Summary
Drug resistance has emerged as a critical challenge in clinical cancer treatment. Here, we present a high-throughput screening protocol to identify therapeutic small-molecule inhibitors against drug-resistant cancer cells. We detail the steps for constructing drug-resistant cell models, executing the chemical screening process, and performing data analysis and validation. This protocol facilitates the rapid identification of therapeutic strategies for different types of drug-resistant cancers and aids in studying mechanisms.
For complete details on the use and execution of this protocol, please refer to Zhang et al.1
Subject areas: cell-based assays, cancer, high-throughput screening
Graphical abstract

Highlights
-
•
Protocol for high-throughput screening of small-molecule inhibitors
-
•
Steps for constructing a drug-resistant cell strain
-
•
Guide to confirming selected small-molecule inhibitors
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Drug resistance has emerged as a critical challenge in clinical cancer treatment. Here, we present a high-throughput screening protocol to identify therapeutic small-molecule inhibitors against drug-resistant cancer cells. We detail the steps for constructing drug-resistant cell models, executing the chemical screening process, and performing data analysis and validation. This protocol facilitates the rapid identification of therapeutic strategies for different types of drug-resistant cancers and aids in studying mechanisms.
Before you begin
Drug resistance in cancer treatment presents a significant challenge, frequently rendering the failure of clinical therapeutic regimens and poor prognosis. Resistance may emerge within a few months after the initiation of therapy. For instance, non-small cell lung cancer can develop resistance to epidermal growth factor receptor (EGFR)-targeting therapies as early as 4 months after treatment begins.2 Similarly, chemotherapeutic resistance can occur within 3–6 months during the regimen.3
Drug resistance allows tumor cells to continue proliferating, ultimately leading to tumor recurrence or metastasis. Once drug resistance is developed, the options of alternative regimens become severely limited. Significant progress has been achieved in recent years toward understanding the mechanisms of resistance and developing new drugs to combat drug resistance, but durable and effective therapeutic strategies are still in desperate need.4,5
Paclitaxel is a first-line drug in the treatment of both early-stage and metastatic triple-negative breast cancer (TNBC), but most patients develop resistance to the treatment within 5–7 months, resulting in disease progression.6 To address this issue, we employed a library of epigenetic small-molecule inhibitors for screening, given that paclitaxel resistance is intertwined with epigenetic dysregulation.7 The protocol outlined below details the specific steps for the chemical screening based on MDA-MB-436 and Hs 578T cell models, which can be readily extended to screen small-molecule inhibitors against other types of drug-resistant cancer cells.
Cell culture and passaging
Timing: 1 week
-
1.Reviving cells.
-
a.Immediately place the cryovial into a pre-warmed 37°C water bath upon retrieval from liquid nitrogen.
 CRITICAL: Rapid thawing is critical for maintaining cell viability, as slow thawing can lead to re-crystallization and cellular damage.
CRITICAL: Rapid thawing is critical for maintaining cell viability, as slow thawing can lead to re-crystallization and cellular damage. -
b.Gently agitate the cryovial to ensure uniform heating.
-
c.Once most of the ice has melted, immediately transfer the cell suspension to a pre-warmed centrifuge tube containing pre-warmed culture medium.
-
a.
-
2.Centrifuge cells and resuspend for culturing.
 CRITICAL: All operations are performed under sterile conditions to avoid contamination.
CRITICAL: All operations are performed under sterile conditions to avoid contamination.-
a.Transfer the cell suspension to a 15 mL sterile conical tube containing 4 mL of suitable culture medium. Centrifuge at 1000 × g for 3 min to pellet the cells and then discard the supernatant.
-
b.Resuspend the cell pellet in an appropriate volume of pre-warmed culture medium and dispense it into a sterile petri dish or flask. Gently rock the dish or flask to distribute the cells evenly.
-
c.Place the petri dish or flask in a 37°C incubator with 5% CO2. Observe the cell morphology after 24 h to assess cell growth status.Note: Different cells have varying growth conditions. Healthy cells should maintain their normal shape and size with smooth edges. Swollen, shrunk, or floating cells may indicate poor growth conditions. If the cell background is unclean, remove the original medium after the cells have attached, add an appropriate volume of 1× phosphate buffered saline (PBS), and gently rock to rinse the cells. Discard the PBS and add fresh medium to continue culturing.
-
d.Regularly monitor the cells and change the medium as needed.Note: The frequency of medium changes largely depends on the proliferation rate of the cultured cells. If the medium contains phenol red indicator, its color can serve as a visual cue for determining when to change it.
-
a.
-
3.Cell passaging.Note: Passage cells when they reach 80%–90% confluence.
-
a.Carefully aspirate and discard the culture medium and then add an appropriate volume of trypsin solution. Incubate the cells at 37°C until they round up and begin to detach.Note: Monitor cells under a microscope; once they appear rounded and detached, proceed to the next step.
-
b.Add an appropriate volume of culture medium to terminate trypsinization. Use a pipette to mix the medium and trypsin thoroughly, detaching the cells from the bottom of the dish to create a cell suspension.
-
c.Centrifuge the cell suspension at 1000 xg for 3 min at room temperature. Carefully aspirate and discard the supernatant. Resuspend the cell pellet in an appropriate volume of culture medium at the optimal seeding density.Note: The optimal seeding density varies depending on the growth rate of the cells. Ideally, passage cells every 2–3 days. Adjust the passaging frequency if cells exhibit morphological changes, slow growth, or other signs of poor cellular conditions.
-
d.Perform experiments when cells are in the logarithmic (Log) phase of growth.
-
a.
Preparing stock solutions of small-molecule inhibitors
Timing: 1–2 h
-
4.
Centrifuge the vials containing the small-molecule inhibitors at 1000 xg for 3 min to pellet the drug.
-
5.
Resuspend the drug in dimethyl sulfoxide (DMSO) to achieve appropriate screening concentrations. Aliquot the solutions and store them in a −20°C freezer.
Note: The storage concentration of small-molecule inhibitors should be lower than their individual solubility in DMSO at room temperature, ensuring the homogeneity of the stocks when preparing working solutions. Given that DMSO is a good solvent for many small molecules, be it hydrophilic or hydrophobic, we empirically recommend a standard storage concentration of 50 mM for most if not all small-molecule inhibitors. For exceptions, reducing the recommended concentration is necessary when precipitation occurs.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| GSK2801 | Sigma | Cat# SML0768 |
| BAZ2-ICR | Sigma | Cat# SML1276 |
| PFI-1 | Sigma | Cat# SML0352 |
| JQ1 | Sigma | Cat# SML1524 |
| JQ1N | Sigma | Cat# SML1525 |
| BAY-299 | Cayman | Cat# 19777 |
| I-BRD9 | Sigma | Cat# SML1534 |
| LP99 | Cayman | Cat# 17661 |
| BI-9564 | Sigma | Cat# SML1655 |
| PFI-4 | Sigma | Cat# PZ0307 |
| OF-1 | Sigma | Cat# SML1184 |
| NI-57 | Sigma | Cat# SML1486 |
| SGC-CBP30 | Sigma | Cat# SML1133 |
| I-CBP112 | Sigma | Cat# SML1134 |
| PFI-3 | Sigma | Cat# SML0939 |
| TP-472 | Sigma | Cat# SML1862 |
| SGC0946 | Sigma | Cat# SML1107 |
| A-395 | Cayman | Cat# 20257 |
| A-395N | Sigma | Cat# SML1879 |
| UNC1999 | Sigma | Cat# SML0778 |
| GSK343 | Sigma | Cat# SML0766 |
| GSK126 | Sigma | Cat# 5.0058 |
| UNC0642 | Sigma | Cat# SML1037 |
| A-366 | Sigma | Cat# SML1410 |
| PFI-2 | Sigma | Cat# SML1408 |
| S-PFI-2 | Sigma | Cat# PZ0312 |
| BAY-598 | Sigma | Cat# SML1603 |
| A-196 | Sigma | Cat# SML1565 |
| OICR-9429 | Sigma | Cat# SML1209 |
| MS023 | Cayman | Cat# 34786 |
| MS094 | Sigma | Cat# SML2548 |
| TP-064 | Cayman | Cat# 20256 |
| SGC707 | Sigma | Cat# SML1242 |
| GSK591 | Cayman | Cat# 18354 |
| UNC1215 | Sigma | Cat# SML0662 |
| UNC1079 | Cayman | Cat# 20566 |
| GSK-J4 | Sigma | Cat# SML0701 |
| GSK-LSD1 | Sigma | Cat# SML1072 |
| IOX2 | Sigma | Cat# SML0652 |
| GSK484 | Sigma | Cat# SML1658 |
| GSK864 | Sigma | Cat# SML1757 |
| LLY283 | MCE | Cat# HY-107777 |
| GSK595 | MCE | Cat# HY-18665 |
| DMSO | Solarbio | Cat#D8371 |
| Penicillin-streptomycin | Gibco | Cat#15140122 |
| Fetal bovine serum | Biological Industries | Cat#04-001-1ACS |
| Na2HPO4·7H2O | Sigma | Cat#S9390-500g |
| NaCl | Affymetrix | Cat#7647-14-5 |
| KCl | Sigma | Cat#746436 |
| KH2PO4 | ChemCruz | Cat#SC-203211B |
| Critical commercial assays | ||
| Cell Counting Kit-8 | Biosharp | Cat#BS350B |
| Experimental models: Cell lines | ||
| MDA-MB-436 | ATCC | Cat#Bio-73324; RRID:CVCL_0623 |
| MDA-MB-436-20RA | The Qin Wu Laboratory | N/A |
| MDA-MB-436-20RB | The Qin Wu Laboratory | N/A |
| MDA-MB-436-20RC | The Qin Wu Laboratory | N/A |
| Hs 578T | ATCC | Cat#Bio-54123; RRID:CVCL_0332 |
| Hs 578T-20RA | The Qin Wu Laboratory | N/A |
| Hs 578T-20RB | The Qin Wu Laboratory | N/A |
| Software and algorithms | ||
| GraphPad Prism 8 | GraphPad Software | https://www.graphpad.com/scientific-software/prism/; RRID:SCR_002798 |
| Other | ||
| Countess 3 automated cell counter | Thermo Fisher Scientific | https://www.thermofisher.cn/cn/zh/home/life-science/cell-analysis/cell-analysis-instruments/automated-cell-counters/models.html |
| Incucyte SX5 | Sartorius | https://github.com/chrisamiller/aml31SuppSite |
| DMEM medium | YESEN | Cat#41401ES76 |
| Trypsin | Cienry | Cat#CR-27250 |
| Penicillin-Streptomycin solution | Biosharp | Cat#BL505A |
| Cell culture dish | NEST | Cat#704001 |
| 1 mL pipette tips | KIRGEN | Cat#KG1616 |
| 10 μL pipette tips | GEB | Cat#FT0010-R-NS |
| 200 μL pipette tips | GEB | Cat#FT0200-R-NS |
| 96-well flat-bottom cell culture plate | WHB | Cat#WHB-96 |
| 96-well round-bottom cell culture plate | NEST | Cat#701101 |
| Cryogenic vials | Thermo Fisher Scientific | Cat#5012-0020 |
| 100 mm Petri dish | Corning | Cat# 430167 |
Materials and equipment
Cell culture medium
| Reagent | Final concentration | Amount |
|---|---|---|
| DMEM | 89% | 445 mL |
| Penicillin-Streptomycin | 1% | 5 mL |
| Fetal Bovine Serum (FBS) | 10% | 50 mL |
| Total | N/A | 500 mL |
Store at 4°C for up to 1 month.
Note: Different cell types require different media.
10× PBS solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Na2HPO4·7H2O | 100 mM | 26.8 g |
| NaCl | 1.37 M | 80 g |
| KCl | 27 mM | 2 g |
| KH2PO4 | 18 mM | 2.4 g |
| ddH2O | N/A | 1L |
Store at 20°C–25°C for up to one year.
Note: Sterilize the solution using a 0.22 μm filter. Dilute freshly in ddH2O to prepare 1x PBS.
Step-by-step method details
Establishing drug-resistant strains
Timing: 3–6 months
This section describes the steps for constructing drug-resistant cell lines. If drug-resistant cells are already available, skip this part.
-
1.Culture parental cell lines and prepare drug stock solutions.
-
a.Select a cell line that is easy to culture and sensitive to the target drug. Initiate the construction of drug-resistant cell lines using cells in the logarithmic growth phase. For example, we select the MDA-MB-436 and Hs 578T cell lines, which are sensitive to paclitaxel.
-
b.Prepare stock solutions of the drug using DMSO and ensure the stability of the drug solution.
-
a.
-
2.Treat parental cells with the target drug.
-
a.When the parental cells reach 80%–90% confluence, replace the culture medium with medium containing the target drug.Note: Treat parental cells with a lower concentration of the drug. Typically, use a concentration of 1/10 or less of the IC50. For example, the IC50 value of paclitaxel against parental MDA-MB-436 was determined to be 16.7 nM in our lab. Based on drug pressure at 1/10 of the IC50 or lower concentrations for the parental cells, we have chosen a starting screening concentration of 1 nM.Note: Prepare the culture medium with the target drug freshly.Note: Determine whether to passage cells based on cell density. Continue treating the cells with a new drug concentration after passaging.
-
b.Replace the medium with medium containing a higher concentration of the target drug approximately every week.Note: The interval depends on cell number and cell state. If the cell count is high, shorten the interval for increasing the drug concentration. If the cell count is low, extend the interval.Note: The incremental escalation of paclitaxel concentrations is essential for gradually selecting resistant cell populations while minimizing excessive cell death. This approach allows cells to adapt to the drug pressure, simulating the gradual development of resistance observed in clinical settings. In our experience, parental cells were initially treated with 1 nM paclitaxel, followed by subsequent passages treated with 2, 4, and 8 nM. This stepwise increase continued until the IC50 value was confirmed to have increased 4.5-fold, as shown in Figure 1.
-
c.Repeat step b multiple times until the cells stably tolerate higher concentrations of the target drug.Note: Regularly observe cell morphology and growth status under a microscope to ensure healthy growth. Change the culture medium regularly based on the cell condition and the phenol red indicator to maintain optimal growth conditions.Note: If the cell count drops sharply after increasing the drug concentration, temporarily decrease the drug concentration.
 CRITICAL: Pay particular attention to cell growth status to avoid cell death due to drug treatment. Adherent cells prefer growing in clusters, and sparse cells can significantly affect growth rates.
CRITICAL: Pay particular attention to cell growth status to avoid cell death due to drug treatment. Adherent cells prefer growing in clusters, and sparse cells can significantly affect growth rates. -
d.Assess cell sensitivity to the drug using the Cell Counting Kit-8 (CCK-8) assay. The resistant cell line is considered to be successfully established if the IC50 increases by more than threefold.Optional: Assess cell sensitivity to the drug using the CCK-8 assay or other cell viability assays at each concentration stage.
-
e.Clone the resistant cells using the limited dilution technique to ensure a homogeneous resistant cell line once the desired resistance level is achieved.
-
i.Utilize a cell counter or microscope to enumerate cells and determine their density.
-
ii.Calculate and dilute the cell suspension based on the desired number of cells per well.
-
iii.Dispense the diluted cell suspension into new cell culture plates, ensuring that the number of cells per well is as minimal as possible to increase the likelihood of monoclonal formation.
-
iv.Place the cell culture plates in a sterile workstation and transfer them to an incubator for growth. Regularly monitor the cell growth and document the progression in each well.
-
v.After the cells begin to grow, replace the medium with drug-containing resistant medium.
-
vi.Following a period of drug treatment, observe and select clones that continue to grow in the presence of the drug. Confirm their stable drug resistance by re-determining the IC50 values.
-
i.
-
f.Assess cell sensitivity to the drug using the CCK-8 assay. See examples Figure 2.
-
a.
Figure 1.
Timeline of the evolution of resistance in drug-resistant clones
This figure illustrates the progression of drug resistance in drug-resistant cell clones over time. Drug concentrations incrementally increase to mimic the gradual development of resistance observed in clinical settings. This stepwise increase continues until the IC50 value has been confirmed to be 4.5-fold higher.
Figure 2.
Determination of resistance degree in resistant clones
Paclitaxel dose-dependent viability curves of parental and paclitaxel-resistant of MDA-MB-436 (A) and Hs 578T (B) (Data are represented as mean ± SD, n = 3). Figure reprinted and adapted with permission from Zhang et al., 2024.
Seeding cells
Timing: 1–2 h
This section details the primary steps involved in seeding cells onto culture plates and the critical factors to consider. Cell seeding density is crucial because it directly affects the subsequent small-molecule inhibitors screening.
-
3.
Determine the seeding quantity for parental and resistant cells on culture plates. Troubleshooting 1.
CRITICAL: Seeding densities vary as different cell types exhibit distinct growth rates. The cells in the DMSO group achieve a confluence of over 90% on the culture plates by the end of the observation period, while we also attempt to avoid overgrowth that could lead to cell layering.
Note: We determine the duration of cell growth observation based on the time needed for the drug to take effect. Accordingly, we observe cells treated with epigenetic small-molecule inhibitors for five days to assess their pharmaceutical effect.
-
4.Prepare cell suspensions at appropriate concentrations and volumes.
-
a.Carefully aspirate the medium, add an appropriate volume of trypsin solution, and incubate at 37°C for 2–3 min.Note: Different cells require different digestion times. Prolonged digestion affects cell viability.
-
b.Add medium to stop the digestion, gently pipette to detach the cells, and create a cell suspension.
-
c.Centrifuge at room temperature at 1000 xg for 3 min to collect cells.
-
d.Carefully remove and discard the supernatant and resuspend the cells in an appropriate volume of medium.
-
e.Count the cells using a cell counter.
-
f.Configure the cell suspension to the requisite concentration and volume.Note: Add 20 μL of cell suspension per well as recommended. Adjust the cell suspension concentration according to the number of cells required per well.Note: Prepare a slightly larger volume of cell suspension than required to account for potential errors during aliquoting.
-
a.
-
5.Prepare cell suspensions at appropriate concentrations and volumes. Troubleshooting 1 and 3.
-
a.Use a pipettor to transfer the cell suspension to the culture plate.Note: Seed both parental and resistant cells on the same plate.Note: Use a multichannel pipette to enhance efficiency.Note: Ensure the use of a transparent 384-well culture plate.
-
b.Gently rock the culture plate from side to side after adding the cells.Note: Ensure the cells are evenly distributed across the bottom of the wells, avoiding clustering.
-
a.
Treatment of cells with small-molecule inhibitors
Timing: 1–2 h
This section outlines the steps for preparing and aliquoting the medium containing the small-molecule inhibitor.
-
6.
Calculate the amount of small-molecule inhibitors required based on the screening concentration and prepare the corresponding stock solution using DMSO. Troubleshooting 4.
Note: Suggest using a screening concentration of 5 μM for the small-molecule inhibitors.
CRITICAL: Accurately calculate the dilution factor and ensure that the stock solution dissolved in DMSO is diluted at least 1,000-fold. High DMSO concentrations can be toxic to cells, impacting their growth and viability.
-
7.
Prepare the medium supplemented with the small-molecule inhibitor and the medium containing an equivalent volume of DMSO.
Note: Set up four replicates for each small-molecule inhibitor treatment group in the 384-well plate. Prepare the total volume of the inhibitor-containing medium for the replicate groups in a 96-well plate.
Note: Add 20 μL of the small molecule inhibitor to the existing culture medium. Account for losses during aliquoting by preparing a slightly larger total volume of solution. Include the volume of the cell-seeding plate when calculating the dilution factor.
-
8.
Add the prepared medium to the culture plate with the seeded cells. Troubleshooting 2.
CRITICAL: Proceed with this step when the cells are in an optimal growth state. Adherent cells usually require 12 hours.
Note: Use a multichannel pipette to enhance efficiency.
Note: Mark the culture plate, excluding the areas above the wells. Avoid marking these areas to prevent interference with Incucyte cell imaging and counting.
-
9.
Gently rock the culture plate from side to side.
Note: Gently rock the culture plate to ensure the small-molecule inhibitor is evenly distributed in the medium, preventing it from adhering to the lid of the culture plate.
Real-time dynamic monitoring of cell proliferation using Incucyte SX5
Timing: 5 days
This section includes the steps for using Incucyte and analyzing the imaging data. If Incucyte is not available, use the CCK-8 assay to detect cell proliferation on the final day of monitoring cell growth.
-
10.Set the timeline according to the Incucyte usage protocol.
-
a.Select the timeline option and create a new experiment.
-
b.Choose the imaging mode. For example, select Standard for a cell proliferation assay.
-
c.Set the imaging channel. For example, choose the phase channel for a cell proliferation assay.
-
d.Select the type of culture plate being used.Note: Use a culture plate that meets the specifications required by Incucyte, and ensure the plate is transparent.
-
e.Select the position of the culture plate within the Incucyte.
-
f.Set the imaging areas.Note: Define the imaging areas based on experimental needs. Multiple positions can be chosen for multi-site scanning to ensure comprehensive information collection.
-
g.Define the experiment timeline.Note: Use the naming to locate the captured images later.Optional: Label each well of the culture plate with the cell line name, the small-molecule inhibitor name, and its concentration at this step.
-
h.Set the imaging cycle and frequency.
-
i.Place the culture plate in the designated area of the Incucyte and start the imaging.Note: Place the culture plate in the Incucyte instrument, which should be located in an incubator set to conditions matching those required for cell culture to maintain normal cell growth.
-
a.
-
11.
Throughout the experiment, the Incucyte automatically captures image data at predetermined time intervals. Troubleshooting 5.
Note: Keep the culture plate stationary during imaging to ensure clear pictures.
-
12.Process and analyze the captured images.
-
a.Open the Incucyte analysis software and navigate to the folder with your named files. Choose the imaging mode. For example, select Standard for a cell proliferation assay.Note: Ensure the Incucyte instrument is connected to the computer.
-
b.Preview the acquired raw images in the software to check the quality of the captured images.
-
c.Select the analysis module by choosing the confluence analysis option from the software menu.
-
d.Choose the appropriate channel for analysis, such as the bright-field channel for cell proliferation.
-
e.Select three images for preview analysis.
-
f.Automatically or manually adjust the threshold to distinguish cells from the background.
 CRITICAL: The software marks the identified cell regions in the images to assess whether the settings are appropriate. Based on the preview image results, manually adjusting options like cell area can improve cell identification.
CRITICAL: The software marks the identified cell regions in the images to assess whether the settings are appropriate. Based on the preview image results, manually adjusting options like cell area can improve cell identification. -
g.Select the time points and well positions for analysis.
-
h.Click the apply or run button to have the software automatically process the image data and output the analysis results.Note: The software marks the identified cell regions in all images and displays the percentage values of confluence.
-
i.Export the analysis results to Excel or another format.
-
a.
Screening of small-molecule inhibitors
Timing: 1–2 h
This section focuses on processing the data exported from Incucyte and selecting small-molecule inhibitors that align with the experimental objectives.
-
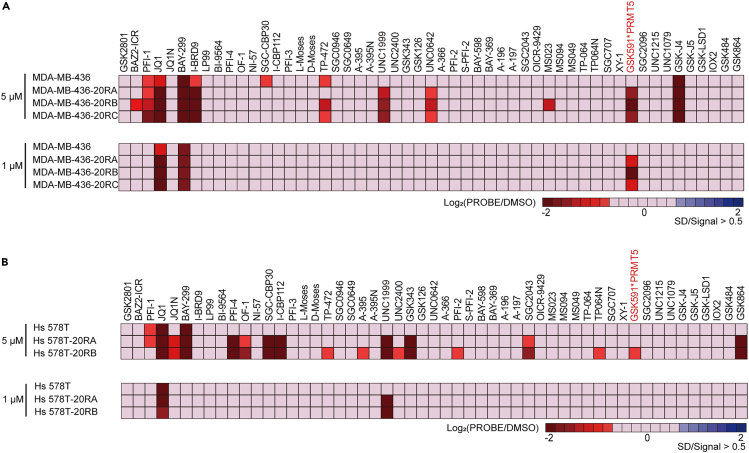
13.Process the exported data. See examples Figure 3.
-
a.Calculate the average values for replicate wells.
-
b.Divide the values from the small-molecule inhibitor treatment groups by the values from the DMSO treated group.
-
c.Take the Log2 values of the aforementioned ratios.
-
a.
-
14.Screen for small-molecule inhibitors that meet the experimental objectives.
-
a.Select small-molecule inhibitors with Log2 fold change values reaching ±1.Note: The software marks the identified cell regions in all images and displays the percentage values of confluence. However, integrate experimental design and statistical analysis for comprehensive evaluation in specific applications.Note: Consider adjusting the screening concentrations if the changes in values are minimal.
-
b.Choose appropriate small-molecule inhibitors based on the experimental objectives. For example, we screen for small-molecule inhibitors that render paclitaxel-resistant cells vulnerable.Note: Some small-molecule inhibitors exhibit significant cytotoxicity, affecting healthy cell growth. Exclude these inhibitors when analyzing the results.
-
a.
Figure 3.
Display of small-molecule inhibitors screening outcomes
Heatmap showing average cell confluence of parental and paclitaxel resistant cell of MDA-MB-436 (A) and Hs 578T (B) lines treated with epigenetic chemical probes at 5 μM and 1 μM after 5 days treatment in (n = 4). Figure reprinted and adapted with permission from Zhang et al., 2024.
Effects of small-molecule inhibitor candidates
Timing: 6 days
This section validates the effects of the screened small-molecule inhibitors at varying concentrations on both parental and resistant cells. Use a 96-well plate, as this part includes less groups than the screening experiment. Many steps are similar to those previously described, but attention should be given to the variables introduced by changing the culture plate.
-
15.
Repeat Steps 3-5. Seed the parental and drug-resistant cells onto a cell culture plate, ensuring peripheral wells are filled with PBS. Troubleshooting 1 and 3.
Note: If using a 96-well plate, a cell suspension volume of 80 μL per well is recommended.
-
16.
Once the cells have adhered, add a series of culture media containing different concentrations of small-molecule inhibitors. Prepare the medium with the inhibitor, starting at 10 μM and decreasing by two-fold for each subsequent concentration, covering a total of nine experimental groups. Use the media immediately after preparation. Troubleshooting 1 and 4.
CRITICAL: Accurately calculate the dilution factor, ensuring the stock solution dissolved in DMSO is diluted at least 1,000-fold. High concentrations can be toxic to cells, impacting their growth and viability.
Note: Add 20 μL to each well, accounting for losses during aliquoting by preparing a slightly larger total volume of solution.
Note: Consider the volume of the cell-seeding plate when calculating the dilution factor.
Note: Set up three replicates in a 96-well plate to enhance data stability.
-
17.
On the final day of monitoring cell growth, use the Incucyte instrument or the CCK-8 kit to detect cell proliferation.
-
18.Process the data and use GraphPad Prism software to plot the fitted dose-response curve.
-
a.Click on New Data Table, select XY, and choose the option corresponding to the replicate values for each group, then click Create.
-
b.Input the concentrations in the X column and enter the corresponding cell confluence values for other groups.
-
c.Click on Analyze in the menu bar, then click Transform, and select the option to transform the X column values to Log10.
-
d.Click on Analyze again, then choose Nonlinear Regression (curve fit) under XY analyses, and click OK.
-
e.In the new dialog box, select Dose-Response Inhibition under Log (inhibitor) vs. Response—Variable slope (four parameter), and click OK.
-
f.Adjust colors and line thickness according to your aesthetic preferences. See examples Figure 4.
-
a.
Figure 4.
Determination of sensitivity to small-molecule inhibitors
Structure of GSK591 (A). Cell confluence curves of parental cells and paclitaxel resistant cells of MDA-MB-436 (B) and Hs 578T (C) treated with GSK591 for 5 days (Data are represented as mean ± SD, n = 3). Figure reprinted and adapted with permission from Zhang et al., 2024.
Expected outcomes
The cell viability assay results confirm the successful establishment of a drug-resistant cell line, showing diminished sensitivity to the drug (Figure 2). By employing a library of 53 epigenetic compound probes, we systematically identify cellular vulnerabilities related to resistance. The screening indicates that numerous small-molecule inhibitors display markedly enhanced sensitivity in the resistant cell lines (Figure 3). In alignment with our experimental goals, we choose candidate drugs for further research and validate the screening results. Via additional cell-based experiments, we verify the sensitivity of TNBC paclitaxel-resistant cells to the chosen inhibitors (Figure 4).
Limitations
This approach provides an efficient streamline for screening small-molecule inhibitors against drug-resistant cancer cells, but it still faces several limitations. First, the screening concentrations set in this protocol may not fully cover the effective doses of all small-molecule inhibitors, as their effects are significantly concentration-dependent. Adherence to the principle of a single variable may lead to the selected concentrations not falling within the optimal activity range for certain inhibitors, possibly omitting some positive results.
The involvement of numerous groups in the screening process increases the risk of confusion during the addition of small-molecule inhibitors. Although the quantity used for each inhibitor is relatively small, the diversity of inhibitors involved renders the overall cost a significant consideration.
Some small-molecule inhibitors may act not only on the intended target proteins but also interact with multiple off-target proteins, leading to off-target effects. These non-specific interactions can interfere with the interpretation of experimental results and lead to false positives and false negatives. Therefore, further validation experiments are necessary to confirm the initial screening results, ensuring that the identified small-molecule inhibitors possess high specificity and efficacy.
In vitro paclitaxel-resistant cell lines and patient-derived resistance share several common mechanisms but also exhibit important differences. Both models often involve increased expression of drug efflux pumps, such as P-glycoprotein, which actively export paclitaxel from cells, contributing to resistance. Additionally, paclitaxel-resistant cells, either induced in vitro or developed from patient samples, can develop altered microtubule dynamics or changes in microtubule-associated proteins, preventing paclitaxel from stabilizing microtubules and inhibiting mitosis. Furthermore, dysregulation of cell cycle checkpoints, such as alterations in p53 or p21, is observed in both models, allowing continued proliferation despite drug treatment. Phenotypically, both resistant cell lines and patient tumors maintain a high proliferation rate or the ability to survive paclitaxel treatment. Resistance is often associated with upregulation of genes involved in drug metabolism and DNA repair in both settings. However, patient-derived resistance is typically more complex due to additional factors that are difficult to replicate in vitro, such as the tumor microenvironment, including hypoxia, acidity, and immune evasion. These factors can modulate drug response and contribute to resistance in ways that are not captured by standard in vitro models.
Furthermore, while resistance can be induced more rapidly in vitro by prolonged drug exposure, in vivo resistance develops more gradually, influenced by a range of factors, including the tumor’s genetic diversity, prior drug exposure, and adaptive responses to treatment. In patients, resistance mechanisms can vary significantly between individuals, reflecting the unique genetic and molecular characteristics of each tumor. In contrast, in vitro models typically use homogeneous cell lines, which may not fully capture the diversity and complexity of resistance mechanisms observed in clinical settings.
There are several alternative methods for generating drug-resistant cells. One approach is to expose cancer cells to gradually increasing concentrations of paclitaxel, leading to the survival of a small fraction of cells that acquire resistance. This method simulates the selective pressure seen in clinical treatments, but it can be time-consuming and may not fully reflect the complexity of resistance mechanisms observed in patients. Another strategy is to use 3D cell culture systems, such as spheroids or organoids, which better mimic the tumor microenvironment. These models create drug gradients and promote cell-cell interactions, providing a more accurate representation of how resistance develops in vivo under sustained drug exposure. In vivo models, such as patient-derived xenografts or genetically engineered mouse models, allow resistance to develop in a more natural context, including the influence of the tumor microenvironment and immune system. These models are particularly useful for studying how tumors adapt to treatment over time. Finally, isolating drug-resistant cancer cells directly from patients who have undergone treatment offers a clinically relevant model. These cells reflect the specific resistance mechanisms present in the patient’s tumor and allow for the study of genetic and molecular changes associated with resistance.
Troubleshooting
Problem 1
Seeding an excessive or insufficient number of cells impacts the comparability between experimental and control groups (Step 3 Seeding cells and Step 15 Confirmation of screened small-molecule inhibitor effects). See examples Figure 5.
Figure 5.
Problems with cell plating
Representative images showing the cells seeded at sparse (A) or excessively high (B) density (Scale bar, 10 μm).
Potential solution
Determine the appropriate number of cells through preliminary experiments. Set up different cell seeding densities and monitor for the duration of the experiment. Observe cell density under the microscope after the monitoring period. Optimal cell seeding density is achieved when the final confluence is above 90% without cell layering.
Problem 2
The total volume in each well is inconsistent after adding the drugs (Step 8 Treatment of cells with small-molecule inhibitors and Step 18 Confirmation of screened small-molecule inhibitor effects).
Potential solution
-
•
Configure a high-precision pipette and calibrate it regularly.
-
•
Pay attention to the volume in the pipette tip when aspirating liquids. Inconsistent volumes in the pipette tips indicate that the tips may be loose.
-
•
Observe the liquid for bubbles during aliquoting. Bubbles indicate inconsistent aliquoting volumes.
-
•
Account for losses during aliquoting by preparing a total volume greater than the required amount.
Problem 3
The volume in the outer wells of the culture plate reduces over time (Step 5 Seeding cells and Step 15 Confirmation of screened small-molecule inhibitor effects).
Potential solution
Water evaporation occurs in the culture medium within the incubator. To avoid variables caused by changes in the total volume of the medium, add an equal volume of PBS to the peripheral wells of the culture plate to maintain surrounding humidity.
Problem 4
The effectiveness of the small-molecule inhibitors decreases after multiple uses (Step 6 Treatment of cells with small-molecule inhibitors and Step 16 Confirmation of screened small-molecule inhibitor effects).
Potential solution
Aliquot and store the stock solutions of small-molecule inhibitors at −20°C to avoid repeated freeze-thaw cycles.
Problem 5
Contamination commonly occurs during Incucyte detection (Step 11 Real-time dynamic monitoring of cell proliferation using incucyte).
Potential solution
-
•
Ensure that the procedures are performed according to standards, maintain a sterile environment, and minimize the likelihood of cell contamination.
-
•
Seal the culture plates before transferring them to prevent contamination that may occur during transfer in non-sterile environments.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Qin Wu (wuqin@ibmc.ac.cn).
Technical contact
Technical questions on executing this protocol should be directed to and will be answered by the technical contact, Qin Wu (wuqin@ibmc.ac.cn).
Materials availability
This study did not generate new unique reagents.
Data and code availability
The published article includes all datasets generated or analyzed during this study.
Acknowledgments
We thank the following organizations for supporting our study: the National Natural Science Foundation of China (grant 82103287 to Q.W. and grant 22304177 to H.-R.J.), the Zhejiang Provincial Natural Science Foundation (grant LR22B050001 to Q.W.), the Zhejiang Provincial Key R&D Program (grant 2023SDYXS0003 to Q.W.), and the Canadian Institutes of Health Research (FDN154328 to Cheryl H. Arrowsmith and FAPESP 20/02006-0 to Katlin B. Massirer). The Structural Genomics Consortium is a registered charity (no. 1097737) that receives funds from Bayer AG, Boehringer Ingelheim, Bristol Myers Squibb, Genentech, Genome Canada through the Ontario Genomics Institute (OGI-196), the EU/EFPIA/OICR/McGill/KTH/Diamond Innovative Medicines Initiative 2 Joint Undertaking (EUbOPEN grant 875510), Janssen, Merck KGaA (aka EMD in Canada and the US), Pfizer, and Takeda.
Author contributions
Q.W. and W.T. designed the project; J.W., X.-Y.Z., and S.-Y.Z. performed most of the experiments; J.W. and Y.W. analyzed the data; and J.W. and H.-R.J. wrote and revised the manuscript. All authors have read and approved the final submitted manuscript.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Hao-Ran Jia, Email: jiahr@ucas.ac.cn.
Qin Wu, Email: wuqin@ibmc.ac.cn.
Weihong Tan, Email: tan@hnu.edu.cn.
References
- 1.Zhang K., Wei J., Zhang S., Fei L., Guo L., Liu X., Ji Y., Chen W., Ciamponi F.E., Chen W., et al. A chemical screen identifies PRMT5 as a therapeutic vulnerability for paclitaxel-resistant triple-negative breast cancer. Cell Chem. Biol. 2024;31:1942–1957.e6. doi: 10.1016/j.chembiol.2024.08.003. [DOI] [PubMed] [Google Scholar]
- 2.de Miguel F.J., Gentile C., Feng W.W., Silva S.J., Sankar A., Exposito F., Cai W.L., Melnick M.A., Robles-Oteiza C., Hinkley M.M., et al. Mammalian SWI/SNF chromatin remodeling complexes promote tyrosine kinase inhibitor resistance in EGFR-mutant lung cancer. Cancer Cell. 2023;41:1516–1534.e9. doi: 10.1016/j.ccell.2023.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abu Samaan T.M., Samec M., Liskova A., Kubatka P., Büsselberg D. Paclitaxel's Mechanistic and Clinical Effects on Breast Cancer. Biomolecules. 2019;9:789. doi: 10.3390/biom9120789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin P., Jiang J., Zhou L., Huang Z., Nice E.C., Huang C., Fu L. Mitochondrial adaptation in cancer drug resistance: prevalence, mechanisms, and management. J. Hematol. Oncol. 2022;15:97. doi: 10.1186/s13045-022-01313-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li P., Shang X., Jiao Q., Mi Q., Zhu M., Ren Y., Li J., Li L., Liu J., Wang C., et al. Alteration of chromatin high-order conformation associated with oxaliplatin resistance acquisition in colorectal cancer cells. Exploration. 2023;3 doi: 10.1002/EXP.20220136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmid P., Turner N.C., Barrios C.H., Isakoff S.J., Kim S.B., Sablin M.P., Saji S., Savas P., Vidal G.A., Oliveira M., et al. First-line ipatasertib, atezolizumab, and taxane triplet for metastatic triple-negative breast cancer: Clinical and biomarker results. Clin. Cancer Res. 2024;30:767–778. doi: 10.1158/1078-0432.CCR-23-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Q., Nie D.Y., Ba-Alawi W., Ji Y., Zhang Z., Cruickshank J., Haight J., Ciamponi F.E., Chen J., Duan S., et al. PRMT inhibition induces a viral mimicry response in triple-negative breast cancer. Nat. Chem. Biol. 2022;18:821–830. doi: 10.1038/s41589-022-01024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The published article includes all datasets generated or analyzed during this study.