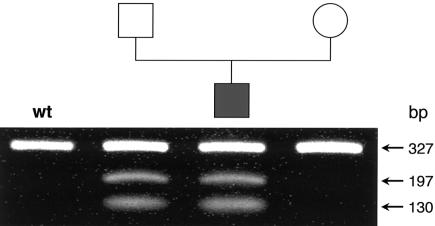
Despite high residual MCC activity in vitro, the pattern of metabolic abnormalities in our patients suggested a partial defect at the level of MCC in vivo. To investigate this possibility, we sequenced the entire ORF of MCCA and MCCB in the products of RT-PCR amplification of fibroblast RNA in patients MCC047 and MCC048. We found that both patients and the father of patient MCC047 are heterozygous for an A→C transversion at position 1155, creating a new restriction site for NheI (GCTAGA→GCTAGC) and substituting aserine for an arginine at position 385 (MCCA-R385S).Patients MCC047 and MCC048 and the mother of MCC047 are heterozygous for the previously described neutral polymorphism 1391C→A (MCCA-P464H). The father of MCC047 does not carry this polymorphism. From this, we conclude that the MCCA-R385S allele encodes a proline at codon 464. We found no other sequence abnormalities over the entire length of the MCCA and MCCB ORFs. We confirmed these results by direct sequencing of PCR-amplified genomic DNA followed by digestion with NheI (fig. 2). We also amplified and sequenced all MCCA exons and flanking intronic sequences in patient MCC047 and found no additional mutations. Because biotin responsiveness in other carboxylase-deficient patients has been shown to be due to deficiency of HCS, we also RT-PCR amplified and sequenced the entire HCS ORF and found no sequence abnormalities.
Figure 2.
Detection of the MCCA 1155A→C (R385S) mutation in the MCC047 nuclear family. The 327-bp fragment amplified from genomic DNA (MCCA exon 11) was subjected to digestion with NheI. The wild-type fragment is not cut, whereas the R385S fragment is cleaved into fragments of 130 bp and 197 bp.
Expression Studies
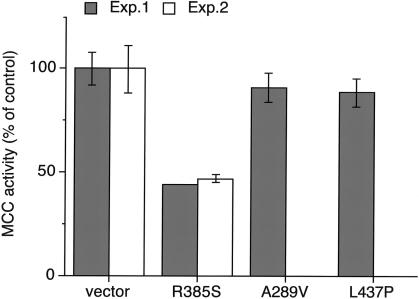
In previous experiments, we showed that MCCA-R385S and several other MCCA missense alleles conferred no MCC activity when transfected into MCCA null cells (Baumgartner et al. 2001). To test if MCCA-R385S also has a dominant negative influence on the activity of MCCA wild type, we cotransfected both alleles into an SV40T-transformed MCCα-deficient reference cell line and measured MCC activity 72 h later. In two separate experiments, cotransfection of MCCA wild-type allele with vector without an insert restored MCC activity to 55% of nontransfected control fibroblasts, whereas cotransfection of MCCA wild type with MCCA-R385S restored MCC activity to 25%—that is, to ∼50% of that obtained with wild type coexpressed with vector without an insert. In contrast, cotransfection of MCCA wild type with either MCCA-A289V or MCCA-L437P restored MCC activity to the same level as that obtained with wild type coexpressed with vector without insert (fig. 3). Thus, the reduction of MCC activity is specific for MCCA-R385S. We repeated the experiment using the MCCA–wild-type construct with histidine instead of a proline at codon 464 (MCCA-P464H) with similar results. Thus, the dominant negative effect of MCCA-R385S when coexpressed with the wild-type allele is independent of the P464H polymorphism.
Figure 3.
The effect of coexpression of MCCA missense alleles and wild-type MCCA. MCC-deficient cells were cotransfected with wild-type MCCA plus MCCA alleles with the indicated missense mutation. Activities represent mean and range obtained in two individual experiments. MCC activity of cells cotransfected with wild-type MCCA and a vector without an insert was set at 100%.
Allelic Variation in Expression
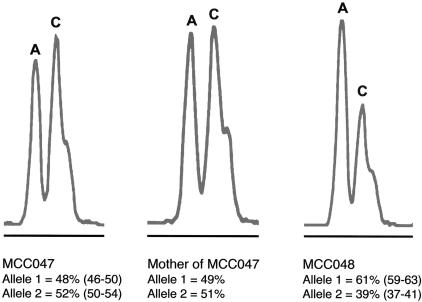
Recent reports have shown variation in expression of apparently wild-type alleles, for as many as 50% of all genes (Yan et al. 2002; Lo et al. 2003). This variation in expression ranges ⩾2–4 fold, segregates with the allele, and could markedly affect the consequences of heterozygosity for a recessive loss-of-function mutation on the other allele. To determine if allele-specific variation in expression was playing a role in the manifestation of MCC deficiency in individuals heterozygous for MCCA-R385S, we used a fluorescent dideoxy-terminator–based method to distinguish the mRNA products of individual alleles in patient MCC047, his mother, and patient MCC048, by use of the MCCA-P464H polymorphism to mark the alleles. In patient MCC047 and his mother, both alleles were expressed at equal levels, whereas, in patient MCC048, the expression of the MCCA-S385 allele (marked by P464 in cis) was reduced to ∼40% of that of the wild-type MCCA allele (marked by H464) (fig. 4). Thus, in these heterozygous individuals, we found no evidence for significant variation in expression of the MCCA wild-type allele.
Figure 4.
Allelic variation in MCCA expression. A sample of electrospherograms of SnuPE products from the sense strand of RT-PCR–amplified DNA from patient MCC047 and his mother and from patient MCC048. Mobility of tetramethyl-6-carboxyrhodamine (TAMRA)–labeled allele-specific SnuPE is shown for the MCCA polymorphic variant c.1391C→A (MCCA-P464H). Each peak represents a specific allelic variant. Allele 1 with an A nucleotide at c.1391 encodes 464H, whereas allele 2 with a C nucleotide at c.1391 encodes 464P and the functionally significant 385S. The relative quantification of each allele represents the mean and range of two independent experiments performed on fibroblast RNA.
Discussion
Both patients described here showed a persistent characteristic organic aciduria, with elevated excretion of 3-MCG and 3-HIVA suggesting MCC deficiency. One suffered from severe neurological symptoms starting at the age of 3 wk, whereas the other was an asymptomatic newborn detected by TMS newborn screening. In patient MCC047, excretion of the disease-specific metabolite 3-MCG was within the range found in patients with severe MCC deficiency (Sweetman and Williams 2001); in patient MCC048, urinary 3-MCG was lower but was persistently elevated. Enzymatic determination of MCC activity in cultured fibroblasts revealed a partial but specific MCC deficiency. In both patients, residual fibroblast MCC activity was not abnormally sensitive to biotin depletion, nor was it enhanced by addition of high concentrations of biotin to the medium. These results rule out HCS deficiency as the cause of reduced MCC activity and are consistent with the clinical features and the persistent organic aciduria in our patients.
Our mutation analysis of the MCCA and MCCB genes revealed that both patients are heterozygous for a previously described missense mutation, MCCA-R385S, and a known polymorphism, MCCA-P464H (Obata et al. 2001). In earlier studies, we showed that MCCA-R385S confers no MCC activity when transfected into reference MCCα-deficient cells (Baumgartner et al. 2001). We found the MCCA-R385S allele in 1 of 132 control chromosomes from white individuals (Baumgartner et al. 2001).
An arginine at position 385 (or the corresponding position) is strictly conserved in mammalian, plant, fungal, and bacterial carboxylases (Samols et al. 1988; Jitrapakdee and Wallace 1999), and the corresponding residue in the biotin carboxylase domain of Escherichia coli acetyl-CoA carboxylase (R338) has been shown to be part of a positively charged pocket for bicarbonate binding (Thoden et al. 2000).
To explain the biochemical abnormalities in both patients and the clinical phenotype in patient MCC047, we hypothesize a dominant negative influence of the mutant S385 MCCα subunit on MCC activity. A dominant negative effect of the protein product of a mutant allele usually involves homomeric or heteromeric proteins. Typically, the protein product of a dominant negative allele is functionally inactive and has the added property of inhibiting the activity of the protein product of the normal allele (Herskowitz 1987). To achieve this, the dominant negative mutant protein product must be stable and must be able to assemble into the normal multimeric protein complex. MCCα S385 fulfills these criteria. In contrast to the protein products of all other MCCA missense alleles, the MCCα S385 protein is stable (Baumgartner et al. 2001; Gallardo et al. 2001). On protein-blot analysis using avidin alkaline phosphatase as a probe, MCCα S385 accumulates to levels equal to or greater than normal (Baumgartner et al. 2001). Despite this, expression studies clearly show that the MCCα S385 is catalytically inactive (Baumgartner et al. 2001; Desviat et al. 2003). Moreover, the results of our cotransfection experiments show that expression of MCCα S385 but not of two other MCCA missense alleles inhibits the function of coexpressed wild-type MCCα protein. Taken together, these data strongly support our hypothesis of a dominant negative effect of MCCα S385 on the wild-type protein and thus on MCC activity.
Additional evidence comes from the recent studies of Sloane and Waldrop (2004), who used the purified homodimer of the biotin carboxylation subunit of E. coli acetyl CoA carboxylase to study kinetic properties of mutant proteins. They showed that E. coli biotin carboxylase-R338S, the missense mutant corresponding to MCCA-R385S, exhibited a dominant negative effect on the function of the wild-type active site, by negative cooperativity with respect to bicarbonate concentration (Sloane and Waldrop 2004). Furthermore, they presented evidence that the degree of negative cooperativity is decreased with increasing concentrations of biotin. These data support our hypothesis of a dominant negative effect of MCCα S385 on the wild-type protein and thus on MCC activity.
The presence of a functionally significant mutation in the second allele would also explain the reduced MCC activity in our patients. However, we searched extensively for a functional mutation on the second allele by RT-PCR of mRNA of the entire MCCA ORF, as well as by amplification and sequencing of all MCCA exons and flanking intronic splice sites from genomic DNA. By direct sequencing of the RT-PCR product, we could clearly see that both patients are heterozygous for the R385S change, which indicates that the second allele produced a stable mRNA transcript, but we found no molecular abnormalities in the MCCA transcript or structural gene that could explain a reduction in the function of its protein product. For all of these reasons, we concluded that MCCα S385 has a dominant negative effect on the wild-type protein.
How do we explain the lack of biochemical abnormalities in other R385S heterozygotes (e.g., the father of MCC047)? The R385S-MCCA heterozygotes with normal urinary organic acids who we have studied were all adults. Thus, one possible explanation is that the metabolic demands on the pathway are lesser in adults than in infants and children. This would also be consistent with the identification of asymptomatic adults with severe MCC deficiency (Gibson et al. 1998; Baumgartner et al. 2001). An alternative and nonexclusive explanation for the phenotypic variation in MCCA-R385S heterozygotes has to do with the amount of functional MCCα produced from the wild-type allele. Recent studies have suggested that alleles of as many as half of all genes examined exhibit ⩾2–4 fold allelic variation in expression and that this characteristic segregates with the alleles from one generation to the next (Yan et al. 2002; Lo et al. 2003). If this is the case for MCCA, then the expression level of the wild-type allele would be an additional variable affecting the consequences of heterozygosity for a nonfunctional allele. A heterozygote with a low-expression wild-type allele might be clinically and/or biochemically symptomatic. Conversely, a heterozygote with a high-expression wild-type allele would be normal. However, our allelic expression studies showed approximately equal expression of both alleles in patient MCC047 and his mother. In patient MCC048, we found slightly reduced expression of the MCCA-R385S allele (marked by P464) as compared with the wild-type allele (fig. 4). This result may explain the less severe biochemical abnormalities and clinical phenotype in this patient.
Additional factors that could influence the phenotypic consequences of MCC deficiency include the extent to which the pathway is stressed by dietary or other environmental factors (e.g., excessive protein breakdown associated with an intercurrent infection). In this regard, the father of patient MCC047 is described as being healthy during infancy and childhood. Finally, normal variation in genes whose protein products have the potential to modify the function of MCC or the demands on the pathway could also influence the phenotype of MCC deficiency. MCCB and HCS are obvious candidate modifier genes for MCCA, but we found no sequence variation in either of these genes in our patients.
It is surprising that the clinical symptoms in patient MCC047 and the biochemical phenotype in both patients appeared to respond to high doses of biotin. A biotin-responsive form of MCC deficiency has not been reported elsewhere (Sweetman and Williams 2001), even in Germany, where MCCA-R385S appears to be a common allele. In part, this may be because of failure to administer high doses of biotin on the order of 2–5 mg/kg/d. Biotin responsiveness in our patients is consistent with the observation that MCCA-R385S produces a stable protein and that other cofactor-responsive disorders are allele specific (e.g., cystathione β-synthase) (Mudd et al. 2001). It also agrees with the findings of Sloane and Waldrop (2004), who showed that biotin decreases the negative cooperativity of E. coli biotin carboxylase with a missense mutation in the residue corresponding to MCCA-R385S. Alternatively, a biotin-dependent increase in the level of MCCA mRNA also could explain biotin responsiveness. A regulatory role for biotin in the control of HCS and carboxylase mRNA levels through a signaling pathway that requires HCS, guanylate cyclase, and cGMP-dependent protein kinase has recently been proposed (Solorzano-Vargas et al. 2002). To provide further evidence for biotin responsiveness in our patients, additional clinical, biochemical, and molecular studies in patients with biotin versus without biotin would be useful. This was not possible, however, because of parental unwillingness (for patient MCC047) and ethical considerations regarding the asymptomatic patient MCC048. For the future, we recommend careful documentation of the consequences of biotin administration in newly diagnosed patients, especially those carrying the MCCA-R385S allele.
Acknowledgments
We thank A. Randolph for technical assistance. A part of this study was supported by a grant from the Swiss National Science foundation (3200-065059). D.V. is an Investigator in the Howard Hughes Medical Institute.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/GenBank/ (for MCCA [accession number BC004214], MCCB [accession number BC065027], and HCS [accession number BC060787])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for MCC deficiency and 3-methylcrotonylglycinuria)
References
- Bannwart C, Wermuth B, Baumgartner R, Suormala T, Weismann UN (1992) Isolated biotin-resistant deficiency of 3-methylcrotonyl-CoA carboxylase presenting as a clinically severe form in a newborn with fatal outcome. J Inherit Metab Dis 15:863–868 [DOI] [PubMed] [Google Scholar]
- Baumgartner MR, Almashanu S, Suormala T, Obie C, Cole RN, Packman S, Baumgartner ER, Valle D (2001) The molecular basis of human 3-methylcrotonyl-CoA carboxylase deficiency. J Clin Invest 107:495–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemer FA, Bartlett K, Duran M, Ghneim HK, Wadman SK, Bruinvis L, Ketting D (1982) Isolated biotin-resistant 3-methylcrotonyl-CoA carboxylase deficiency in two sibs. Eur J Pediatr 138:351–354 [DOI] [PubMed] [Google Scholar]
- Braverman N, Steel G, Obie C, Moser A, Moser H, Gould SJ, Valle D (1997) Human PEX7 encodes the peroxisomal PTS2 receptor and is responsible for rhizomelic chondrodysplasia punctata. Nat Genet 15:369–376 [DOI] [PubMed] [Google Scholar]
- Desviat LR, Pérez-Cerdá C, Pérez B, Esparza-Gordillo J, Rodríguez-Pombo P, Peñalva MÁ, Rodríguez de Cordoba S, Ugarte M (2003) Functional analysis of MCCA and MCCB mutations causing methylcrotonylglycinuria. Mol Genet Metab 80:315–320 [DOI] [PubMed] [Google Scholar]
- Gallardo ME, Desviat LR, Rodríguez JM, Esparza-Gordillo J, Pérez-Cerdá C, Pérez B, Rodríguez-Pombo P, Criado O, Sanz R, Morton DH, Gibson MK, Le TP, Ribes A, Rodríguez de Córdoba S, Ugarte M, Peñalva MÁ (2001) The molecular basis of 3-methlcrotonylglycinuria, a disorder of leucine catabolism. Am J Hum Genet 68:334–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson KM, Bennett MJ, Naylor EW, Morton DH (1998) 3-methylcrotonyl-coenzyme A carboxylase deficiency in Amish/Mennonite adults identified by detection of increased acylcarnitines in blood spots of their children. J Pediatr 132:519–523 [DOI] [PubMed] [Google Scholar]
- Herskowitz I (1987) Functional inactivation of genes by dominant negative mutations. Nature 329:219–222 [DOI] [PubMed] [Google Scholar]
- Holzinger A, Röschinger W, Lagler F, Mayerhofer PU, Lichtner P, Kattenfeld T, Thuy LP, Nyhan WL, Koch HG, Muntau AC, Roscher AA (2001) Cloning of the human MCCA and MCCB genes and mutations therein reveal the molecular cause of 3-methylcrotonyl-CoA: carboxylase deficiency. Hum Molec Genet 10:1299–1306 [DOI] [PubMed] [Google Scholar]
- Jitrapakdee S, Wallace JC (1999) Structure, function and regulation of pyruvate carboxylase. Biochem J 340:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurecki E, Packman S (1992) Nutritional therapy for β-methylcrotonylglycinuria. Metab Curr 5:9–12 [Google Scholar]
- Koeberl DD, Millington DS, Smith WE, Weavil SD, Muenzer J, McCandless SE, Kishnani PS, McDonald MT, Chaing S, Boney A, Moore E, Frazier DM (2003) Evaluation of 3-methylcrotonyl-CoA carboxylase deficiency detected by tandem mass spectrometry newborn screening. J Inherit Metab Dis 26:25–35 [DOI] [PubMed] [Google Scholar]
- Lau EP, Cochran BC, Fall RR (1980) Isolation of 3-methylcrotonyl-coenzyme A carboxylase from bovine kidney. Arch Biochem Biophys 205:352–359 [DOI] [PubMed] [Google Scholar]
- Lehnert W, Niederhoff H, Suormala T, Baumgartner ER (1996) Isolated biotin-resistant 3-methylcrotonyl-CoA carboxylase deficiency: long-term outcome in a case with neonatal onset. Eur J Pediatr 155:568–572 [DOI] [PubMed] [Google Scholar]
- Lo H, Wang Z, Hu Y, Yang H, Gere S, Buetow K, Lee M (2003) Allelic variation in gene expression is common in the human genome. Genome Res 13:1855–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mátyás G, Giunta C, Steinmann B, Hossle JP, Hellwig R (2002) Quantification of single nucleotide polymorphisms: a novel method that combines primer extension assay with capillary electrophoresis. Hum Mutat 19:58–68 [DOI] [PubMed] [Google Scholar]
- Mourmans J, Bakkeren J, de Jong J, Wevers R, van Diggelen OP, Suormala T, Baumgartner R, Wendel U (1995) Isolated (biotin-resistant) 3-methylcrotonyl-CoA carboxylase deficiency: four sibs devoid of pathology. J Inherit Metab Dis 18:643–645 [DOI] [PubMed] [Google Scholar]
- Mudd SH, Levy HL, Kraus JP (2001) Disorders of transsulfuration. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B (eds) The metabolic and molecular basis of inherited disease. McGraw-Hill, New York, pp 2007–2056 [Google Scholar]
- Naylor EW, Chace DH (1999) Automated tandem mass spectrometry for mass newborn screening for disorders in fatty acid, organic acid and amino acid metabolism. J Child Neurol 14:S4–S8 [DOI] [PubMed] [Google Scholar]
- Obata K, Fukuda T, Morishita R, Abe S, Asakawa S, Yamaguchi S, Yoshino M, Ihara K, Murayama K, Shigemoto K, Shimizu N, Kondo I (2001) Human biotin-containing subunit of 3-methylcrotonyl-CoA carboxylase gene (MCCA): cDNA Sequence, genomic organization, localization to chromosomal band 3q27, and expression. Genomics 72:145–152 [DOI] [PubMed] [Google Scholar]
- Roscher AA, Liebl B, Fingerhut R, Olgemöller B (2000) Prospective study of MS-MS newborn screening in Bavaria, Germany. J Inherit Metab Dis 23:4 [Google Scholar]
- Samols D, Thornton CG, Murtif VL, Kumar GK, Haase FC, Wood HG (1988) Evolutionary conservation among biotin enzymes. J Biol Chem 263:6461–6464 [PubMed] [Google Scholar]
- Schulze A, Lindner M, Kohlmüller D, Olgemöller K, Mayatepek E, Hoffmann G (2003) Expanded newborn screening for inborn errors of metabolism by electropsray ionization-tandem mass spectrometry: results, outcome, and implications. Pediatrics 111:1399–1406 [DOI] [PubMed] [Google Scholar]
- Sloane V, Waldrop GL (2004) Kinetic characterization of mutations found in propionic acidemia and methylcrotonylglycinuria: evidence for cooperativity in biotin carboxylase. J Biol Chem 279:15772–15778 [DOI] [PubMed] [Google Scholar]
- Solorzano-Vargas RS, Pacheco-Alvarez D, León-Del-Rio A (2002) Holocarboxylase synthetase is an obligate participant in biotin-mediated regulation of its own expression and of biotin-dependent carboxylases mRNA levels in human cells. PNAS 99:5325–5330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suormala T, Fowler B, Jakobs C, Duran M, Lehnert A, Raab K, Wick H, Baumgartner ER (1997) Five patients with a biotin-responsive defect in holocarboxylase formation: evaluation of responsiveness to biotin therapy in vivo and comparative biochemical studies in vitro. Pediatr Res 41:666–673 [DOI] [PubMed] [Google Scholar]
- Suormala T, Wick H, Bonjour J-P, Baumgartner ER (1985) Rapid differential diagnosis of carboxylase deficiencies and evaluation for biotin responsiveness in a single blood sample. Clin Chim Acta 145:151–162 [DOI] [PubMed] [Google Scholar]
- Sweetman L, Williams JC (2001) Branched chain organicacidurias. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B (eds) The metabolic and molecular basis of inherited disease. McGraw-Hill, New York, pp 2125–2163 [Google Scholar]
- Thoden JB, Blanchard CZ, Holden HM, Waldrop GL (2000) Movement of biotin carboxylase B-domain as a result of ATP binding. J Biol Chem 275:16183–16190 [DOI] [PubMed] [Google Scholar]
- Tuchman M, Berry SA, Thuy LP, Nyhan WL (1993) Partial methylcrotonyl-coenzyme A carboxylase deficiency in an infant with failure to thrive, gastrointestinal dysfunction and hypertonia. Pediatrics 91:664–666 [PubMed] [Google Scholar]
- Wiesmann UN, Suormala T, Pfenninger J, Baumgartner ER (1998) Partial 3-methylcrotonyl-CoA carboxylase deficiency in an infant with fatal outcome due to progressive respiratory failure. Eur J Pediatr 157:225–229 [DOI] [PubMed] [Google Scholar]
- Wilcken B, Wiley V, Hammond J, Carpenter K (2003) Screening newborns for inborn errors of metabolism by tandem mass spectrometry. N Engl J Med 348:2304–2312 [DOI] [PubMed] [Google Scholar]
- Wolf B (2001) Disorders of biotin metabolism. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B (eds) The metabolic and molecular basis of inherited disease. McGraw-Hill, New York, pp 3935–3962 [Google Scholar]
- Yan H, Yuan W, Vleculescu V, Vogelstein B, Kinzler KW (2002) Allelic variation in human gene expression. Science 297:1143 [DOI] [PubMed] [Google Scholar]
- Yan H, Zhou W (2003) Allelic variations in gene expression. Curr Opin Oncol 16:39–43 [DOI] [PubMed] [Google Scholar]