Abstract
Purpose
Despite recent advancements in the treatment of atypical meningioma, control rates in high-risk patients continue to be suboptimal. Stereotactic radiosurgery (SRS) offers the ability to achieve improved local control (LC) with a low toxicity profile. However, available data are limited. We aimed to conduct a comprehensive review of a consecutive cohort of patients diagnosed with high-risk atypical meningioma who underwent SRS, either as a single-fraction SRS or in the hypofractionated SRS (hf-SRS), and evaluate the LC rates (LCR) with a specific emphasis on patterns of treatment failure.
Methods and Materials
We identified consecutive patients diagnosed with high-risk World Health Organization grade 2 meningioma treated with SRS at a single institution between 2014 and 2021. High-risk meningioma was defined as a residual disease or recurrence after initial gross total resection. Follow-up data were analyzed to evaluate LCRs and patterns of treatment failure. We defined local failure as tumor recurrence wthin the prescription isodose line, marginal failure as recurrence within 5 mm but outside the prescription isodose line, and distant/regional failure as recurrence beyond 5 mm of the prescription isodose line but within 2 cm of the surgical cavity.
Results
We identified 45 pathologically confirmed atypical meningiomas in 25 patients. Thirty-three tumors underwent single-fraction SRS, and 12 tumors received hf-SRS. The median follow-up was 36 months (range, 2-86 months). The 3-year LCR was 84.6%, and overall survival was 96.0%. Four patients with a total of 7 tumors experienced treatment failure. Failures were either local (3 patients and 3 lesions) or marginal (3 patients and 4 lesions). Patients treated with hf-SRS did not exhibit local, marginal, or distant failures.
Conclusions
Our institutional data on atypical patients with meningioma treated with radiosurgery compare favorably to existing literature using fractionated radiation therapy. SRS offers a promising strategy to improve LC in this patient population, and the occurrence of marginal failure plays a role in creating clinical target volume margins.
Introduction
Meningioma, the most prevalent primary intracranial tumor, accounted for approximately 37,020 cases in the United States in 2022.1 While most meningiomas are benign (World Health Organization [WHO] grade 1) with favorable long-term outcomes, atypical (WHO grade 2) and anaplastic (WHO grade 3) meningiomas exhibit distinct clinical behavior characterized by aggressiveness, frequent treatment failures, and higher risk of dying of tumor progression.2, 3, 4, 5, 6
Because of the relative rarity of high-grade meningioma, available data predominantly stem from retrospective reviews. While it is well-established that recurrence risk is substantial following initial surgery, there remains a lack of consensus regarding optimal adjuvant treatment strategies. Addressing this gap, the Radiation Therapy Oncology Group (RTOG) 0539 trial emerged as the first prospective effort to address this issue.7,8
In the RTOG 0539 trial, patients were stratified into risk groups based on factors such as tumor grade, extent of resection, and recurrent disease. An established adjuvant algorithm was applied accordingly. High-risk patients, defined as those with subtotal resected WHO grade 2 meningioma, recurrent WHO grade 2 meningioma, or any grade 3 meningioma, received dose-escalated intensity modulated radiation therapy (RT). The prescribed dose was 60 Gy to a 1 cm margin and 54 Gy to a 2 cm margin. This approach yielded a reported 3-year local control (LC) rate (LCR) of 68.9% and an overall survival (OS) of 78.6%, demonstrating favorable outcomes compared with historical controls. However, despite these promising results, concerns regarding suboptimal LCRs remain.
One technique that has been explored to try to improve LC is stereotactic radiosurgery (SRS). Data exploring its role in patients with higher-grade meningiomas are based on limited retrospective studies, with widely varying rates reported in the literature ranging from 5-year progression-free survival (PFS) of 20% to 64.4%.9,10 The pattern of failure with SRS appears to be different than that with fractionated RT. While the most common pattern of failure after fractionated RT is within the radiation field (92.9% in the RTOG 0539 trial, high-risk group), the predominant pattern of recurrence after SRS seems to be along the margin of the prescribed dose.11,12 This observation suggests that failures less frequently occurred in the high-dose region and more commonly occurred at the edge of the field, possibly because of overly conformal treatment or the region of sharp dose fall-off where the dose may be lower.13
In this study, we presented outcomes for a cohort of high-risk WHO grade 2 patients with meningioma, as per the RTOG 0539 trial stratification, treated with either single-fraction SRS (sf-SRS) or hypofractionated SRS (hf-SRS). Our primary focus is on assessing control rates and, in patients that do develop a local or marginal failure, evaluating clinical and dosimetric parameters that may be associated with such treatment failures.
Methods and Materials
This study was conducted under the approval of the institutional review board, and patient consent was waived because of its retrospective nature, following our institutional guidelines. We retrospectively identified and reviewed patients with intracranial atypical meningioma (WHO grade 2) from our institutional database treated between 2014 and 2021. To ensure consistency and focus on high-risk cases, we excluded patients with a history of prior cranial radiation or neurofibromatosis-2.
All patients included in this study met the high-risk criteria as defined by the RTOG 0539 trial.7 We collected comprehensive clinical data, including patient demographics such as age, sex, ethnicity, and race. The pretreatment clinical course and lesion characteristics, including the location of the tumor, the volume of the tumor, previous gross/subtotal surgical resection, and proximity to critical structures, were recorded.
Patients were treated with the Leksell Gamma Knife Perfexion from 2014-2017 and the Leksell Gamma Knife ICON after 2018 (Elekta AB). Before 2018, patients received exclusively sf-SRS, whereas after 2018, they received either sf-SRS or hf-SRS in 3 to 5 fractions. The determination of the treatment technique was at the discretion of the treating physicians. Typically, treatment-planning magnetic resonance imaging (MRI) was obtained within 1 week of treatment delivery. The targeting technique included treatment to the residual/recurrent meningioma alone or the surgical cavity plus residual/recurrent meningioma, with no use of planning target volume (PTV) expansions.
To assess tumor control and treatment-related side effects, we used patient clinical and radiographic data. We considered interval MRIs that showed either interval growth of any residual tumor ≥ 20% along any dimension or the presence of a new tumor as indicative of tumor progression. Representative MRIs were imported into the Leksell GammaPlan system and fused with a treatment-planning MRI for further review. The site of progression was further categorized as local, defined as tumor recurrence within the prescription isodose line; marginal, characterized by recurrence outside the prescription isodose line but within a 5 mm margin; and tumor growth beyond 5 mm of the prescription isodose line was classified as a distant failure. In patients where the entire surgical cavity was not targeted in treatment, distant failures were further classified based on their proximity to the surgical cavity. Follow-up duration was calculated from the date of radiosurgery to the date of the last clinic follow-up or death.
Statistical analysis
All statistical analyses were performed using SPSS version 28 (IBM Corp). The primary endpoint of this study was LC at 3 years, with secondary endpoints, including OS as failure patterns. Control rates were calculated by considering patients who had either a local or marginal failure. Kaplan-Meier curves were used to calculate LC, OS, and PFS at the specified 3-year time point. Further statistical analyses, such as subgroup analysis and Cox proportional hazards modeling, were performed to explore potential prognostic factors and their associations with study endpoints. This analysis aimed to provide a deeper understanding of the factors influencing treatment outcomes. Appropriate statistical tests, such as chi-square tests, Fisher's exact tests, and log-rank tests, were used as appropriate to assess the significance of observed differences and associations. Statistical significance was set at a P-value < .05. Multivariate logistic regression with independent parameters, such as age, gender, target technique, the intent of SRS, lesion PTV, location of meningioma, number of fractions, conformity index, dose per lesion, and type of lesion progression, was used to identify significant predictors of disease progression, OS, and disease-free survival.
Results
Patient characteristics
We identified 45 WHO high-risk grade 2 meningiomas in 25 patients who were treated with SRS in the postoperative setting and had not previously received RT. The median age at the time of radiosurgery treatment was 63 years (range, 33-87) (Table 1). Eleven patients received adjuvant SRS following subtotal resection (STR), accounting for 12 meningiomas. We treated 14 patients in the recurrent setting, which included 33 meningiomas. Two of these patients had a STR of the recurrent disease prior to SRS.
Table 1.
Demographic characteristics of 25 patients receiving stereotactic radiosurgery (SRS) for atypical meningioma
| Characteristic | Value |
|---|---|
| Sex, n (%) | |
| Male | 9 (36.0) |
| Female | 16 (64.0) |
| Age at treatment, mean (range), (y) | 63 (33-87) |
| Ethnicity, n (%) | |
| Asian | 3 (12.0) |
| American Indian or Alaska Native | 2 (8.0) |
| Black/African American | 3 (12.0) |
| White | 12 (48.0) |
| Other/not specified | 5 (20.0) |
Lesion characteristics and treatment details
The most common location of the treated meningioma was the convexity (46.7%), followed by parasagittal/parafalcine (37.8%) and skull base (13.3%) (Table 2). The majority of lesions (n = 33, 73%) were treated with sf-SRS, while the remaining 27% (n = 12) lesions were treated with hf-SRS. The median dose for sf-SRS was 16 Gy (range, 12-18 Gy), while for hf-SRS it was 25 Gy (range, 21-27.5 Gy) delivered in 3 or 5 fractions. The treatment target was to the residual disease in 35 lesions and to the surgical cavity plus residual disease in 10 lesions.
Table 2.
Details of stereotactic radiosurgery (SRS) treated meningiomas and their treatment characteristics
| Characteristic | Value | |
|---|---|---|
| Location, n (%) | ||
| Convexity | 21 (46.7) | |
| Parasagittal/parafalcine | 17 (37.8) | |
| Skull base | 6(13.3) | |
| Intraventricular | 1 (2.2) | |
| Indication for SRS, n (%) | ||
| Salvage therapy | 33 (73.3) | |
| Adjuvant therapy after STR | 12 (26.7) | |
| SRS does n (%) | 33 (73.3) | |
| Single fraction | ||
| Prescribed dose Gy, median (range) | 16 (12-18) | |
| Hypofractionated n (%) | 12 (26.7) | |
| Prescribed dose Gy, median (range) | 25 (21-27.5) | |
| Number of fractions | 3-5 | |
| Treatment target, n (%) | ||
| Residual disease | 35 (77.8) | |
| Surgical cavity | 10 (22.2) | |
| PriorRT | Number of patients | 12 |
| Number of lesions | 17 | |
| Focality | Unifocal disease | 17 patients |
| Multifocal disease | 8 patients (28 lesions) | |
| PTV (range 0.31-18.59) | Median PTV | 4.10 |
| Average PTV | 8.69 | |
| Conformity index | Average | 0.97 |
| Follow-up | Duration | No.of lesions (n) |
| <6 mon | 2 | |
| >6 mon | 43 |
Abbreviations: PTV = planning target volume; RT = radiation therapy; STR = subtotal resection.
Patient outcomes
The median follow-up for the entire cohort was 36 months (range, 2-86 months). Median follow-up of >6 months was available for 93.5% of lesions (n = 43, in 23 patients). The 3-year LCR was 84.6%, and the 3-year OS was 96.0% (Fig. 3).
Figure 3.
Kaplan-Meier curves showing 3-year (A) progression-free survival and (B) relapse-free survival for the entire cohort.
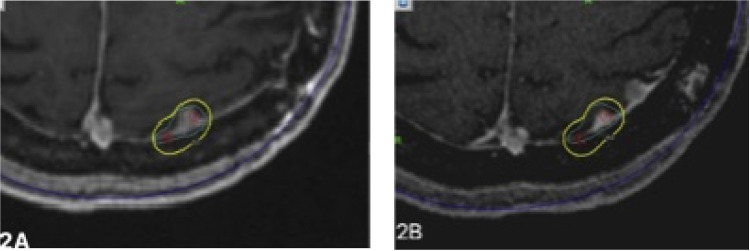
There were a total of 3 meningiomas that had progression of disease whose failure was local (Fig. 1), defined as within the prescription isodose line (Table 3). There were an additional 4 meningiomas that developed marginal failures (Fig. 2), defined as outside the prescription isodose line but within 5 mm. No failures were seen in patients in whom the treatment targeted the surgical cavity and residual disease (n = 5 sf-gamma knife radiosurgery [GKRS], n = 7 hf-GKRS) or who received hf-GKRS. Prescription dose (P = .23) did not correlate with failure.
Figure 1.
Representative magnetic resonance imaging (MRI) images illustrating patterns of local failure. (A) Pretreatment image (coronal view) showing a falcine meningioma with a 50% isodose line. (B) Local failure demonstrated in the posttreatment follow-up image in coronal section. (C) Pretreatment image (sagittal view) demonstrating the 50% isodose line around the entire lesion. (D) Demonstrating the local failure in sagittal view of the posttreatment follow-up image.
Table 3.
Characteristics of patients with failure patterns after radiosurgery treatment
| Patient | Total lesions | Treatment setting | SRS dose | Total lesion failures | Local failure n (location-dose) | Marginal failure n (location-dose) |
|---|---|---|---|---|---|---|
| 1 | 5 | Adjuvant | 14 Gy (4 lesions); 16 Gy (1 lesion) | 2 | 1 (periventricular lesion- received 14 Gy) | 1 (frontal convexity lesion-received 14 Gy) |
| 2 | 2 | Recurrent (prior surgery and RT) | 16 Gy (2 lesions) | 1 | 1 (Frontal convexity lesion- received16 Gy) | |
| 3 | 1 | Adjuvant | 16 Gy (1 lesion) | 1 | 1 (Sphenoid Wing lesion-received 16 Gy) | |
| 4 | 7 | Recurrent (prior surgery and RT) | 16 Gy (6 lesions) 14 Gy (1 lesion) | 3 | 1 (falcine lesion-received 16 Gy) | 2 (frontal convexity received 14 Gy; parietal convexity lesion-received 16 Gy) |
Abbreviations: RT = radiation therapy; SRS = stereotactic radiosurgery.
Figure 2.
Representative magnetic resonance imaging (MRI) images demonstrating the pattern of marginal failure after stereotactic radiosurgery (SRS). (A) Axial view of MRI showing 50% isodose line around the lesion in the parietal convexity and (B) posttreatment follow-up MRI images demonstrating marginal failure within 5 mm of isodose.
The multivariate logistic regression analysis did not reveal any significant correlation between dependent variables like OS, PFS, and disease progression and independent variables, such as age, gender, target technique, intent of SRS, lesion PTV, location of meningioma, number of fractions, conformity index, dose per lesion, and type of lesion progression. A plausible reason for this might be the small subset of patients we have in our cohort.
Toxicity
Sf-SRS and hf-SRS were both well tolerated by patients. In the sf-SRS group, 1 patient developed a common terminology criteria for adverse events grade 2 seizure and severe intractable headaches. She was hospitalized and required the use of steroids and long-term antiepileptic drugs. She progressed at 28 months after treatment and died. In the hf-SRS group, 1 patient had transient grade 2 dizziness and grade 2 seizures requiring the use of steroids and antiepileptic. After 1 year of treatment, he was back to his baseline and had discontinued antiepileptic drugs.
Discussion
In this study, we investigated consecutive patients with high-risk meningiomas treated with SRS to evaluate LC, toxicity, and patterns of failure, with the aim of comparing our findings with published data and assessing the feasibility of this treatment strategy.
The current SRS literature presents mixed results, with some studies suggesting outcomes similar to conventional RT and others hinting at potentially inferior control rates.14, 15, 16, 17, 18 In our study, patients treated with sf-RS or hypofractionated SRS hf-SRS fared favorably in comparison with the RTOG 0539 trial high-risk cohort, and they exhibited acceptable toxicity profiles. Our data revealed a 3-year LC of 84.6%, surpassing the 3-year LCR of 68.9% reported in the RTOG 0539 study. It is worth noting that our series exclusively included high-risk grade 2 meningiomas, while 40% of patients in the RTOG 0539 trial presented with either de novo or recurrent WHO grade 3 meningiomas. However, the difference in control rates between the 2 studies is unlikely to be reflective of this difference in patient population because post hoc analysis of the RTOG 0539 trial demonstrated that initial grade 3 patients exhibited better PFS, OS, and time to progression compared to recurrent grade 2 patients.7 Furthermore, our 3-year PFS of 77.6% compared very favorably with the SRS literature. Kowalchuk et al19 presented a multicenter retrospective study showing outcomes after SRS for atypical meningioma, representing the largest contemporary experience to our knowledge. They presented outcomes on 233 patients treated at 12 institutions, of which 21% of patients had received prior RT. They report a 3-year PFS of 53.9% with 49% of patients experiencing tumor progression at a median of 18.5 months, similar to the RTOG 0539 trial. The study further suggested a good prognostic group that includes patients with no previous RT, aged >50 years, and when aged <50 years, a treatment volume < 11.5 cc. The good prognostic group had a 3-year PFS of 63.1% compared with 41.9% in the poor prognostic group. In our study, patients with previous RT were excluded, suggesting a more favorable cohort of patients. We also tended to have higher marginal doses (mean dose 16 Gy) and did use hf-SRS in 27% of the lesions that were treated.
While these data suggest that SRS may be an appropriate treatment approach for patients with high-risk atypical meningiomas, several unanswered questions necessitate further investigation.
First, SRS is intriguing because of its potential for dose escalation (higher relative biological effective dose) and its capacity to leverage radiobiological properties to overcome radioresistance and provide LC. The principle that dose escalation can improve LC has been established with fractionated RT. Schuring-Pereira et al15 reported infield recurrence rates reduced from 50% to 21% when biologically effective doses ≥ 60 Gy were prescribed. More recently, Zeng et al20 presented consecutive patients with WHO grade 2 or 3 meningiomas treated with dose-escalated RT (> 66 Gy equivalent dose in 2-Gy fractions) compared with the standard-dose cohort. Despite the higher dose group representing a higher risk group of patients, the reported PFS in the high-dose group at 3 years was 78.9% compared with 57.2% in the standard-dose group.20 While the optimal dose with SRS is less established, some studies suggested that higher doses may improve LC. Attia et al13 suggested doses > 14 Gy, as well as a lower conformity index, were associated with improvement in tumor control, while Hanakita et al21 reported that margin doses > 18 Gy were associated with a better outcome. Kano et al22 reported a 5-year PFS of 63.1% for grade 2 meningiomas treated with a marginal dose exceeding 20 Gy compared with 29.4% for those receiving < 20 Gy. In our study, the median dose of SRS prescribed was 16 Gy. Except for 3 meningiomas, we obtained LC in all other patients. This suggests that radioresistance to the prescribed dose may be related to biological variation between meningiomas that warrants further exploration.19 It is plausible that the optimal dose may be a function of the volume of disease and biological characteristics of the tumor, such as genetic profile and/or proliferative index.12
Another technique to achieve dose escalation with SRS is hf-SRS. Emerging data indicate that hf-SRS is associated with good LC of tumor volume, lower rates of toxicities, and symptomatic edema compared to sf-SRS, which may allow for further dose escalation safely.23, 24, 25, 26 The median hf-SRS dose in this study was 25 Gy in 5 fractions, and we have begun to dose escalate to 27.5 to 30 Gy in 5 fractions. We did not observe any local failures in the 12 patients treated with this approach. However, more data are needed to observe control rates with hf-SRS and the role and need for dose escalation using these techniques. We believe that hf-SRS may be advantageous in treating patients with higher-grade lesions because the cumulative effect of higher dose/fraction works on the principle of facilitating reoxygenation and accruing sublethal DNA damage in tumor cells. Marchetti et al26 showed 86% 3-year PFS, considering only infield progressions and LC of 92% in this subset of 24 patients treated with multisession radiosurgery. Simonetti et al27 demonstrated a beneficial effect of PFS with adjuvant SRS after gross total resection in high-grade meningiomas.
The second variable to consider is target delineation, specifically the volume targeted within the SRS fields. Radiosurgical volumes traditionally encompass the gross tumor volume without a margin, in contrast to fractionated RT, which typically includes dural margins that can extend beyond 1 cm. The poor control rates in previous SRS data sets may reflect inadequate coverage of microscopic disease. Previous SRS studies have identified that marginal failure is a common pattern of recurrence following SRS.28,29 Attia et al13 reported that expanding the margin by 8 to 10 mm could account for 80% of the missed targets. Based on these data, we are using larger treatment volumes when using SRS techniques. We believed that marginal coverage of 3 to 5 mm clinical target volume at a minimum to adjacent dura should be considered when using SRS. This is based on the philosophy of treating any viable tumor in the dural tail region and thus preventing marginal recurrences. We have also been incorporating 68-gallium DOTATATE positron emission tomography (PET) (DOTATATE-PET) imaging into our treatment-planning algorithm to help us better delineate the tumor and allow for more accurate contouring precision.30,31
This practice is based on previous studies that have shown a 50% reduction in PTV with accurate tumor delineation using PET scan and reflected by the recent Response Assessment in Neuro-Oncology Working Group guidelines, which recommend DOTATATE-PET imaging for RT planning in meningioma.30, 31, 32, 33
The third variable of consideration is whether the target volume should comprise the visible disease alone ± immediate adjacent dura or include the entire surgical cavity. In the adjuvant setting following STR, our current strategy has typically been to encompass the entire surgical cavity, often with an hf-SRS approach rather than an sf-SRS approach.31 This approach is a logical extension of the data published in the recent RTOG and European Organization for Research and Treatment of Cancer studies that advocate for adjuvant treatment in the entire surgical cavity after a gross total resection.8,29,30 For patients treated in the salvage setting with a local failure >3 years postoperatively, we typically will image with a DOTATATE-PET scan, and if an isolated failure is confirmed, we will treat the recurrent tumor alone.34
Limitations
Our study is limited in the way that most single-institution retrospective studies are. Although we have a small number of patients and a variable, short follow-up duration, we were able to analyze and provide granular, patient-level data regarding treatment response and failure. Our patient population may not resemble the larger multicenter database in terms of the number of patients, but we were able to tightly regulate treatment paradigms and follow-up intervals to achieve significant results. A longer follow-up duration will be able to project control rates closer to the real-world scenario. Hence, our results reflect those seen in larger, prospective studies with similar patients. Furthermore, because many recurrences were diagnosed on MRI, rather than with surgical excision, pathologic confirmation of true progression versus radiation-induced necrosis is impossible. DOTATATE-PET imaging has been incorporated as a routine component of imaging for SRS in previous studies as well.31 Molecular information such as Ki-67 and genetic analysis such as NF2 gene mutation were not available for the majority of the patients in our cohort; hence we were unable to include discussion correlating clinical outcomes and molecular data.
Conclusion
SRS provides a unique opportunity to improve the outcomes for patients with high-risk atypical meningiomas. Questions remain regarding optimal dose, SRS technique, and target volume, which may account for the large variation in results that have been published to date. Our experience suggests there may be a role for DOTATATE-PET imaging in addition to MRI to help improve target delineation and the use of a clinical target volume expansion to help minimize marginal misses with consideration of treating the entire surgical cavity. Hf-SRS may be an approach to help reduce morbidity while maintaining LC.
Disclosures
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Sources of support: This work had no specific funding.
All data generated and analyzed during this study are included in this published article (and its supplementary information files). Any other data required can be made available upon reasonable request to the corresponding author.
References
- 1.Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014-2018. Neuro-Oncol. 2021;23(12 Suppl 2):iii1–iii105. doi: 10.1093/neuonc/noab200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marchetti M, Sahgal A, De Salles AAF, et al. Stereotactic radiosurgery for intracranial noncavernous sinus benign meningioma: International stereotactic radiosurgery society systematic review, meta-analysis and practice guideline. Neurosurgery. 2020;87:879–890. doi: 10.1093/neuros/nyaa169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers L, Barani I, Chamberlain M, et al. Meningiomas: Knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg. 2015;122(1):4–23. doi: 10.3171/2014.7.JNS131644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garzon-Muvdi T, Yang W, Lim M, Brem H, Huang J. Atypical and anaplastic meningioma: Outcomes in a population based study. J Neurooncol. 2017;133:321–330. doi: 10.1007/s11060-017-2436-6. [DOI] [PubMed] [Google Scholar]
- 5.Chao S, Rogers L. External beam radiation therapy for meningioma. Handb Clin Neurol. 2020;170:259–278. doi: 10.1016/B978-0-12-822198-3.00046-X. [DOI] [PubMed] [Google Scholar]
- 6.Vagnoni L, Aburas S, Giraffa M, et al. Radiation therapy for atypical and anaplastic meningiomas: An overview of current results and controversial issues. Neurosurg Rev. 2022;45:3019–3033. doi: 10.1007/s10143-022-01806-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers CL, Won M, Vogelbaum MA, et al. High-risk meningioma: Initial outcomes from NRG Oncology/RTOG 0539. Int J Radiat Oncol Biol Phys. 2020;106:790–799. doi: 10.1016/j.ijrobp.2019.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber DC, Ares C, Villa S, et al. Adjuvant postoperative high-dose radiotherapy for atypical and malignant: A phase-II parallel non-randomized and observation study (EORTC 22042-26042) Radiother Oncol. 2018;128:260–265. doi: 10.1016/j.radonc.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Bulthuis VJ, Hanssens PE, Lie ST, van Overbeeke JJ. Gamma Knife radiosurgery for intracranial meningiomas: Do we need to treat the dural tail? A single-center retrospective analysis and an overview of the literature. Surg Neurol Int. 2014;5(Suppl 8):S391–S395. doi: 10.4103/2152-7806.140192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang WH, Lee CC, Yang HC, et al. Gamma Knife radiosurgery for atypical and anaplastic meningiomas. World Neurosurg. 2016;87:557–564. doi: 10.1016/j.wneu.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 11.Park CK, Jung NY, Chang WS, Jung HH, Chang JW. Gamma knife radiosurgery for postoperative remnant meningioma: Analysis of recurrence factors according to World Health Organization grade. World Neurosurg. 2019;132:e399–e402. doi: 10.1016/j.wneu.2019.08.136. [DOI] [PubMed] [Google Scholar]
- 12.Shepard MJ, Xu Z, Kearns K, et al. Stereotactic radiosurgery for atypical (World Health Organization II) and anaplastic (World Health Organization III) meningiomas: results from a multicenter, international cohort study. Neurosurgery. 2021;88:980–988. doi: 10.1093/neuros/nyaa553. [DOI] [PubMed] [Google Scholar]
- 13.Attia A, Chan MD, Mott RT, et al. Patterns of failure after treatment of atypical meningioma with gamma knife radiosurgery. J Neurooncol. 2012;108:179–185. doi: 10.1007/s11060-012-0828-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helis CA, Hughes RT, Cramer CK, et al. Stereotactic radiosurgery for atypical and anaplastic meningiomas. World Neurosurg. 2020;144:e53–e61. doi: 10.1016/j.wneu.2020.07.211. [DOI] [PubMed] [Google Scholar]
- 15.Schuring-Pereira M, Hagenbeek R, Mast M, et al. Atypical meningioma: Patterns of postradiotherapy recurrences. Br J Neurosurg. 2021;35:591–596. doi: 10.1080/02688697.2021.1922606. [DOI] [PubMed] [Google Scholar]
- 16.Fatima N, Meola A, Pollom EL, Soltys SG, Chang SD. Stereotactic radiosurgery versus stereotactic radiotherapy in the management of intracranial meningiomas: A systematic review and meta-analysis. Neurosurg Focus. 2019;46:E2. doi: 10.3171/2019.3.FOCUS1970. [DOI] [PubMed] [Google Scholar]
- 17.Gagliardi F, De Domenico P, Snider S, et al. Efficacy of radiotherapy and stereotactic radiosurgery as adjuvant or salvage treatment in atypical and anaplastic (WHO grade II and III) meningiomas: A systematic review and meta-analysis. Neurosurg Rev. 2023;46:71. doi: 10.1007/s10143-023-01969-7. [DOI] [PubMed] [Google Scholar]
- 18.Unterberger A, Nguyen T, Duong C, Kondajji A, Kulinich D, Yang I. Meta-analysis of adjuvant radiotherapy for intracranial atypical and malignant meningiomas. J Neurooncol. 2021;152:205–216. doi: 10.1007/s11060-020-03674-7. [DOI] [PubMed] [Google Scholar]
- 19.Kowalchuk RO, Shepard MJ, Sheehan K, et al. Treatment of WHO grade 2 meningiomas with stereotactic radiosurgery: Identification of an optimal group for SRS using RPA. Int J Radiat Oncol Biol Phys. 2021;110:804–814. doi: 10.1016/j.ijrobp.2021.01.048. [DOI] [PubMed] [Google Scholar]
- 20.Zeng KL, Soliman H, Myrehaug S, et al. Dose-escalated radiation therapy is associated with improved outcomes for high-grade meningioma. Int J Radiat Oncol Biol Phy. 2024;118:662–671. doi: 10.1016/j.ijrobp.2023.09.026. [DOI] [PubMed] [Google Scholar]
- 21.Hanakita S, Koga T, Igaki H, et al. Role of gamma knife surgery for intracranial atypical (WHO grade II) meningiomas. J Neurosurg. 2013;119:1410–1414. doi: 10.3171/2013.8.JNS13343. [DOI] [PubMed] [Google Scholar]
- 22.Kano H, Takahashi JA, Katsuki T, et al. Stereotactic radiosurgery for atypical and anaplastic meningiomas. J Neurooncol. 2007;84:41–47. doi: 10.1007/s11060-007-9338-y. [DOI] [PubMed] [Google Scholar]
- 23.Iwai Y, Yamanaka K, Nakajima H, Shiotani M, Uyama T. Radiosurgery of acoustic neuromas: Results of low dose treatment. J Neurosurg. 2000;93:19–22. doi: 10.1227/01.neu.0000073416.22608.b3. [DOI] [PubMed] [Google Scholar]
- 24.Pendl G, Eustacchio S, Unger F. Radiosurgery as alternate treatment for skull base meningiomas. J Clin Neurosci. 2001;1:12–14. doi: 10.1054/jocn.2001.0869. [DOI] [PubMed] [Google Scholar]
- 25.Jahanbakhshi A, Najafi M, Gomar M, et al. Radiosurgery in grade II and III meningiomas: A systematic review and meta-analysis. J Pers Med. 2024;14:802. doi: 10.3390/jpm14080802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchetti M, Pinzi V, Iezzoni C, et al. Multisession radiosurgery for grade 2 (WHO), high risk meningiomas. A phase II clinical trial. J Neurooncol. 2022;157:397–403. doi: 10.1007/s11060-022-03978-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simonetti G, Silvani A, Tramacere I, et al. Long term follow up in 183 high grade meningioma: A single institutional experience. Clin Neurol Neurosurg. 2021;207 doi: 10.1016/j.clineuro.2021.106808. [DOI] [PubMed] [Google Scholar]
- 28.Bagshaw HP, Burt LM, Jensen RL, et al. Adjuvant radiotherapy for atypical meningiomas. J Neurosurg. 2017;126:1822–1828. doi: 10.3171/2016.5.JNS152809. [DOI] [PubMed] [Google Scholar]
- 29.Masalha W, Heiland DH, Franco P, et al. Atypical meningioma: Progression-free survival in 161 cases treated at our institution with surgery versus surgery and radiotherapy. J Neurooncol. 2018;136:147–154. doi: 10.1007/s11060-017-2634-2. [DOI] [PubMed] [Google Scholar]
- 30.Galldiks N, Albert NL, Sommerauer M, et al. PET imaging in patients with meningioma-report of the RANO/PET Group. Neuro Oncol. 2017;19:1576–1587. doi: 10.1093/neuonc/nox112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perlow HK, Siedow M, Gokun Y, et al. 68Ga-DOTATATE PET-based radiation contouring creates more precise radiation volumes for patients with meningioma. Int J Radiat Oncol Biol Phys. 2022;113:859–865. doi: 10.1016/j.ijrobp.2022.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Jenkinson MD, Javadpour M, Haylock BJ, et al. The ROAM/EORTC-1308 trial: radiation versus observation following surgical resection of atypical meningioma: Study protocol for a randomised controlled trial. Trials. 2015;16:519. doi: 10.1186/s13063-015-1040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.NRG Oncology Phase III Trial of Observation Versus Irradiation for a Gross Totally Resected Grade II Meningioma. clinicaltrials.gov. 2023 https://clinicaltrials.gov/ct2/show/NCT03180268 Accessed April 10, 2023. [Google Scholar]
- 34.Hintz Eric B., Park, et al. Using 68Ga-DOTATATE PET for postoperative radiosurgery and radiotherapy planning in patients with meningioma: A case series. Neurosurgery. 2023;93:95–101. doi: 10.1227/neu.0000000000002377. [DOI] [PubMed] [Google Scholar]