Abstract
Catechol-O-methyltransferase (COMT) is a key enzyme in the elimination of dopamine in the prefrontal cortex of the human brain. Genetic variation in the COMT gene (MIM 116790) has been associated with altered prefrontal cortex function and higher risk for schizophrenia, but the specific alleles and their functional implications have been controversial. We analyzed the effects of several single-nucleotide polymorphisms (SNPs) within COMT on mRNA expression levels (using reverse-transcriptase polymerase chain reaction analysis), protein levels (using Western blot analysis), and enzyme activity (using catechol methylation) in a large sample (n = 108) of postmortem human prefrontal cortex tissue, which predominantly expresses the -membrane-bound isoform. A common coding SNP, Val158Met (rs4680), significantly affected protein abundance and enzyme activity but not mRNA expression levels, suggesting that differences in protein integrity account for the difference in enzyme activity between alleles. A SNP in intron 1 (rs737865) and a SNP in the 3′ flanking region (rs165599)—both of which have been reported to contribute to allelic expression differences and to be associated with schizophrenia as part of a haplotype with Val—had no effect on mRNA expression levels, protein immunoreactivity, or enzyme activity. In lymphocytes from 47 subjects, we confirmed a similar effect on enzyme activity in samples with the Val/Met genotype but no effect in samples with the intron 1 or 3′ SNPs. Separate analyses revealed that the subject's sex, as well as the presence of a SNP in the P2 promoter region (rs2097603), had small effects on COMT enzyme activity. Using site-directed mutagenesis of mouse COMT cDNA, followed by in vitro translation, we found that the conversion of Leu at the homologous position into Met or Val progressively and significantly diminished enzyme activity. Thus, although we cannot exclude a more complex genetic basis for functional effects of COMT, Val is a predominant factor that determines higher COMT activity in the prefrontal cortex, which presumably leads to lower synaptic dopamine levels and relatively deleterious prefrontal function.
Introduction
Since its discovery in 1958 (Axelrod and Tomchick 1958), catechol-O-methyltransferase (COMT) has been an important enzyme in catecholamine biochemistry and pharmacology and, more recently, in genetic mechanisms of variation in catechol metabolism and its clinical implications. COMT catalyzes the transfer of a methyl group from S-adenosyl-methionine (SAM) to a hydroxyl group on a catechol nucleus (e.g., dopamine, norepinephrine, or catechol estrogen) (Weinshilboum et al. 1999). Genetic variation in COMT has been associated with diverse clinical phenotypes, from anxiety to estrogen-related cancer (Enoch et al. 2003; Ahsan et al. 2004), and it has been studied particularly extensively in relation to risk for schizophrenia (Harrison and Weinberger 2004). The COMT gene is located in chromosome 22q11, one of the principal loci linked to schizophrenia (Badner and Gershon 2002), and in the DiGeorge–velocardiofacial syndrome hemideletion. There are two promoters, P1 and P2, (Tenhunen et al. 1993), that control transcription of two different mRNAs. A longer mRNA from the P2 promoter encodes mainly a membrane-bound COMT (MB-COMT), and a shorter mRNA from the P1 promoter encodes a soluble COMT (S-COMT). The MB-COMT has higher substrate affinity but lower catalytic activity than S-COMT (Lotta et al. 1995). The MB-COMT is predominantly expressed in brain neurons (Matsumoto et al. 2003), and S-COMT is predominantly expressed in other tissues, such as liver, blood, and kidney (Tenhunen et al. 1993; Lundstrom et al. 1995). Human COMT contains a common functional polymorphism, a G→A substitution in exon 4 that alters the amino acid codon at position 108 (Val108Met) (rs165688) in S-COMT or position 158 (Val158Met) in the MB-COMT protein (Lotta et al. 1995). This common polymorphism has been shown to result in a significant change in enzyme activity in peripheral blood and in liver (Scanlon et al. 1979; Weinshilboum and Dunnette 1981; Boudikova et al. 1990; Lotta et al. 1995).
Although COMT is expressed widely throughout the brain, it appears to play a particularly important role in dopamine flux in the prefrontal cortex. In the cortex, the dopamine transporter, which has a 1,000-fold higher affinity for dopamine than does COMT, is expressed at very low levels and does not appear to affect extracellular dopamine levels (Garris et al. 1993; Sesack et al. 1998; Lewis et al. 2001; Huotari et al. 2002b). Thus, inactivation of dopamine in the prefrontal cortex appears to rely preferentially on catabolic enzymes, including COMT. This notion is supported by pharmacological evidence (Garris et al. 1993; Tunbridge et al. 2004a) and by studies that found that dopamine tissue levels (Gogos et al. 1998) or turnover (Huotari et al. 2002a) were significantly increased in the frontal cortices but not in the striata of COMT knockout mice, as compared with wild-type mice. Also, COMT mRNA expression in the prefrontal cortex of the human brain appears to be greater than in the striatum (Matsumoto et al. 2003). Given the established role of prefrontal dopamine in working memory (Goldman-Rakic et al. 2004), it is not surprising that COMT would play a role in prefrontally mediated cognitive function. Indeed, clinical studies of COMT have revealed that the Met allele, which encodes the enzyme with lower activity, is associated with better performance on tests of prefrontally mediated cognition (Egan et al. 2001; Goldberg et al. 2003; Mattay et al. 2003; Diamond et al. 2004; Nolan et al. 2004). The effect of the Val/Met polymorphism on dopamine-mediated frontal cortical function may be the neurobiological mechanism behind the clinical associations with CNS disorders (Weinberger et al. 2001).
Most studies of COMT enzyme activity have focused on the effect of the Val/Met polymorphism at the 108th/158th codon. The Val allele encodes an enzyme with higher stability at a variety of temperatures from 37°C to 56°C (Scanlon et al. 1979; Spielman and Weinshilboum 1981; Weinshilboum and Dunnette 1981; Lotta et al. 1995). However, the actual difference in enzyme activity between the Val and Met proteins is controversial: different temperatures were used for testing enzyme stability, and high variations were observed among the various samples tested (Scanlon et al. 1979; Spielman and Weinshilboum 1981; Weinshilboum and Dunnette 1981; Lotta et al. 1995). The high variations raise the possibility that there might be other SNPs that affect enzyme activity. Indeed, other SNPs in COMT, particularly a SNP in intron 1 (rs737865) of the MB-COMT isoform and a SNP in the 3′ flanking region (rs165599), have been reported to be associated with risk for schizophrenia and possibly to affect COMT expression (Shifman et al. 2002). Bray et al. (2003) suggested, on the basis of evidence of differential expression of Val and Met chromosomes, that these other SNPs may represent cis elements that have greater effects on enzyme activity than the Val/Met polymorphism. Another SNP in the 5′ flanking region (rs2097603)—and potentially contained within the P2 promoter—has been proposed as a functional SNP; linkage disequilibrium (LD) analysis suggests that this SNP may account for the association of the intron 1 SNP with schizophrenia (Palmatier et al. 2004). However, it is unknown whether any variation in COMT, other than Val/Met, affects protein integrity or enzyme activity. In addition, COMT activity in human dorsolateral prefrontal cortex (DLPFC), a brain region critical to cognitive function and presumably especially impacted by COMT activity, has never been examined.
In this study, we analyzed samples of human postmortem DLPFC and human lymphocytes to examine the effects of SNPs implicated in schizophrenia on COMT enzyme activity. We also measured COMT gene and protein expression in the brain samples. The intent of this study was to test for potential functional effects of these SNPs. Thus, functional SNPs in the P2 promoter region and SNPs reflecting cis-acting factors might be expected to particularly affect mRNA abundance, consistent with the data of Bray et al. (2003). In contrast, the coding Val/Met SNP might be expected to have a greater impact on protein integrity (Shield et al. 2004). For any of these genetic mechanisms to penetrate to the level of clinical phenotype, an effect on enzyme activity would be expected. Our results demonstrate that the enzyme activity of COMT-Val is ∼40% higher than that of COMT-Met in postmortem human DLPFC at 37°C, a normal physiological temperature, and that an analogous effect on the level of protein immunoreactivity is evident for the COMT-Val variant. The P2 promoter SNP may have a small effect on COMT expression, as reflected in enzyme activity in lymphocytes, whereas the intron 1 and 3′ flanking SNPs have no significant effects on COMT mRNA expression, protein immunoreactivity, or enzyme activity in the human DLPFC or on enzyme activity in lymphocytes. A comparison of human and mouse COMT confirms that the amino acid at the Val/Met locus is important for COMT activity and suggests that COMT activity has decreased during the course of evolution. These results suggest that the Val/Met SNP is the predominant genetic factor that determines COMT activity in human DLPFC and its effect on prefrontal dopamine signaling.
Material and Methods
Human Postmortem Tissue
Postmortem DLPFC tissue (one hemisphere) from 108 brains was collected at the Clinical Brain Disorders Branch, National Institute of Mental Health (NIMH), from 77 normal controls (19 females, 58 males; 26 whites, 51 African Americans; mean age 40.6 ± 14.9 years; postmortem interval [PMI] 30.7 ± 13.6 h; pH 6.60 ± 0.24) and 31 individuals with schizophrenia (11 females, 20 males; 15 whites, 16 African Americans; mean age 45.9 ± 15.4 years; PMI 37.1 ± 14.8 h; pH 6.47 ± 0.24). Informed consent of the next of kin was obtained as described in detail elsewhere (Kleinman et al. 1995), in accordance with a protocol approved by the NIMH institutional review board. The tissues were pulverized and stored at −80°C. The brains were selected from a large pool of postmortem specimens in an effort to optimize mRNA and protein quality without prior knowledge of COMT genotypes. Cases with histories of other psychiatric or neurological disorders or of substance abuse were excluded from the normal control series. Demographic characteristics of this cohort, including causes of death, are shown in table A1 (online only). In general, the cohort characteristics, such as PMI, pH, and age, are similar to those in our previous studies (e.g., Matsumoto et al. 2003; Halim et al. 2003); although samples in our cohort have higher mean pH values in comparison with another study of COMT mRNA expression (Tunbridge et al. 2004b), the cases are similar in terms of PMI and age. The only other study of brain COMT mRNA expression (Bray et al. 2003) did not provide information about pH or PMI.
Protein Extraction
DLPFC (50–130 mg) tissue samples were homogenized in a protease inhibitor-Tris-glycerol extraction buffer (0.024% AEBSF, 0.005% aprotinin, 0.001% leupeptin, 0.001% pepstatin A, 50% glycerol, and 0.6% Tris) (1 g tissue:10 mL buffer) (Halim et al. 2003). The samples were then aliquoted and stored at −80°C. Protein concentration was determined by use of the Bradford assay.
RNA Extraction
Total RNA was extracted from ∼300 mg of tissue by use of the TRIzol reagent (Life Technologies), as described elsewhere (Hashimoto et al. 2004). The yield of total RNA was determined by absorbance at 260 nm. RNA quality was assessed with high-resolution capillary electrophoresis (Agilent Technologies), and only samples showing clearly defined, sharp 18S and 28S ribosomal peaks and 28S:18S ratios >1.2 were included. Total RNA (4 μg) was used in 50 μl of a reverse transcriptase reaction to synthesize cDNA by use of a SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen), in accordance with the manufacturer’s protocol.
Immunoblotting
Homogenates of tissue samples from normal controls (n=73) containing 1.5 μg of total protein content per 5 μl were prepared for immunoblotting by diluting the homogenized samples with water and adding LDS sample buffer (1.25 μl of 4× buffer per sample) (Invitrogen). Samples were then heated to 90°C for 5 min to denature proteins. For each sample, 5 μl were loaded onto precast 10% Bis-Tris polyacrylamide gels (Invitrogen), and proteins were separated by electrophoresis at 175 V for 0.5 h by use of a single power source. Each gel contained a molecular-weight marker ladder (SeeBlue Plus 2 [Invitrogen]) and pooled samples from controls, which were used for normalization in triplicate. Seven separate gels were used in one experiment. Gels were transferred onto nitrocellulose membranes for 2 h at room temperature at 85 V in NuPAGE transfer buffer with 20% methanol. Membranes were blocked for 1 h in 10% goat serum in Tris-buffered saline (TBS) with 0.1% Tween-20 (TBS-T) and then were incubated with the polyclonal rabbit anti-COMT antibody (1:7,000 dilution [Chemicon]) and a monoclonal mouse anti-actin antibody (1:3,000 dilution [Chemicon]). Blots were rinsed in TBS-T, incubated in a peroxidase-conjugated goat anti-rabbit and goat anti-mouse antibody (1:10,000 dilution [Chemicon]) for 2 h in 5% normal goat serum in TBS-T and rinsed in TBS-T. Blots were developed in ECL Plus (Amersham) and were exposed to Kodak BioMax film. Films were digitized using a scanner, and the resulting images were analyzed using National Institutes of Health Image gel plotting macros on a Macintosh computer (G4). The values for all samples were expressed as a percentage of a mean of controls on the same gel.
Human Lymphocytes
Blood was collected from normal subjects, and lymphocytes were transformed into lymphoblast cultures by standard methods. Lymphocytes were selected from 47 individuals who were homozygous at the Val/Met locus, and the samples were matched by sex. The methods for recruitment and screening of volunteers are described in detail elsewhere (Egan et al. 2001). The DNA collection protocol was approved by the NIMH institutional review board, and all donors gave informed consent. Low-speed centrifugation was used to collect 100,000,000 cells. The cell pellets were lysed in 300 μl of 1× lysis buffer (5 mM Tris-HCl [pH 7.4], 0.2% Triton X-100, and 1 mM dithiothreitol [DTT]) with a sonicator. The total protein concentrations of the lysates were measured by the Bradford method. Immunoblotting from lymphocytes was performed using the method described above.
Real-Time Quantitative PCR
COMT mRNA expression levels in brain were measured by real-time quantitative RT-PCR by use of an ABI Prism 7900 sequence detection system with 384-well format. TaqMan probes and primers spanning exons 5–6 were purchased from Applied Biosystems (Assay-on-Demand [catalog number Hs_00241349m1]). Each 20 μl PCR contained 6 μl of cDNA, 1 μl of 20× primer/probe mixture, and 10 μl of RT-PCR MasterMix Plus (Eurogentec) containing Hot GoldStar DNA polymerase, dNTPs with dUTP, uracil-N-glycosylase, passive reference, and optimized buffer components. PCR cycling conditions were 50°C for 2 min, 95°C for 10 min, 40 cycles of 95°C for 15 s, and 59°C or 60°C for 1 min. PCR data were obtained with the Sequence Detection Software (SDS, version 2.0 [Applied Biosystems]) and quantified by a standard curve method by use of serial dilutions of pooled cDNA derived from RNA obtained from DLPFC of 12 normal control subjects. SDS plotted real-time fluorescence intensity. The threshold was set within the linear phase of the amplicon profiles. All measurements were performed in triplicate.
SNP Genotyping
All genotypes were determined using the 5′ exonuclease TaqMan assay. The sequences of primers and TaqMan probes for the SNP genotyping were as follows: (1) P2 promoter SNP (rs2097603), GCCGTGTCTGGACTGTGAGT (forward)/GGGTTCAGAATCACGGATGTG (reverse) and 6FAM-AACAGACAGAAAAGTTTCCCCTTCCCA/VIC-CAGACAGAAAAGCTTCCCCTTCCCATA; (2) intron 1 SNP (rs737865), GCTTGGAGGGTCACTTTAAACAATA (forward)/TGCTAACAGACCTGCTTTTTGG (reverse) and 6FAM-CAGGACACAAAAAcCCCTGGCTG/VIC-CAGGACACAAAAAtCCCTGGCTGG; (3) Val/Met SNP (rs4680), TCGAGATCAACCCCGACTGT (forward)/AACGGGTCAGGCATGCA (reverse) and 6FAM-CCTTGTCCTTCACGCCAGCGA/VIC-ACCTTGTCCTTCATGCCAGCGAAAT; (4) 3′ flanking SNP (rs165599), CAGCCACAGTGGTGCAGAG (forward)/AAGGTGTGAATGCTGGCTGA (reverse) and 6FAM-TTTCCCAGGCtGGCAGTCGTC/VIC-CGTTTCCCAGGCcGGCAGT.
Site-Directed Mutagenesis of Mouse COMT cDNA
Mutations from mouse Leu codon (CTA) to human Val codon (GTG) or Met codon (ATG) were introduced into mouse COMT cDNA (acquired from the Mammalian Gene Collection [see the Mammalian Gene Collection Web site]) by PCR-based site-directed mutagenesis (Ailenberg and Silverman 1997). Primers containing mutations at the Leu site were synthesized and incorporated into mouse COMT cDNA by PCR. The primers for synthesis of the mouse COMT-Met and COMT-Val mutants in the first round of PCR were GATGCTGTTGGCTGCTGTCTCA (forward)/CTGCATGCCTGCGAAGTCCAGCATTTG (reverse) and GAAATGCTGGACTTCGCAGGCATGCAG (forward)/GCACTGTGATCTACTGCATCTCT (reverse) for the COMT-Met mutant and GATGCTGTTGGCTGCTGTCTCA (forward)/CTGCACGCCTGCGAAGTCCAGCATTTG (reverse) and CAAATGCTGGACTTCGCAGGCGTGCAG (forward)/GCACTGTGATCTACTGCATCTCT (reverse) for the COMT-Val mutant. The PCR products that contain the specific mutations were used as templates for the second round of PCR with specific primers (CGTAATACGACTCACTATAGGGCGACCCACCATGGGTGGCACAAAGGAGCA [forward]/GCACTGTGATCTACTGCATCTCT [reverse]), which amplified the mutant mouse COMT cDNA and incorporated a T7 promoter to the 5′ end of the full length of mouse COMT cDNAs. The PCR products from the second-round PCR were purified using a PCR product purification kit (Qiagen) and were used as templates for in vitro synthesis of mouse mutant COMT proteins.
In Vitro Translation
Full-length MB human COMT-Val, COMT-Met, and mouse COMT-Leu cDNAs were obtained from the Mammalian Gene Collection (see Mammalian Gene Collection Web site). The DNA templates for in vitro translation were prepared by PCR with specific forward primers that contained the T7 promoter and the backward primers. The DNA templates were purified using a PCR product purification kit (Qiagen). Human and mouse COMT proteins were translated from the templates by use of an in vitro transcription-coupled protein translation kit (Promega), and enzyme activity of the translated COMT was measured.
Modified COMT Activity Assay
The COMT enzyme activity assay uses the organic solvent extraction method that separates the radioactive product, the methylated catechol, and the free radioactive coenzyme, 3H-SAM (Zurcher and Da Prada 1982). From each sample, 100 μg of human DLPFC or lymphocyte protein at a concentration of 5 μg/μl was transferred to a fresh microcentrifuge tube and equilibrated to room temperature shortly before the enzyme assay. To each tube, we added 500 μl of the substrate mixture, which contained 10 mM Tris (pH 7.4), 1 mM MgCl2, 1.5 μCi of 3H-SAM, 10 μM of catechol, and 1 μM of DTT. The tubes were then incubated at 37°C for 20 min. The reactions were immediately terminated by the addition of 500 μl of 1M HCl. The radioisotope-labeled catechol products from the reactions were extracted by adding 10 ml of scintillation fluid (Flow I [Molecular Diagnosis]) to the reaction mixture and then were measured for the radioactivity of the mixture in a scintillation counter. The relative COMT enzyme activity is presented as disintegrations per minute (DPM) per mg total protein. To establish a baseline control for nonspecific reactions that do not depend on COMT, 5 μl of the specific COMT inhibitor, tolcapone (10 mg/ml), was added to a tube containing 100 μg of the human DLPFC sample. The high concentration of potent inhibitor blocked the specific reaction catalyzed by COMT, and the radioactivity from this reaction served as a baseline. To determine COMT activity in the in vitro–translated protein samples, the relative COMT activity was normalized with the levels of 35S-labeled COMT protein products. A time-course analysis of COMT stability was prepared by either treating protein samples at 37°C (heat-treated) or keeping them on ice (cold-treated) for 0–120 min; the COMT activity in the heat-treated and cold-treated samples was then measured to establish stability.
Statistical Analysis
Two-tailed Student’s t tests were used to examine whether diagnostic groups (normal controls and individuals with schizophrenia) differed in variables such as brain pH, PMI, and age. Separate two-tailed Student’s t tests were also used to test whether mRNA expression levels, protein levels, and enzyme activity differed between the two groups (normal controls and individuals with schizophrenia). Spearman’s coefficients of correlation were calculated to examine whether enzyme activity correlated with protein immunoreactivity or mRNA expression or whether these measurements were associated with age, pH, or PMI. In the case of significant correlations, one-way analyses of covariance (ANCOVAs) were performed covarying for a correlated factor. To test the effects of sex or race on dependent measures, two-way analyses of variance (ANOVAs) were used with diagnosis (controls or individuals with schizophrenia) or genotype and sex as independent factors. One-way ANOVAs, followed by Fisher's protected least significant difference post hoc tests, when appropriate, were used to test the effects of genotype on enzyme activity, protein immunoreactivity, and COMT mRNA expression. To control for possible effects on enzyme activity of genotype at one locus related to LD with another locus, analyses were also performed conditioned on homozygote backgrounds at each locus.
Results
COMT mRNA Expression in Brain by RT-PCR
mRNA expression levels (either without normalization or normalized by β-2-microglobulin or β-actin) were not significantly correlated with age, pH, or PMI (r<0.1; P>.05) (table 1). There was no effect of disease on COMT mRNA expression normalized by β-2-microglobulin or β-actin (t<1.5 [110 df]; P>.5) or on the expression of β-2-microglobulin or β-actin mRNAs (t<1.0 [110 df]; P>.5). There were no significant differences between the genotypic groups in the expression of both reference genes, β-2-microtubulin and β-actin (P>.2), and none of the genotypes examined (Val/Met, P2 promoter, the intron 1 SNP, or the 3′ SNP) significantly affected COMT mRNA expression normalized by β-2-microglobulin or β-actin (P>.5) (table 2), in either of the clinical groups or in the entire sample.
Table 1.
Relationships in Brain with Age, PMI, and pH
|
Spearman's CorrelationCoefficient (ρ) for |
|||
| Measurement | Age | PMI | pH |
| COMT enzyme activity (DLPFC) | .194a | −.141 | −.151 |
| S-COMT immunoreactivity | .085 | −.023 | .077 |
| MB-COMT immunoreactivity | .297b | −.006 | −.091 |
| COMT mRNA levels | −.112 | −.016 | .097 |
P<.05.
P<.01.
Table 2.
Effects of Genotype, Sex, and Race[Note]
|
F Value for |
|||||||
| Genotype |
|||||||
| Measurement | Val/Met | P2 Promoter | 3′ SNP | Intron 1 SNP | Haplotype | Sex | Race |
| COMT enzyme activity (DLPFC) | 7.95a | 1.47 | 1.70 | .57 | 2.24 | 3.96b | .04 |
| COMT enzyme activity (lymphocytes) | 16.29a | 7.31c | .02 | 3.37b | NAd | 2.18 | NAe |
| S-COMT immunoreactivity | 5.96c | .65 | .53 | .61 | 1.11 | .07 | .86 |
| MB-COMT immunoreactivity | 2.25f | .89 | .70 | 1.28 | .55 | .34 | .50 |
| COMT mRNA levels | .10 | 2.10 | .77 | .99 | .11 | 1.05 | .26 |
Note.— The table displays the results of one-way ANOVAs. NA = not applicable.
P<.001.
P<.05.
P<.01.
Data unavailable because groups were small.
Only whites were tested.
P=.09.
COMT Immunoreactivity
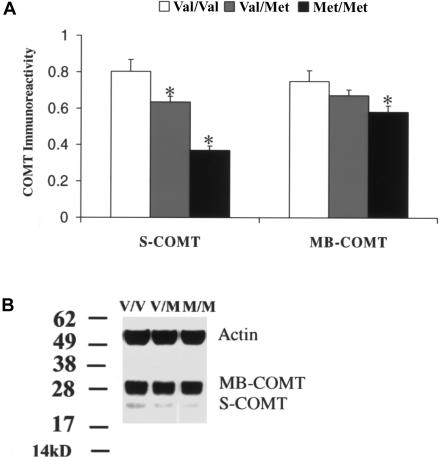
Immunoblotting analysis of DLPFC samples revealed that COMT proteins migrated with a double band approximately at the predicted molecular weights of 25 kDa and 28 kDa, corresponding to the soluble and MB forms of COMT, respectively (fig. 1). The band corresponding to MB-COMT was markedly more prominent than that corresponding to S-COMT. There was a significant effect of the Val/Met genotype on immunoreactivity of the S-COMT isoform (F[2,71]=5.96; P<.01) (table 2). Individuals with the Met/Met genotype showed 54% less S-COMT immunoreactivity than individuals with the Val/Val genotype (P=.001) and 21% less than individuals with the Val/Met genotype (P=.02). Although there was only a trend effect of genotype on MB-COMT immunoreactivity (F[2,71]=2.25; P=0.09) (table 2), post hoc testing revealed that—like S-COMT—individuals with the Met/Met genotype showed significantly lower immunoreactivity than subjects with the Val/Val genotype (P=.03). S-COMT and MB-COMT immunoreactivities were highly correlated with each other (r>0.7; P<.0001). Although there were no significant correlations of S-COMT or MB-COMT immunoreactivity with pH or PMI (r<0.1; P>.1) and no correlation of S-COMT with age (r<0.1; P>.1), there was a positive correlation of MB-COMT with age (r>0.2; P<.01) (table 1). Reanalysis of the effect of genotype on MB-COMT protein immunoreactivity, covarying for age, revealed a similar effect (F[2,71]=2.1; P=.1). There was no significant effect of any other genotype on S-COMT or MB-COMT immunoreactivity, either in the entire sample or in subgroups homozygous for Val or Met (P>.2) (table 2) The effects of sex on S-COMT and MB-COMT immunoreactivity were not significant (F<0.5; P>.4) (table 2). There was no correlation between either S-COMT or MB-COMT and the expression of COMT mRNA (r<0.1; P>.5).
Figure 1.
Effect of the Val/Met SNP on S-COMT and MB-COMT protein immunoreactivity. A, Comparison of protein immunoreactivity (analyzed by Western blotting with the use of a specific anti-human COMT antibody) for S-COMT and MB-COMT proteins in postmortem DLPFC tissues with genotypes Val/Val, Val/Met, and Met/Met. The asterisk (*) indicates difference from Val/Val at a significance level of P<.05. B, A representative Western blotting image with MB-COMT, S-COMT, and internal control (Actin) bands.
Effects of Val/Met on COMT Activity in Human DLPFC at 37°C
Our assay showed a linear relationship between COMT enzyme activity and total protein concentration across a wide range (fig. 2A), which suggests that we can detect differences in COMT activity among human DLPFC samples with large differences in enzyme activity. Under normal physiological temperatures without heat pretreatment, the activity of homozygous COMT-Val was ∼38% higher than that of homozygous COMT-Met in the DLPFC (F[2,102]=7.95; P<.001) (fig. 2B). This difference in enzyme activity between Val and Met is smaller than the 2–4-fold difference reported elsewhere by several other groups; it may reflect the lower temperatures used in our study, confounders associated with postmortem tissue, or differences in assay methodology. Enzyme activity was positively, although weakly, correlated with age (r=0.19; P<.05) (table 1). We therefore reanalyzed the effect of the Val/Met genotype on enzyme activity covarying for age, and the effect was still highly significant (ANCOVA F[2,102]=6.4; P<.01). The effect of the Val/Met polymorphism on COMT activity was confirmed in lymphoblast cultures (F[1,45]=16.288; P<.001) (fig. 2C), where the effect size of the difference was comparable with that of DLPFC, suggesting that the observed effect of the Val/Met polymorphism in brain was not due to confounders of postmortem tissues.
Figure 2.
Significantly higher activity of COMT-Val than of COMT-Met in human DLPFC tissue and in lymphocytes at 37°C. A, High correlation of the relative COMT activity with protein concentration (r=0.999) in a linear relationship. B, COMT activity in protein samples from human postmortem DLPFC tissues with genotypes Val/Val, Val/Met, and Met/Met. C, COMT activity in protein samples from lymphoblast cultures from subjects with genotypes Val/Val and Met/Met. N = sample size. Two asterisks (**) indicate difference from Val/Val at a significance level of P<.01.
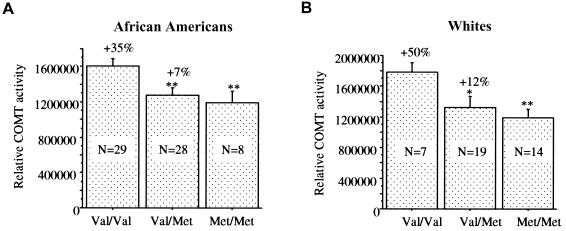
The enzyme activity in DLPFC was significantly correlated with both S-COMT and MB-COMT immunoreactivities (r>0.4; P<.01). To examine whether the effect of the Val/Met genotype on enzyme activity was at least in part independent of its effect on the integrity of the protein, we used MB-COMT immunoreactivity as a covariate in the analyses and found that the effect of genotype was still significant (F[2,67]=4.12; P<.02). This suggests that differences in the abundance or integrity of COMT protein, per se, do not account for the entire difference in enzyme activity between individuals with different Val/Met genotypes. We also analyzed the effect of the Val/Met SNP on enzyme activity in two ethnic populations, African Americans and whites (F[2,96]=6.55; P<.01) (fig. 3). The effects are similar; there was no significant effect of race (F[1,97]=1.662; P>.05) (table 2) and no significant interaction between the Val/Met genotype and race (F[2,96]=0.389; P>.05) (table 3), which suggests that the different haplotypes within COMT that differentiate these populations (DeMille et al. 2002) do not impact the Val/Met effect.
Figure 3.
Effect of the Val/Met genotype on COMT activity in human postmortem DLPFC tissue from two different populations (African Americans and whites). The effects of the Val/Met genotype in African Americans (A) and whites (B) were similar. N = sample size. One asterisk (*) indicates difference from Val/Val at a significance level of P<.05; two asterisks (**) indicate difference from Val/Val at a significance level of P<.001.
Table 3.
Interaction between Genotype and Sex and Race[Note]
|
Interaction (F) between |
||||||||
| Val/Met and |
P2 Promoter and |
3′ SNP and |
Intron 1 SNP and |
|||||
| Measurement | Sex | Race | Sex | Race | Sex | Race | Sex | Race |
| COMT enzyme activity (DLPFC) | .04 | .39 | .08 | .46 | .06 | .4 | 1.19 | 2.27 |
| COMT enzyme activity (lymphocytes) | 5.32a | NAb | 1.51 | NAb | .03 | NAb | 1.27 | NAb |
| S-COMT immunoreactivity | .36 | .88 | .33 | .32 | 1.32 | .92 | .49 | .51 |
| MB-COMT immunoreactivity | .62 | .06 | 1.32 | .65 | 1.55 | 1.71 | .16 | .33 |
| COMT mRNA levels | .03 | 1.96 | .62 | 2.67 | .77 | 1.33 | .48 | 1.69 |
Note.— The results of two-way ANOVAs. NA = not applicable.
P<.05.
Only whites were tested.
Validation of the Val/Met Effect by Use of In Vitro Translation and Site-Directed Mutagenesis
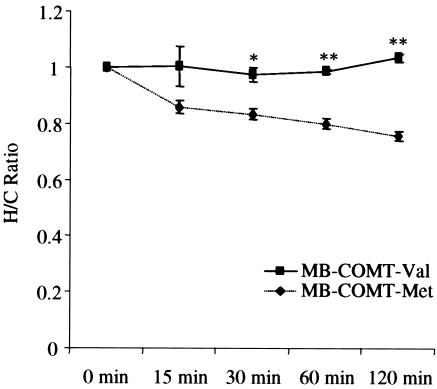
To further characterize the differential Val/Met effect, we synthesized MB-COMT-Val and MB-COMT-Met by use of an in vitro protein synthesis system. We tested the thermostability of the COMT proteins, and the results showed that MB-COMT-Val and MB-COMT-Met responded differently to heat pretreatment at 37°C (F[1,28]=23.57; P<.001) (fig. 4). The ratio of the enzyme activity between the heat-treated and cold-treated MB-COMT-Met decreased significantly as the duration of heat treatment increased; however, this effect was not detected in MB-COMT-Val samples. Thus, there was an interaction between genotype and time (F[4,25]=3.18; P<.05). MB-COMT-Val was significantly more stable than MB-COMT-Met, as has been shown elsewhere for S-COMT (Lotta et al. 1995). This might represent a mechanism that underlies the protein and activity differences in the brains of subjects with the Val and Met genotypes.
Figure 4.
Comparison of the ratio of the enzyme activity between the heat-treated and cold-treated MB-COMT-Met and MB-COMT-Val. MB-COMT-Val is more stable than MB-COMT-Met at 37°C. The ratios (± SE) of COMT activity in the synthesized MB-COMT-Val and MB-COMT-Met samples exposed to a temperature of 37°C for 0–120 min (H) or kept on ice at 4°C (C), measured in triplicate. One asterisk (*) indicates difference from COMT-Met at a significance level of P<.05; two asterisks (**) indicate difference from MB-COMT-Met at a significance level of P<.01.
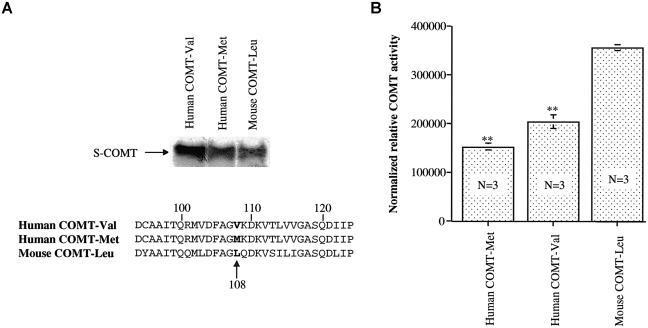
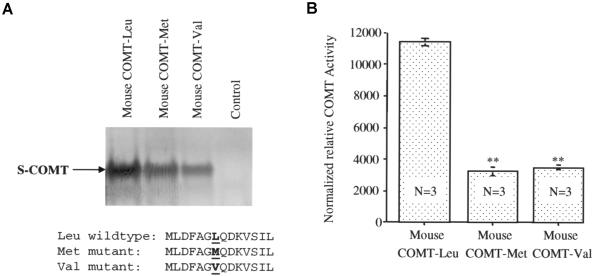
Comparison of the human and mouse cDNA sequences revealed that the mouse has leucine (Leu) at the Val/Met position of the human COMT protein (fig. 5A). To explore the potential consequence of this mutation in evolution, we synthesized the soluble form of human and mouse COMT proteins by use of the in vitro translation system and analyzed the activity of the synthesized proteins (fig. 5B). There were dramatic differences in enzyme activity between these variant COMTs (F[2,6]=128.19; P<.0001); the mouse COMT-Leu had much higher activity than the human COMTs, and the human COMT-Val had greater activity than COMT-Met. Because the Val allele appears to be the ancestral human allele found in primates (Palmatier et al. 2004), these various observations suggest that the mutations from Leu to Val to Met at this polymorphic site in the COMT gene resulted in progressively lower COMT activity. To further confirm the importance of the amino acid at this polymorphic site, we introduced human Val and Met mutations into the mouse COMT gene by use of a PCR-based mutagenesis procedure and synthesized the wild-type and mutant mouse COMT proteins by use of the in vitro synthesis system (fig. 6A). The results again indicate that the human Val or Met mutations at the Leu site dramatically decreased the activity of the COMT proteins (F[2,6]=265.08; P<.0001) (fig. 6B).
Figure 5.
Comparison of the human and mouse cDNA sequences and COMT activity from three assays. The activity of mouse COMT-Leu is higher than that of human COMT-Val and COMT-Met. S-COMT proteins from human COMT-Val and COMT-Met, as well as mouse COMT-Leu, were synthesized using an in vitro transcription-coupled transcription system with 35S-methionine as a radioactive substrate. A, Representative autoradiogram of the synthesized proteins with radioactive 35S-methionine residue and the amino acid sequences of the three COMT proteins. B, Averaged COMT activity from three independent assays, normalized with the radioactivity of the 35S-labeled COMT proteins. The activity of mouse COMT-Leu is significantly higher than that of human COMT-Val or COMT-Met. Two asterisks (**) indicate difference from mouse COMT-Leu at a significance level of P<.0001.
Figure 6.
Comparison of the activity of the proteins in mouse COMT-Leu, COMT-Met, and COMT-Val. Mutations from Leu to Val and Met in mouse COMT resulted in a dramatic reduction in COMT activity. The Leu codon in mouse COMT cDNA was mutated into Val or Met by site-directed mutagenesis, and the wild-type and mutant mouse COMT proteins were synthesized using an in vitro protein synthesis system. A, Representative autoradiogram of the wild-type and mutant mouse S-COMT proteins labeled with 35S-methionine and partial protein sequences. B, Averaged COMT activity from three independent assays, normalized with COMT protein levels. COMT activity differs significantly between the wild-type and mutant proteins. Two asterisks (**) indicate difference from wild-type COMT-Leu at a significance level of P<.0001.
Effect of the P2 Promoter SNP on COMT Activity
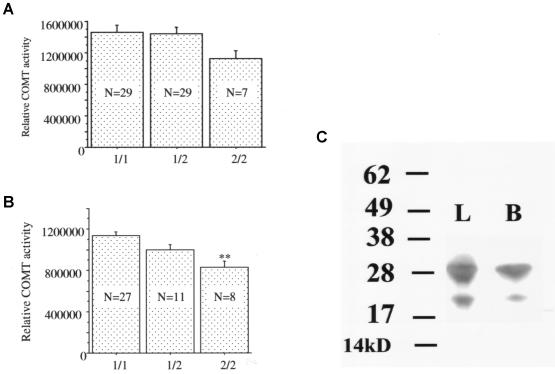
A small effect of the SNP in the P2 promoter region was detected in the DLPFC samples from the African American subjects, but this effect was not statistically significant (F[2,62]=1.465; P=.24) (fig. 7A). To avoid potential confounders from postmortem tissues, including the possibility that alleles might show differential postmortem stability, we analyzed the effect of differences in genotype at this SNP in lymphocyte cultures (fig. 7B). In the lymphocytes, differences in genotype had a statistically significant effect (F[2,43]=7.313; P<.01) (table 2); the P2 promoter SNP effect was also found in individuals homozygous for Met. A Western blot analysis of the COMT protein in lymphocytes confirmed abundant expression of the MB form of the protein (fig. 7C), which would be under the control of the P2 promoter.
Figure 7.
Effect of the P2 promoter SNP on COMT activity in human DLPFC (A) and lymphoblast cell cultures (B) of different genotypes at the promoter SNP site. Two asterisks (**) indicate difference from genotype 1/1 at a significance level of P<.01. Similar effects were seen in lymphocytes of subjects with the homozygous Met/Met genotype. C, Abundant MB-COMT protein, detected by Western blotting with the use of a specific anti-human COMT antibody, in lymphocytes (L) and brain (B) protein samples.
Effects of Other SNPs on COMT Activity
The 3′ flanking SNP near the polyadenylation site has been reported to be strongly associated with schizophrenia and to affect differential expression of Val/Met alleles (Shifman et al. 2002; Bray et al. 2003). We found no significant effects of this SNP on mRNA or protein expression or on COMT activity in DLPFC tissue in the entire sample or in each Val/Met genotype group (F[2,100]=1.70; P>.05) (table 2). The intron 1 SNP has also been identified and linked to risk for schizophrenia, as well as to differential expression of Val/Met alleles (Shifman et al. 2002; Bray et al. 2003). However, differences in genotype at this SNP appeared to have no effect on COMT mRNA or protein expression or enzyme activity in DLPFC in the entire sample (F[2,100]=0.57; P>.5) (table 2) or in each Val/Met genotype group (F[4,94]=0.50; P>.5). Finally, because of the possibility that COMT activity or the Val/Met effect could be modified by a cis-acting haplotype, we constructed likely haplotypes in our samples on the basis of our four polymorphisms by use of SNPHAP, version 1.0 (see the Software and Course Materials Web site), and we reanalyzed COMT activity in the brain on the basis of the common haplotypes. No effects were seen other than those attributable to the Val/Met polymorphism (table 2).
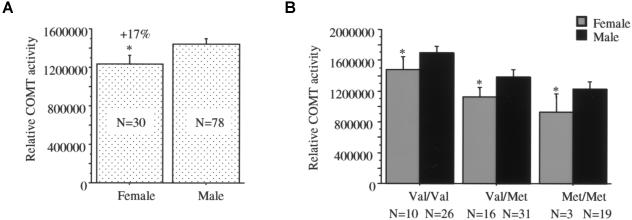
Effect of Sex on COMT Activity
Estrogen has been shown to be a regulator of COMT promoter activity, and COMT activity in blood is lower in females (Weinshilboum et al. 1999). To test the effects of sex on COMT activity, we analyzed enzyme activity of the DLPFC samples from females and males (fig. 8). The results showed that females have lower COMT activity than males (F[1,106]=3.96; P<.05) (fig. 8A), and the effect of sex was independent of the Val/Met effect in the DLPFC (fig. 8B). There was no effect of sex on COMT activity in lymphocytes (F[1,43]=0.58; P>.05) (table 2). However, the interaction between sex and the Val/Met genotype was significant in lymphocytes (F[1,43]=5.32; P<.05) (table 3), indicating that the effect of genotype was more pronounced in males than in females.
Figure 8.
Effect of the subject's sex on COMT activity in human DLPFC. Females had significantly lower COMT activity in the samples as a whole (A) as well as in each of the Val/Met genotypes (B). The asterisk (*) indicates difference from males at a significance level of P<.05.
Discussion
The major findings of this study are (1) the confirmation of earlier work that suggested that the Val/Met SNP in the human COMT gene is a critical genetic factor in determining variation in COMT enzyme activity and (2) the demonstration that this effect is germane to human DLPFC at normal physiological temperature. COMT activity assayed using standard laboratory methods in postmortem DLPFC is ∼40% higher in human subjects with the COMT-Val allele than in those with the COMT-Met allele. Similar results were confirmed in lymphocytes by use of the same assay, which indicates that the findings in postmortem tissue are not artifacts of potential differences in postmortem protein stability related to genotype. Our data represent the first demonstration that there is a significant difference in COMT enzyme activity in the human brain and, specifically, that there is a significant difference between the Val and Met genotypes in the DLPFC. Our results also demonstrate that MB-COMT-Val appears to show higher stability than MB-COMT-Met at 37°C. Analyses of the human Val and Met sites and the mouse Leu site reveal a pattern of progressively lower COMT enzyme activity in the course of these evolutionary changes. Although a SNP in intron 1 and another in the 3′ flanking region have been associated with a greater risk for schizophrenia and with differential expression of Val and Met chromosomes in an in vitro assay, these SNPs—alone or in combination—showed no significant effects on COMT mRNA expression levels, protein immunoreactivity, or enzyme activity in DLPFC or on enzyme activity in lymphocytes. Thus, although we cannot rule out a more complex genetic basis for functional effects of COMT, we doubt that the evidence based on differential expression assays (Bray et al. 2003) translates into functional changes in COMT protein expression or enzyme activity in tissue.
The Mechanism of the Val/Met Effect
The amino acid at the Val/Met polymorphism site has been localized to the surface of the protein by analysis of the crystal structure of COMT (Vidgren et al. 1994; Zubieta et al. 2001). Protein engineering studies have shown that an enzyme is more stable if it has a more hydrophobic amino acid residue on its surface (Liu and Wang 2003; Machius et al. 2003). Since the Val residue is more hydrophobic than Met, it would be predicted that Val would be more stable than Met. Our protein measurements also support this prediction. Likewise, since Leu is more hydrophobic than Val and Met, it would be predicted that mouse COMT-Leu would be more stable than human COMT-Val and COMT-Met. Indeed, our results are consistent with these predictions and with a recent study that measured COMT immunoreactivity in cell culture and in liver and found similar differences between the Val and Met alleles (Shield et al. 2004). However, the sequence homology between mouse and human COMT proteins is only ∼76%. Thus, the difference in COMT activity between mouse and human COMT proteins is probably not solely due to the Val/Met polymorphism site. The difference in enzyme activity between human COMT-Val and COMT-Met is ∼40% in our assay, which is much lower than the 2–4-fold differences reported by other groups (Weinshilboum and Raymond 1977; Scanlon et al. 1979; Lotta et al. 1995). Heat pretreatment has been used to test the thermostability of Val and Met in many studies, and the remaining COMT activities after heat pretreatment were then used to calculate the differences between these alleles. Since we did not use heat pretreatment to estimate the COMT activity difference in the DLPFC samples, the 40% difference for prefrontal COMT activity may be more representative of the real difference under normal physiological conditions. However, we do not know the degree to which our in vitro assay conditions capture the in vivo functional state of COMT or the relative contributions of the S and MB isoforms to overall enzyme activity. Furthermore, we have no basis for estimating how much total in vivo activity is retained in the postmortem tissue.
Single SNP Has a Predominant Effect on Final COMT Activity
Like most human genes, COMT has multiple SNPs. Among them, the Val/Met SNP is the only common nonconservative change in a coding region with known function. Our results demonstrate that the effect of the Val/Met SNP on COMT activity is independent of the three other SNPs in the COMT gene that have been the subject of several recent studies. Those SNPs—in the P2 promoter region and in the intron 1 and 3′ ends of the gene—do not have clear effects independent of their LD relationship with Val/Met. The possible exception is the P2 promoter SNP, which was significant in the lymphocytes of individual homozygotes for the Met or Val alleles. It is interesting that our Western blot analysis of lymphocyte COMT protein indicates that the MB isoform is the predominant expressed protein in lymphocytes, which would be necessary for variation in the P2 promoter region to effect expression. This is consistent with an earlier study that also observed MB-COMT expression in lymphocytes (Sladek-Chelgren and Weinshilboum 1981).
We cannot explain previous reports that the intron 1 and 3′ flanking SNPs affect expression of COMT alleles (Bray et al. 2003). It is conceivable that the allelic expression approach highlights a biological property of the gene or of its mRNA that is not recognized by our method. However, in the same study that reported these differences in allelic expression, Val/Met also showed differential allele expression, and the effects of Val/Met and of the other SNPs were not significantly different from each other in terms of potential transcriptional effects. This finding by Bray et al. (2003) is also in conflict with that of two other studies, which examined the effect of the Val/Met genotype on the expression level of COMT mRNA and found no relationship (Matsumoto et al. 2003; Tunbridge et al. 2004b). Our RT-PCR analysis, which represents the largest tissue sample examined to date, also shows no effect. Clearly, our data suggest that, of the SNPs tested, only Val/Met and possibly the P2 promoter SNP affect enzyme activity in cells and tissues. This would seem to be the critical biological end point of genetic variation in this gene. However, as stated, we cannot rule out complex genetic effects—for example, cis effects within COMT—that only operate under specific conditions (e.g., during activation of other genes or during specific developmental windows). It is also worth emphasizing that our results do not speak directly to the clinical associations with these other SNPs, which were reported by Shifman et al. (2002).
The frequencies of the Val and Met alleles are substantially different among several populations, varying by as much as 4-fold (DeMille et al. 2002), and multimarker haplotypes in COMT also show marked differences across populations (Palmatier et al. 2004). The results in our relatively small population samples are consistent with these earlier data in showing that whites of European ancestry have a lower frequency of the Val allele than do African Americans. It is interesting that we found no difference in the effect of the Val/Met SNP on COMT activity in these two populations, further suggesting that potential cis effects within COMT, which might be expected to vary across these populations because of differences in LD within COMT, do not dilute the Val/Met effect. Consistent with the study by DeMille et al. (2002), we also observed that Val chromosomes predominantly have G alleles at the promoter locus, whereas Met chromosomes have almost equal frequencies of P2 promoter SNP alleles. This suggests that there may be two populations of Met alleles with respect to enzyme activity and that chromosomes with both Met and P2 promoter A (P2A) alleles would have especially low COMT activity. Thus, to the extent that the P2A allele may be the more recent variation at this locus, it suggests another evolutionary change leading to lower COMT activity.
Higher Activity of COMT in DLPFC and Increased Risk for Schizophrenia
Comparative genomics is a useful strategy to elucidate the biological implications of variations in a gene. By comparing human COMT with mouse COMT, we confirmed amino acid differences in the polymorphism site as well as differences in enzyme activity. Mouse COMT has Leu at the position of Val/Met in human COMT and is more active than either human Val or Met. Since COMT is enriched in liver and plays an important role in chemical detoxification, it might be an advantage for the mouse to have a highly active version of COMT. Other species such as rabbit and pig also have the Leu codon at this locus in the COMT gene. However, nonhuman primates carry the Val allele (Palmatier et al. 2004), which is less active than Leu but more active than Met. Met appears to be a unique mutation in the human COMT gene, which has not been seen thus far in any other species. These observations suggest that there has been evolutionary pressure to lower COMT activity, perhaps correlating with the emergence of higher cortical function. Although the absolute difference in COMT activity between the Val and Met alleles appears to be relatively small, at least based on our in vitro assay, this difference may be biologically critical for regulation of dopamine signaling in prefrontal cortex (Goldman-Rakic et al. 2004). Those studies that have shown a positive association between COMT and schizophrenia have primarily pinpointed the Val allele as the risk allele (Harrison and Weinberger 2004). Thus, although our data do not clarify reports that Val alleles on specific haplotype backgrounds may account for greater risk of disease than Val itself (e.g., Shifman et al. 2002), our results do suggest that this putative effect is not because of simple consequences of additional variation in COMT protein abundance or enzyme activity. Therefore, our findings support the hypothesis that higher COMT activity results in lower prefrontal dopamine signaling and, by this mechanism, leads to relatively impaired prefrontal cortical function and an increased risk of clinical disorders in which prefrontal cortical function contributes to disease pathogenesis.
Acknowledgments
We thank Yeva Snitkovsky, for histopathological technical support; Amy Deep and Llewellyn Bigelow, for help with postmortem diagnosis; Cynthia Shannon-Weickert, for help with tissue processing; Cara Horowitz and Tricia Peters, for help with RT-PCR; and Richard Straub, for help with genotyping and haplotype analysis.
Appendix A
Table A1.
Demographic Data on Brains Used in Expression and Enzyme Activity Experiments[Note]
| Brain Reference No. | Cause of Death | Sex | Diagnosisa | pH | Age at Death(years) |
| 836 | GSW to back with massive internal hemorrhage | M | C | 6.46 | 20 |
| 840 | Stab wound of chest with internal hemorrhage | M | C | 6.91 | 35 |
| 859 | Cardiomegaly (dilated cardiomyopathy) associated with severe HCVD; obesity | M | C | 6.52 | 52 |
| 885 | Multiple GSWs | M | C | 6.93 | 19 |
| 914 | IDDM with marked hypoglycemia | F | C | 6.98 | 19 |
| 922 | Erosion and laceration of gastroesophageal junction associated with significant blood loss | M | C | 6.63 | 51 |
| 942 | Adenocarcinoma of colon with hepatic metastasis and subsequent surgical resection | M | C | 6.27 | 61 |
| 946 | Oropharyngeal carcinoma with metastasis | M | C | 6.76 | 54 |
| 1012 | Multiple GSWs | M | C | 6.85 | 19 |
| 1023 | Massive spinal cord injury | M | C | 6.2 | 63 |
| 1034 | Three vessel occlusive coronary atherosclerosis with ischemic changes | M | C | 6.29 | 57 |
| 1039 | Mitral valve prolapse | F | C | 6.79 | 51 |
| 1040 | Multiple GSWs | M | C | 6.41 | 20 |
| 1052 | GSW to abdomen | M | C | 6.98 | 26 |
| 1058 | Exsanguination due to stab wounds of torso (through heart) | M | C | 7.01 | 19 |
| 1068 | Multiple GSWs | M | C | 6.87 | 22 |
| 1073 | Acute MI due to 3-vessel occlusive coronary atherosclerosis | M | C | 6.67 | 47 |
| 1096 | Stab wound of chest with internal hemorrhage | M | C | 6.27 | 58 |
| 1107 | GSW to chest | F | C | 6.57 | 78 |
| 1122 | Cardiomegaly | M | C | 6.67 | 48 |
| 1134 | Complications of asthma | F | C | 6.29 | 36 |
| 1135 | Acute MI due to severe ASCVD | M | C | 6.59 | 60 |
| 1143 | GSW to chest and right leg | M | C | 6.62 | 18 |
| 1146 | GSW to chest | F | C | 6.72 | 20 |
| 1148 | Multiple GSWs | M | C | 6.88 | 29 |
| 1167 | HCVD | M | C | 6.49 | 45 |
| 1179 | Ruptured dissecting thoracic aortic aneurysm | F | C | 6.43 | 45 |
| 1187 | Dilated cardiomyopathy; obesity | F | C | 6.59 | 21 |
| 1191 | Multiple blunt force injuries | M | C | 6.55 | 36 |
| 1214 | Coronary atherosclerosis | M | C | 6.6 | 61 |
| 1216 | GSW to torso | M | C | 6.76 | 18 |
| 1225 | Intra-abdominal intrathoracic bleed during paraesophageal hernia operation | M | C | 6.38 | 32 |
| 1231 | GSW to chest with injuries to lung, liver, pancreas | M | C | 6.32 | 20 |
| 1243 | Coronary artery atherosclerosis | M | C | 6.65 | 49 |
| 1249 | HCVD | F | C | 6.13 | 51 |
| 1250 | HCVD | M | C | 6.61 | 22 |
| 1263 | Rheumatic heart disease (S/P placement of mitral and aortic valve prostheses) | F | C | 6.84 | 41 |
| 1271 | Thrombosis of coronary artery due to ASCVD | M | C | 6.93 | 47 |
| 1275 | ASHCVD | M | C | 6.69 | 60 |
| 1276 | GSW to torso and upper extremity injuring lung, heart, liver, spleen, kidney, and radial vessels | M | C | 6.76 | 24 |
| 1279 | Hypertrophic cardiomyopathy | M | C | 6.84 | 18 |
| 1281 | GSW to torso and upper extremity with injury of lung and blood vessels | M | C | 6.6 | 24 |
| 1285 | Poorly differentiated pulmonary adenocarcinoma | M | C | 6.42 | 58 |
| 1297 | Acute bronchopneumonia due to uncontrolled DM; ASHCVD | M | C | 6.79 | 62 |
| 1304 | Undetermined | M | C | 6.87 | 58 |
| 1306 | GSWs to torso with injuries to spleen, lungs, liver, and bowel | M | C | 6.49 | 21 |
| 1314 | Acute bronchiopneumonia due to DM | F | C | 6.5 | 53 |
| 1324 | HCVD | F | C | 6.58 | 38 |
| 1325 | Metastatic squamous cell carcinoma of cervix | F | C | 6.74 | 45 |
| 1331 | GSW to chest with perforation of heart, lungs, liver, and spleen | M | C | 6.87 | 20 |
| 1340 | Acute bronchial asthma | F | C | 6.92 | 26 |
| 1346 | HCVD due to morbid obesity | M | C | 6.78 | 29 |
| 1348 | ASHCVD | F | C | 6.33 | 42 |
| 1350 | Bronchial asthma with acute exacerbation | M | C | 6.57 | 24 |
| 1351 | ASHCVD | M | C | 6.17 | 53 |
| 1356 | ASCVD | M | C | 6.95 | 49 |
| 1376 | ASCVD | M | C | 6.58 | 40 |
| 1385 | Chest blunt force injuries | M | C | 6.05 | 35 |
| 1390 | Pulmonary sarcoidosis | F | C | 6.34 | 43 |
| 1405 | Myocarditis, remote | F | C | 6.91 | 31 |
| 1407 | GSW to back with injury of lung | M | C | 6.37 | 37 |
| 1409 | Viral myocarditis | F | C | 6.1 | 39 |
| 1431 | ASHCVD | M | C | 6.54 | 51 |
| 1432 | Calcific coronary artery sclerosis | M | C | 6.26 | 45 |
| 1442 | Sudden cardiorespiratory arrest during strenuous exertion associated with dilated cardiomyopathy due to remote viral myocarditis | M | C | 6.48 | 42 |
| 1443 | ASHCVD | M | C | 6.69 | 56 |
| 1446 | ASCVD | M | C | 6.71 | 34 |
| 1456 | HCVD; DM | F | C | 6.4 | 56 |
| 1463 | Cardiomyopathy | M | C | 6.57 | 49 |
| 1469 | ASCVD | M | C | 6.85 | 28 |
| 1473 | Acute exacerbation of chronic bronchial asthma | M | C | 6.77 | 44 |
| 1504 | Acute pulmonary thromboembolism due to DVT due to immobility after right inguinal herniorrhaphy | M | C | 6.57 | 64 |
| 1517 | Pending | F | C | 6.64 | 44 |
| 1518 | Drowning | M | C | 6.98 | 24 |
| 1522 | Cardiac tamponade due to acute dissecting aneurysm of aortic arch due to ASHCVD | F | C | 6.83 | 64 |
| 1525 | Pending | M | C | 6.83 | 46 |
| 888 | Blunt force injuries with fractures of the skull, spine, ribs, and left lower extremity with internal hemorrhage | M | S | 6.8 | 25 |
| 1016 | Asphyxia due to hanging | M | S | 6.65 | 20 |
| 1021 | ASCVD with hypertension | M | S | 6.76 | 63 |
| 1041 | Complications of ethanol abuse | F | S | 6.41 | 36 |
| 1084 | Acute peritonitis due to intestinal obstruction due to chronic inflammatory pelvic disease | F | S | 6.64 | 48 |
| 1093 | HCVD | F | S | 6.28 | 71 |
| 1178 | Acute intoxication by the combined effects of amitriptyline and nortriptyline | F | S | 6.55 | 41 |
| 1183 | Cervical spinal injury due to blunt impact trauma | F | S | 6.72 | 26 |
| 1189 | ASCVD associated with chronic obstructive airway disease | F | S | 6.49 | 70 |
| 1203 | Combined drug poisoning— clozapine and venlafaxine | M | S | 6.62 | 24 |
| 1235 | Intestinal obstruction due to fecal impaction | F | S | 6.88 | 48 |
| 1265 | ASHCVD | M | S | 6.6 | 68 |
| 1269 | Stab wound of neck with injuries to thyroid gland, trachea, esophagus, and aspiration of blood | M | S | 6.63 | 36 |
| 1280 | Pulmonary thromboembolism due to deep venous thrombosis of lower extremities, etiology undetermined | F | S | 6.36 | 62 |
| 1283 | Bronchial asthma | M | S | 6.63 | 37 |
| 1298 | ASHCVD | M | S | 6.68 | 60 |
| 1315 | Pending | F | S | 6.25 | 61 |
| 1320 | Non–small cell carcinoma of lung with erosion of primary branch of pulmonary artery | M | S | 6.52 | 53 |
| 1321 | ASHCVD; chronic bronchitis and pulmonary emphysema | F | S | 5.86 | 57 |
| 1416 | Hanging | M | S | 6.76 | 37 |
| 1418 | Seizure disorder, undetermined etiology | M | S | 6.25 | 43 |
| 1425 | Drowning | M | S | 6.32 | 48 |
| 1427 | ASCVD | M | S | 6.84 | 66 |
| 1434 | Choking with airway obstruction by sausage due to paranoid schizophrenia | M | S | 6.48 | 35 |
| 1436 | Combined drug poisoning— clozapine and sertraline | M | S | 6.32 | 39 |
| 1441 | HCVD | M | S | 6.11 | 48 |
| 1447 | Acute bronchopneumonia due to ASCHVD | M | S | 6.11 | 53 |
| 1448 | Positional asphyxia due to inverted body position and blankets wrapped around head | M | S | 6.68 | 18 |
| 1453 | Quetiapine poisoning | F | S | 6.35 | 32 |
| 1487 | Bilateral squamous cell carcinoma of the lung and chronic obstructive pulmonary disease | M | S | 6.48 | 61 |
| 1497 | MI | M | S | 6.18 | 44 |
| 1503 | Pending | M | S | 6.33 | 41 |
Note.— GSW = gunshot wound; HCVD = hypertensive cardiovascular disease; IDDM = insulin-dependent diabetes mellitus; MI = myocardial infarction; ASCVD = arteriosclerotic cardiovascular disease; ASHCVD = arteriosclerotic hypertensive cardiovascular disease; DM = diabetes mellitus; and DVT = deep-vein thrombosis.
C = control; S = schizophrenia.
Electronic-Database Information
The URLs for data presented herein are as follows:
- Mammalian Gene Collection, http://mgc.nci.nih.gov/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for the COMT gene)
- Software and Course Materials, http://www-gene.cimr.cam.ac.uk/clayton/software/
References
- Ahsan H, Chen Y, Whittemore AS, Kibriya MG, Gurvich I, Senie RT, Santella RM (2004) A family-based genetic association study of variants in estrogen-metabolism genes COMT and CYP1B1 and breast cancer risk. Breast Cancer Res Treat 85:121–131 [DOI] [PubMed] [Google Scholar]
- Ailenberg M, Silverman M (1997) Site-directed mutagenesis using a PCR-based staggered re-annealing method without restriction enzymes. Biotechniques 22:624–626, 628, 630 [DOI] [PubMed] [Google Scholar]
- Axelrod J, Tomchick R (1958) Enzymatic O-methylation of epinephrine and other catechols. J Biol Chem 233:702–705 [PubMed] [Google Scholar]
- Badner JA, Gershon ES (2002) Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol Psychiatry 7:405–411 [DOI] [PubMed] [Google Scholar]
- Boudikova B, Szumlanski C, Maidak B, Weinshilboum R (1990) Human liver catechol-O-methyltransferase pharmacogenetics. Clin Pharmacol Ther 48:381–389 [DOI] [PubMed] [Google Scholar]
- Bray NJ, Buckland PR, Williams NM, Williams HJ, Norton N, Owen MJ, O’Donovan MC (2003) A haplotype implicated in schizophrenia susceptibility is associated with reduced COMT expression in human brain. Am J Hum Genet 73:152–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMille MM, Kidd JR, Ruggeri V, Palmatier MA, Goldman D, Odunsi A, Okonofua F, Grigorenko E, Schulz LO, Bonne-Tamir B, Lu RB, Parnas J, Pakstis AJ, Kidd KK (2002) Population variation in linkage disequilibrium across the COMT gene considering promoter region and coding region variation. Hum Genet 111:521–537 [DOI] [PubMed] [Google Scholar]
- Diamond A, Briand L, Fossella J, Gehlbach L (2004) Genetic and neurochemical modulation of prefrontal cognitive functions in children. Am J Psychiatry 161:125–132 [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR (2001) Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA 98:6917–6922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Xu K, Ferro E, Harris CR, Goldman D (2003) Genetic origins of anxiety in women: a role for a functional catechol-O-methyltransferase polymorphism. Psychiatr Genet 13:33–41 [DOI] [PubMed] [Google Scholar]
- Garris PA, Collins LB, Jones SR, Wightman RM (1993) Evoked extracellular dopamine in vivo in the medial prefrontal cortex. J Neurochem 61:637–647 [DOI] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M (1998) Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci USA 95:9991–9996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Goldman D, Weinberger DR (2003) Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry 60:889–896 [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV (2004) Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology (Berl) 174:3–16 [DOI] [PubMed] [Google Scholar]
- Halim ND, Weickert CS, McClintock BW, Hyde TM, Weinberger DR, Kleinman JE, Lipska BK (2003) Presynaptic proteins in the prefrontal cortex of patients with schizophrenia and rats with abnormal prefrontal development. Mol Psychiatry 8:797–810 [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR (2004) Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. http://www.nature.com/cgi-taf/DynaPage.taf?file=/mp/journal/vaop/ncurrent/abs/4001558a.html&dynoptions=doi1095366210 (electronically published July 20, 2004; accessed September 16, 2004) [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Straub RE, Weickert CS, Hyde TM, Kleinman JE, Weinberger DR (2004) Expression analysis of neuregulin-1 in the dorsolateral prefrontal cortex in schizophrenia. Mol Psychiatry 9:299–307 [DOI] [PubMed] [Google Scholar]
- Huotari M, Gogos JA, Karayiorgou M, Koponen O, Forsberg M, Raasmaja A, Hyttinen J, Mannisto PT (2002a) Brain catecholamine metabolism in catechol-O-methyltransferase (COMT)-deficient mice. Eur J Neurosci 15:246–256 [DOI] [PubMed] [Google Scholar]
- Huotari M, Santha M, Lucas LR, Karayiorgou M, Gogos JA, Mannisto PT (2002b) Effect of dopamine uptake inhibition on brain catecholamine levels and locomotion in catechol-O-methyltransferase-disrupted mice. J Pharmacol Exp Ther 303:1309–1316 [DOI] [PubMed] [Google Scholar]
- Kleinman JE, Hyde TM, Herman MM (1995) Methodological issues in the neuropathology of mental illness. In: Bloom FE, Kupfer DJ (eds) Psychopharmacology, the fourth generation of progress. Raven Press, New York, pp 859–864 [Google Scholar]
- Lewis DA, Melchitzky DS, Sesack SR, Whitehead RE, Auh S, Sampson A (2001) Dopamine transporter immunoreactivity in monkey cerebral cortex: regional, laminar, and ultrastructural localization. J Comp Neurol 432:119–136 [DOI] [PubMed] [Google Scholar]
- Liu HL, Wang WC (2003) Protein engineering to improve the thermostability of glucoamylase from Aspergillus awamori based on molecular dynamics simulations. Protein Eng 16:19–25 [DOI] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, Taskinen J (1995) Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry 34:4202–4210 [DOI] [PubMed] [Google Scholar]
- Lundstrom K, Tenhunen J, Tilgmann C, Karhunen T, Panula P, Ulmanen I (1995) Cloning, expression and structure of catechol-O-methyltransferase. Biochim Biophys Acta 1251:1–10 [DOI] [PubMed] [Google Scholar]
- Machius M, Declerck N, Huber R, Wiegand G (2003) Kinetic stabilization of Bacillus licheniformis α-amylase through introduction of hydrophobic residues at the surface. J Biol Chem 278:11546–11553 [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Weickert CS, Beltaifa S, Kolachana B, Chen J, Hyde TM, Herman MM, Weinberger DR, Kleinman JE (2003) Catechol O-methyltransferase (COMT) mRNA expression in the dorsolateral prefrontal cortex of patients with schizophrenia. Neuropsychopharmacology 28:1521–1530 [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR (2003) Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA 100:6186–6191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan KA, Bilder RM, Lachman HM, Volavka J (2004) Catechol O-methyltransferase Val158Met polymorphism in schizophrenia: differential effects of Val and Met alleles on cognitive stability and flexibility. Am J Psychiatry 161:359–361 [DOI] [PubMed] [Google Scholar]
- Palmatier MA, Pakstis AJ, Speed W, Paschou P, Goldman D, Odunsi A, Okonofua F, Kajuna S, Karoma N, Kungulilo S, Grigorenko E, Zhukova OV, Bonne-Tamir B, Lu RB, Parnas J, Kidd JR, DeMille MM, Kidd KK (2004) COMT haplotypes suggest P2 promoter region relevance for schizophrenia. Mol Psychiatry 9:859–870 [DOI] [PubMed] [Google Scholar]
- Scanlon PD, Raymond FA, Weinshilboum RM (1979) Catechol-O-methyltransferase: thermolabile enzyme in erythrocytes of subjects homozygous for allele for low activity. Science 203:63–65 [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI (1998) Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci 18:2697–2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shield AJ, Thomae BA, Eckloff BW, Wieben ED, Weinshilboum RM (2004) Human catechol O-methyltransferase genetic variation: gene resequencing and functional characterization of variant allozymes. Mol Psychiatry 9:151–160 [DOI] [PubMed] [Google Scholar]
- Shifman S, Bronstein M, Sternfeld M, Pisante-Shalom A, Lev-Lehman E, Weizman A, Reznik I, Spivak B, Grisaru N, Karp L, Schiffer R, Kotler M, Strous RD, Swartz-Vanetik M, Knobler HY, Shinar E, Beckmann JS, Yakir B, Risch N, Zak NB, Darvasi A (2002) A highly significant association between a COMT haplotype and schizophrenia. Am J Hum Genet 71:1296–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek-Chelgren S, Weinshilboum RM (1981) Catechol-o-methyltransferase biochemical genetics: human lymphocyte enzyme. Biochem Genet 19:1037–1053 [DOI] [PubMed] [Google Scholar]
- Spielman RS, Weinshilboum RM (1981) Genetics of red cell COMT activity: analysis of thermal stability and family data. Am J Med Genet 10:279–290 [DOI] [PubMed] [Google Scholar]
- Tenhunen J, Salminen M, Jalanko A, Ukkonen S, Ulmanen I (1993) Structure of the rat catechol-O-methyltransferase gene: separate promoters are used to produce mRNAs for soluble and membrane-bound forms of the enzyme. DNA Cell Biol 12:253–263 [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Banneman D, Sharp T, Harrison PJ (2004a) Catechol-o-methyltransferase (COMT) inhibition improves set shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci 24:5331–5335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge EM, Burnet PW, Sodhi MS, Harrison PJ (2004b) Catechol-o-methyltransferase (COMT) and proline dehydrogenase (PRODH) mRNAs in the dorsolateral prefrontal cortex in schizophrenia, bipolar disorder, and major depression. Synapse 51:112–118 [DOI] [PubMed] [Google Scholar]
- Vidgren J, Svensson LA, Liljas A (1994) Crystal structure of catechol O-methyltransferase. Nature 368:354–358 [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, Berman KF, Goldberg TE (2001) Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry 50:825–844 [DOI] [PubMed] [Google Scholar]
- Weinshilboum R, Dunnette J (1981) Thermal stability and the biochemical genetics of erythrocyte catechol-O-methyl-transferase and plasma dopamine-β-hydroxylase. Clin Genet 19:426–437 [DOI] [PubMed] [Google Scholar]
- Weinshilboum RM, Otterness DM, Szumlanski CL (1999) Methylation pharmacogenetics: catechol O-methyltransferase, thiopurine methyltransferase, and histamine N-methyltransferase. Annu Rev Pharmacol Toxicol 39:19–52 [DOI] [PubMed] [Google Scholar]
- Weinshilboum RM, Raymond FA (1977) Inheritance of low erythrocyte catechol-o-methyltransferase activity in man. Am J Hum Genet 29:125–135 [PMC free article] [PubMed] [Google Scholar]
- Zubieta C, He XZ, Dixon RA, Noel JP (2001) Structures of two natural product methyltransferases reveal the basis for substrate specificity in plant O-methyltransferases. Nat Struct Biol 8:271–279 [DOI] [PubMed] [Google Scholar]
- Zurcher G, Da Prada M (1982) Rapid and sensitive single-step radiochemical assay for catechol-O-methyltransferase. J Neurochem 38:191–195 [DOI] [PubMed] [Google Scholar]