Abstract
Background:
Treatment options for patients with advanced neuroendocrine tumors (NET) are limited. This randomized, double-blinded, phase 3 trial evaluated efficacy of cabozantinib in patients with previously treated, progressive extra-pancreatic or pancreatic NET.
Methods:
The trial enrolled two independent cohorts of patients, including 203 patients with extra-pancreatic NET and 95 patients with pancreatic NET, who had received peptide receptor radionuclide therapy and/or targeted therapy. Patients were randomized 2:1 to receive cabozantinib 60 mg daily or placebo. The primary endpoint was progression-free survival by blinded independent central review. Secondary endpoints were objective response rate, overall survival, and safety.
Results:
In patients with extra-pancreatic NET, median progression-free survival with cabozantinib was 8.4 months compared to 3.9 months with placebo (stratified hazard ratio [HR], 0.38; 95% confidence interval [CI] 0.25–0.59, p<0.001). In patients with pancreatic NET, median progression-free survival was 13.8 months compared to 4.4 months with placebo (stratified HR, 0.23; 95% CI 0.12–0.42, p<0.001). Confirmed objective response rates with cabozantinib were 5% and 19% in patients with extra-pancreatic and pancreatic NET, respectively, compared to 0% with placebo. Grade 3 or higher adverse events were noted in 62–65% of patients treated with cabozantinib compared to 23–27% with placebo. Common grade 3 or higher treatment-related adverse events included hypertension, fatigue, diarrhea, and thromboembolic events.
Conclusions:
Cabozantinib significantly improves progression-free survival in patients with previously treated, progressive advanced extra-pancreatic or pancreatic NET. Adverse events were consistent with the known safety profile of cabozantinib.
Introduction
Neuroendocrine tumors (NET) are a heterogeneous group of malignancies that arise most commonly in the gastrointestinal tract, lung, and pancreas.1 Treatment of advanced NET is guided by features including primary tumor location, pathologic differentiation and grade, presence of symptoms related to hormone secretion or extent of disease, and somatostatin receptor expression. Depending on these factors, treatment options for extra-pancreatic NET (epNET) include somatostatin analogs (SSAs), lutetium Lu-177 dotatate, or everolimus.2–6 For pancreatic NET (pNET), sunitinib or alkylating agent chemotherapy also can be considered.7–9 Most patients eventually experience progression on available therapies.
Angiogenesis plays a key role in pathogenesis of NET.10,11 Antiangiogenic agents targeting vascular endothelial growth factor (VEGF) and its receptors have demonstrated activity in the treatment of NET. Cabozantinib is an oral small-molecule inhibitor of multiple tyrosine kinases including VEGF receptors, MET, AXL, and RET.12 Clinical activity of cabozantinib was demonstrated in a phase II trial that enrolled patients with advanced epNET or pNET.13 Based on these results, we conducted a randomized, double-blinded, placebo-controlled study to evaluate the efficacy of cabozantinib in patients with previously treated, progressive epNET or pNET (CABINET; Alliance A021602).
Methods
Patients
Eligible patients were 18 years of age or older and had histologically confirmed locally advanced or metastatic well- or moderately-differentiated, WHO grades 1–3 NET of extra-pancreatic or pancreatic origin. Patients with poorly differentiated neuroendocrine carcinomas and high-grade neuroendocrine carcinoma without specification of differentiation status were not eligible. Patients were required to have progressive disease according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 in the 12 months before enrollment and an Eastern Cooperative Oncology Group (ECOG) performance status score of 0–2 (a 5-point scale where higher numbers reflect greater disability). Disease progression or intolerance leading to treatment discontinuation of at least one FDA-approved line of therapy depending on primary tumor site was required. Because of changes in the treatment landscape and approval of Lu-177 dotatate, a protocol amendment in November 2020 expanded eligibility related to prior therapy to include Lu-177 dotatate or everolimus for patients with epNET (excluding lung NET); everolimus for lung NET; Lu-177 dotatate, everolimus or sunitinib for pNET. Before this amendment, prior therapy with everolimus was required for all patients. Continuation of somatastatin analogues was allowed if dose was stable for at least 2 months before enrollment.
Trial Design and Treatments
The CABINET trial was a multicenter, phase 3 trial designed and conducted by the Alliance for Clinical Trials in Oncology (Alliance), a member of the National Clinical Trials Network sponsored by the National Cancer Institute (NCI). The trial was activated in July 2018 and enrolled patients with epNET or pNET in two independent cohorts. Within each cohort, patients were randomly assigned in a 2:1 ratio to receive cabozantinib or placebo. Randomization was stratified by concurrent somatostatin analogue use and primary tumor site (midgut gastrointestinal and unknown primary versus non-midgut gastrointestinal, lung, other sites) in the epNET cohort and by concurrent somatostatin analogue use and prior sunitinib in the pNET cohort. Patients received cabozantinib (60 mg) or placebo orally once daily. Interruptions in treatment and dose reductions for cabozantinib (40 mg, then 20 mg) and placebo were specified for management of adverse events. Patients continued blinded treatment until disease progression, unacceptable toxicity, or withdrawal of consent. A protocol amendment activated in November 2020 permitted patients receiving placebo to crossover to open-label cabozantinib after real-time central confirmation of progressive disease.
Endpoints and Assessment
Progression-free survival has been recommended as the primary endpoint for phase 3 trials evaluating novel systemic therapies in NET.14,15 The primary endpoint was defined as the time from randomization to radiographic progressive disease per RECIST 1.1, as determined retrospectively by blinded independent central review (BICR), or death from any cause. Participants were evaluated every 12 weeks by radiographic imaging for tumor response and progression. If investigator assessments of progressive disease were confirmed by blinded real-time central review, patients were unblinded to treatment assignment; if progression was not confirmed, patients continued assigned therapy without unblinding. Secondary endpoints included confirmed objective response rate, overall survival, and safety. Confirmed response rate was defined as having two consecutive scans showing complete or partial response per RECIST 1.1 as assessed by BICR. Overall survival was defined as the time from randomization to death of any cause. Adverse event severity and perceived attribution to study treatment were assessed by investigators using the NCI Common Terminology Criteria for Adverse Events version 5.0. An optional correlative study assessed health-related quality of life (HRQL) before treatment and every 12 weeks until progression or start of new cancer treatment primarily using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (QLQ-C30) and QLQ-GINET21 (see Supplement for more details).16,17
Statistical Analysis
The trial was designed with targeted sample sizes of 210 and 185 patients in the epNET and pNET cohorts, respectively, to provide 90% power using stratified one-sided log-rank tests to detect true hazard ratios of 0.583 and 0.568 reflecting superior progression-free survival in patients treated with cabozantinib versus placebo, with an overall significance level of 0.025 (corresponding to a two-sided significance level of 0.05). This statistical design required a total of 164 and 149 progression events for the epNET and pNET cohorts, respectively. Two interim analyses for futility were included in each cohort after 33% and 66% of the projected numbers of progression events had occurred. Alpha spending of 0.001 for efficacy was included per interim analysis (corresponding to 0.002 in a two-sided setting). Although the trial protocol design and analysis plan were based on one-sided tests for the primary endpoint, two-sided P values are reported in accordance with Journal policy (the protocol is available at nejm.org).
In May 2023, the independent Alliance Data Safety Monitoring Board (DSMB) recommended that the protocol-specified interim analyses of progression-free survival be performed using local assessments due to a lag in batched BICR tumor assessments and the double-blinded nature of the trial. Based on interim analyses demonstrating superior efficacy with cabozantinib (Supplementary Table 1), the DSMB recommended early termination of the trial. Unblinding of treatment assignment and crossover of patients in the placebo arm to cabozantinib treatment occurred on August 24, 2023, which was used for the data cutoff for the final analyses of the study endpoints. A prespecified analysis of overall survival was conducted based on significance of the primary analysis of progression-free survival, where hazard ratios and corresponding 95% confidence intervals for overall survival between treatment arms were calculated and reported.
Efficacy analyses were conducted following the intention-to-treat principle. Hazard ratios (HR) were estimated through stratified Cox regression models and stratified log-rank tests calculated using stratification factors at randomization per the protocol design. Median progression-free survival and corresponding 95% confidence intervals (CI) for each treatment arm were estimated using Kaplan-Meier methodology. Subgroup analyses were performed by including treatment assignment, key demographic factors, and their interaction using Cox models. Response rate was calculated as the number of patients with confirmed response divided by the total number of patients in that arm with corresponding 95% exact binomial confidence intervals. Two-sided Chi-square p-values were calculated to compare the rates between arms. Descriptive means and 95% confidence intervals were computed per arm per time point for the HRQL summary score (protocol-specified main correlative endpoint) which combines function and symptom scale scores.18 Full statistical analysis of the HRQL data will be published separately.
Trial Oversight
The protocol was approved by the NCI central institutional review board (CIRB). The trial was conducted in accordance with the principles of the Declaration of Helsinki and the International Council for Harmonisation Good Clinical Practice guidelines. All participants provided written informed consent before enrollment. The trial was regularly monitored by the Alliance DSMB. Data were collected by the Alliance, reviewed by the study chair (J.A.C), and analyzed in collaboration with the authors. The authors wrote the manuscript, vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol. No one who is not an author contributed to writing the manuscript.
Results
Patients
Between October 2018 and August 2023, 203 patients were randomized in the epNET cohort and 95 patients randomized in the pNET cohort at 62 sites in the United States (Tables 1A and 1B; Supplemenatry Tables 2A and 2B). The demographics of enrolled patients are representative and generalizable to patients diagnosed with NET in the United States and elsewhere (Supplementary Table 3). The intent-to-treat cohorts included 7 patients with pancreatic NET who were misallocated to the extra-pancreatic NET cohort and 3 patients with extra-pancreatic NET who were misallocated to the pancreatic NET cohort by the registering site. Treatment groups were balanced for baseline demographic and disease characteristics in both cohorts, except more patients receiving cabozantinib compared with placebo in the epNET cohort had tumors of unknown primary site. In the epNET cohort, patients in both treatment arms had received a median of 2 prior lines of therapy not including SSAs; the majority had received Lu-177 dotatate and everolimus. In the pNET cohort, patients had a median of 3 and 2 prior lines of therapy in the cabozantinib and placebo treatment groups, respectively; most patients had received Lu-177 dotatate, everolimus, and temozolomide-based therapy.
Table 1A:
Baseline demographic and clinical characteristics of all patients enrolled in the extra-pancreatic NET cohort
| Cabozantinib (n=134) | Placebo (n=69) | |
|---|---|---|
| Age, years, median (range) | 66 (28–86) | 66 (30–82) |
| Female Sex, n (%) | 74 (55) | 31 (45) |
| ECOG PS, n (%) | ||
| 0 | 49 (37) | 32 (46) |
| 1 | 84 (63) | 36 (52) |
| Primary tumor site, n (%)* | ||
| Gastrointestinal | 70 (52) | 46 (67) |
| Lung | 27 (20) | 12 (17) |
| Thymus | 6 (5) | 4 (6) |
| Unknown | 22 (16) | 2 (3) |
| Tumor grade, n (%) | ||
| Grade 1 | 37 (28) | 15 (22) |
| Grade 2 | 86 (64) | 48 (70) |
| Grade 3 | 8 (6) | 5 (7) |
| Unknown | 3 (2) | 1 (1) |
| Hormone syndrome present (functional tumor), n (%) | 41 (31) | 25 (36) |
| Concurrent SSA, n (%) | 92 (69) | 48 (70) |
| Number of prior systemic therapies not including SSA, median (range) | 2 (1–6) | 2 (1–6) |
| Prior systemic therapy, n (%) | ||
| SSA, n (%) | 125 (93) | 64 (93) |
| Lu-177 dotatate | 80 (60) | 41 (59) |
| Everolimus | 96 (72) | 44 (64) |
| Temozolomide +/− Capecitabine | 43 (32) | 20 (29) |
| Cisplatin or carboplatin + etoposide | 11 (8) | 8 (12) |
Abbreviations: ECOG PS: Eastern Cooperative Oncology Group Performance Status
The intent-to-treat cohort includes 7 patients with pancreatic neuroendocrine tumors (4 in the cabozantinib group and 3 in the placebo group) who were misallocated to the extra-pancreatic neuroendocrine tumor cohort by the registering site.
Table 1B:
Baseline demographic and clinical characteristics of all patients enrolled in the pancreatic NET cohort
| Cabozantinib (n=64) | Placebo (n=31) | |
|---|---|---|
| Age, years, median (range) | 60 (29–79) | 64 (39–79) |
| Female Sex, n (%) | 27 (42) | 13 (42) |
| ECOG PS, n (%) | ||
| 0 | 35 (55) | 15 (48) |
| 1 | 28 (44) | 16 (52) |
| Primary site, n (%)* | ||
| Pancreas | 62 (97) | 30 (97) |
| Tumor grade, n (%) | ||
| Grade 1 | 14 (22) | 7 (23) |
| Grade 2 | 39 (61) | 19 (61) |
| Grade 3 | 8 (13) | 3 (10) |
| Unknown | 3 (5) | 2 (7) |
| Hormone syndrome present (functional tumor), n (%) | 11 (17) | 5 (16) |
| Concurrent SSA, n (%) | 35 (55) | 17 (55) |
| Number of prior systemic therapies not including SSA, median (range) | 3 (1–9) | 2 (1–7) |
| Prior systemic therapy, n (%) | ||
| SSA, n (%) | 63 (98) | 30 (97) |
| Lu-177 dotatate | 38 (59) | 18 (58) |
| Everolimus | 51 (80) | 25 (81) |
| Sunitinib | 18 (28) | 7 (23) |
| Temozolomide +/− capecitabine | 43 (67) | 16 (52) |
Abbreviations: ECOG PS: Eastern Cooperative Oncology Group Performance Status
The intent-to-treat cohort includes 3 patients with extra-pancreatic neuroendocrine tumors (2 in the cabozantinib group and 1 in the placebo group) who were misallocated to the pancreatic neuroendocrine tumor cohort by the registering site.
As of the data cutoff date of August 24, 2023, 21 (16%) and 14 (22%) patients treated with cabozantinib and 7 (10%) and 2 (6%) patients treated with placebo were still receiving study treatment in the epNET and pNET cohorts, respectively. The most common reason for discontinuing therapy in both treatment groups of both cohorts was disease progression (Supplementary Figures 1A and 1B).
Efficacy
Extra-pancreatic NET Cohort
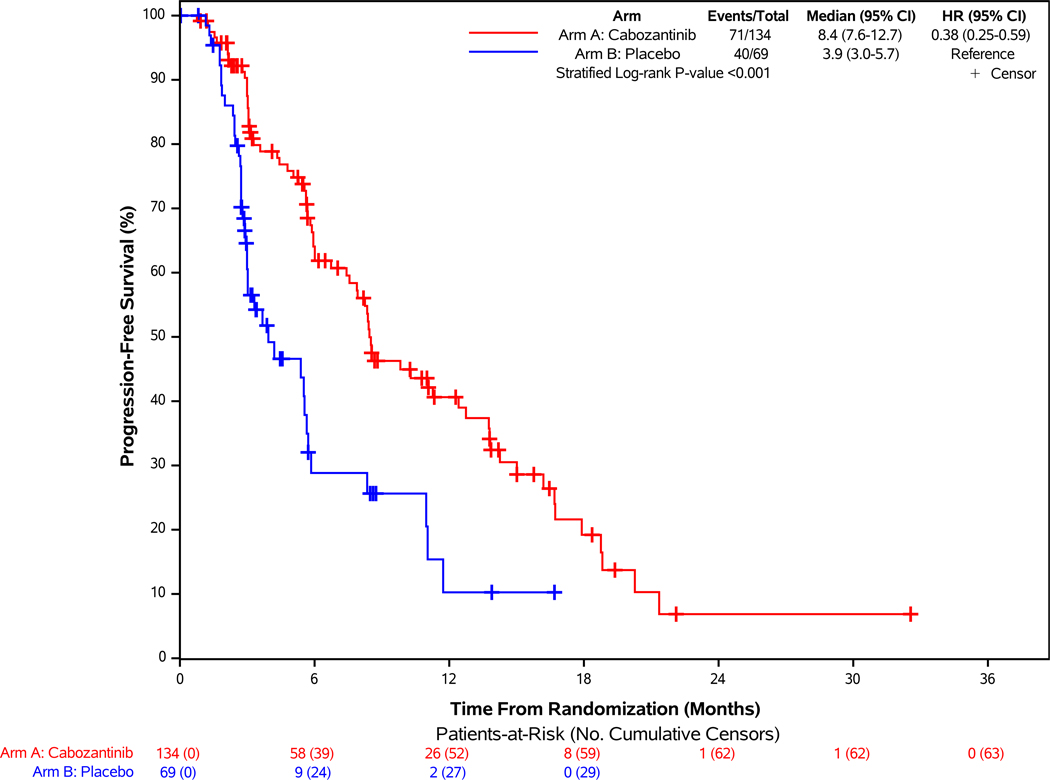
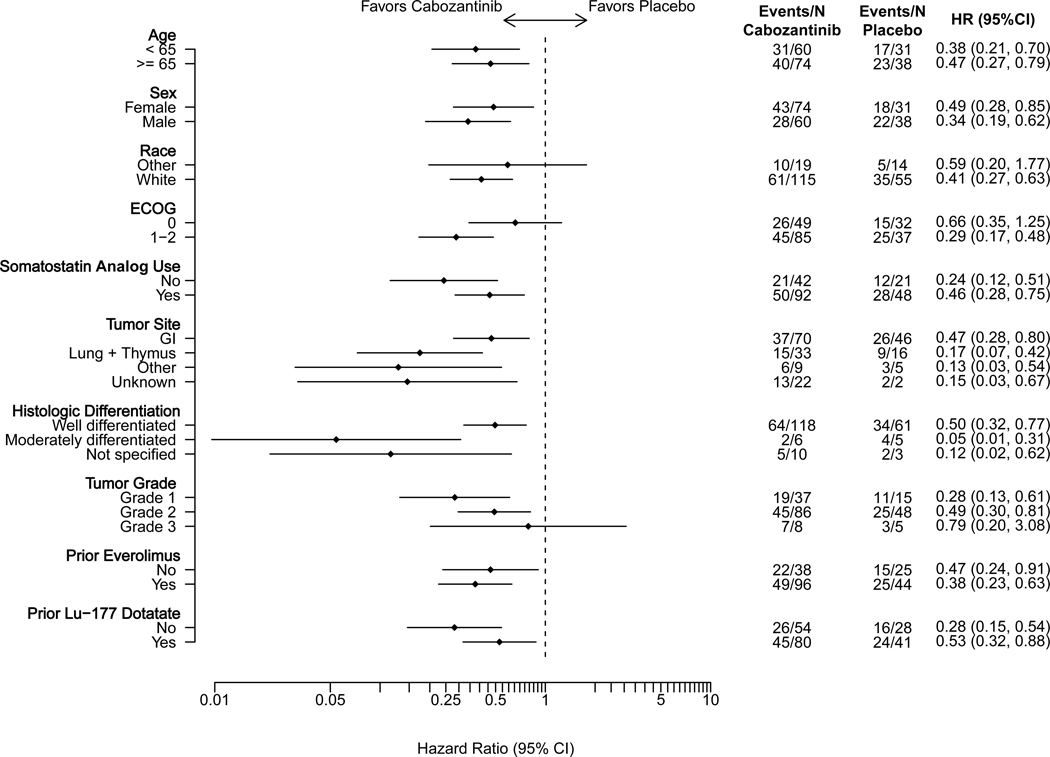
Of the 203 patients in this cohort, 111 progression events by BICR were reported with median follow-up of 10.2 months (95% CI: 8.2 – 13.8 months). At the data cutoff date, 71 participants (53%) in the cabozantinib group and 40 (58%) in the placebo group had disease progression or had died. Cabozantinib was associated with a 62% lower risk of disease progression or death on average compared with placebo (stratified HR = 0.38, 95% CI: 0.25–0.59; p<0.001; Figure 1A). Median progression-free survival was 8.4 months (95% CI: 7.6–12.7 months) with cabozantinib and 3.9 months (95% CI: 3.0–5.7 months) with placebo. Subgroup analyses by stratification factors and key factors including primary tumor site, grade, and prior treatment are shown in Figure 1C. Results from sensitivity analyses, including progression-free survival by investigator assessment and analyses accounting for misallocation of patients into the incorrect disease cohort, were consistent with the primary analysis (Supplementary Figures 2A and 3A).
Figure 1. Progression-free Survival by Blinded Independent Central Review and Overall Survival of Patients Enrolled in the Extra-pancreatic NET Cohort.
Panel A demonstrates the results of progression-free survival (PFS), as assessed retrospectively per RECIST 1.1 criteria by blinded independent central review (BICR), for patients enrolled in the epNET cohort. For the comparison between treatment groups, two-sided stratified log-rank P-value <0.001. Panel B demonstrates the results of overall survival of patients enrolled in the epNET cohort. Panel C demonstrates the results of PFS according to stratification factors and selected clinical subgroups of patients enrolled in the epNET cohort. For the Tumor Grade subgroup analysis, the “Unknown” subgroup is not displayed in the figure due to having zero PFS events in at least one treatment arm.
Partial responses were observed in 5% of patients in the cabozantinib group (95% CI: 2%−10%) compared with 0% (95% CI: 0%−5%) in the placebo group (p=0.05). Best response of stable disease occurred in 65% with cabozantinib versus 54% with placebo, and of progressive disease in 11% with cabozantinib versus 35% with placebo (Table 2). Any reduction in target lesions was observed for 67% and 29% of the cabozantinib and placebo groups, respectively (Supplementary Figure 4A).
Table 2:
Objective Tumor Response by Blinded Independent Central Review
| Extra-pancreatic NET Cohort | Pancreatic NET Cohort | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Tumor response | Cabozantinib | Placebo | p-value | Cabozantinib | Placebo | p-value |
| (N=134) | (N=69) | (N=64) | (N=31) | |||
| Objective response rate (95% CI) | 5% (2%−10%) | 0% (0%−5%) | 0.05 | 19% (10%−30%) | 0% (0%−11%) | 0.010 |
|
| ||||||
| Best overall response, n (%) | ||||||
|
| ||||||
| Partial Response | 7 (5%) | 0 | 12 (19%) | 0 | ||
|
| ||||||
| Stable Disease | 87 (65%) | 37 (54%) | 39 (61%) | 17 (55%) | ||
|
| ||||||
| Progressive Disease | 15 (11%) | 24 (35%) | 5 (8%) | 12 (39%) | ||
|
| ||||||
| Not Evaluable | 25 (19%) | 8 (12%) | 8 (13%) | 2 (6%) | ||
Abbreviations: CI: confidence interval
Objective response rate was defined as the percentage of patients who had a confirmed complete or partial response by blinded independent central review according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. All responses were partial responses. Chi-square tests were used to compare the response rates between the two groups.
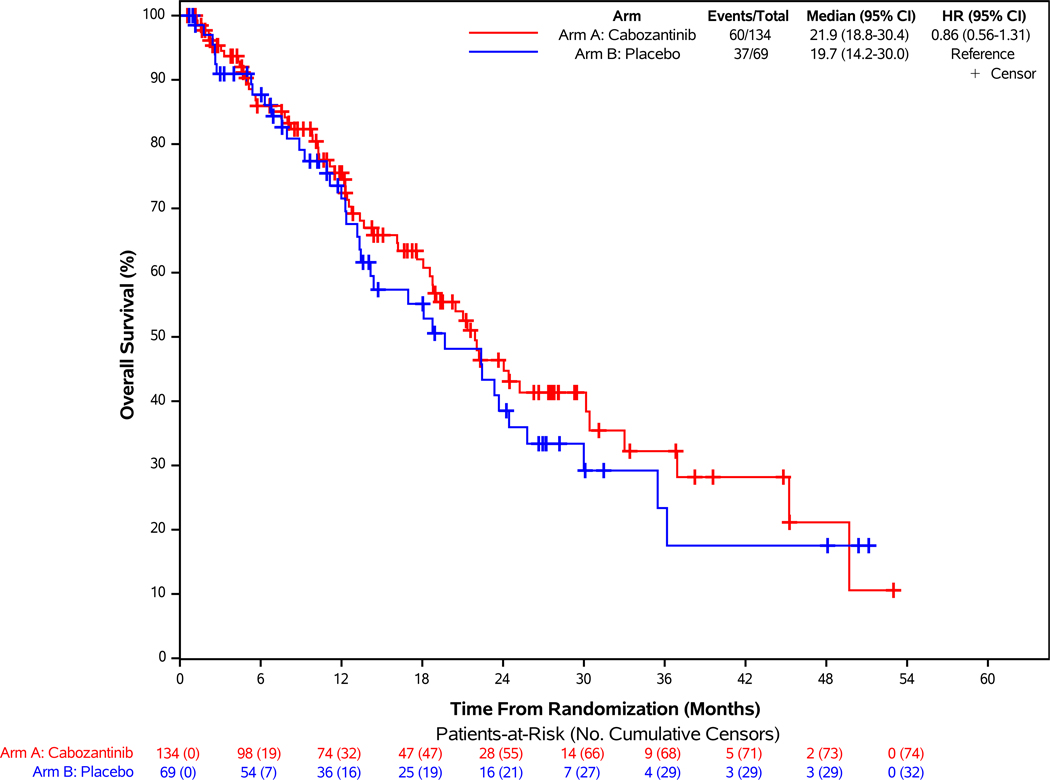
Median survival follow-up time was 24.2 months, with 60 (45%) and 37 (54%) deaths in the cabozantinib and placebo groups, respectively. Median overall survival was 21.9 months with cabozantinib versus 19.7 months with placebo (HR= 0.86; 95% CI: 0.56–1.31; Figure 1B). Subsequent anticancer therapy was received by 45% of patients in the cabozantinib group and 67% of patients in the placebo group, including 20 (33%) who crossed over to open-label cabozantinib on protocol (Supplementary Table 4). Overall HRQL remained stable over time and similar in both arms among those completing questionnaires (Supplementary Figure 5A).
Pancreatic NET Cohort
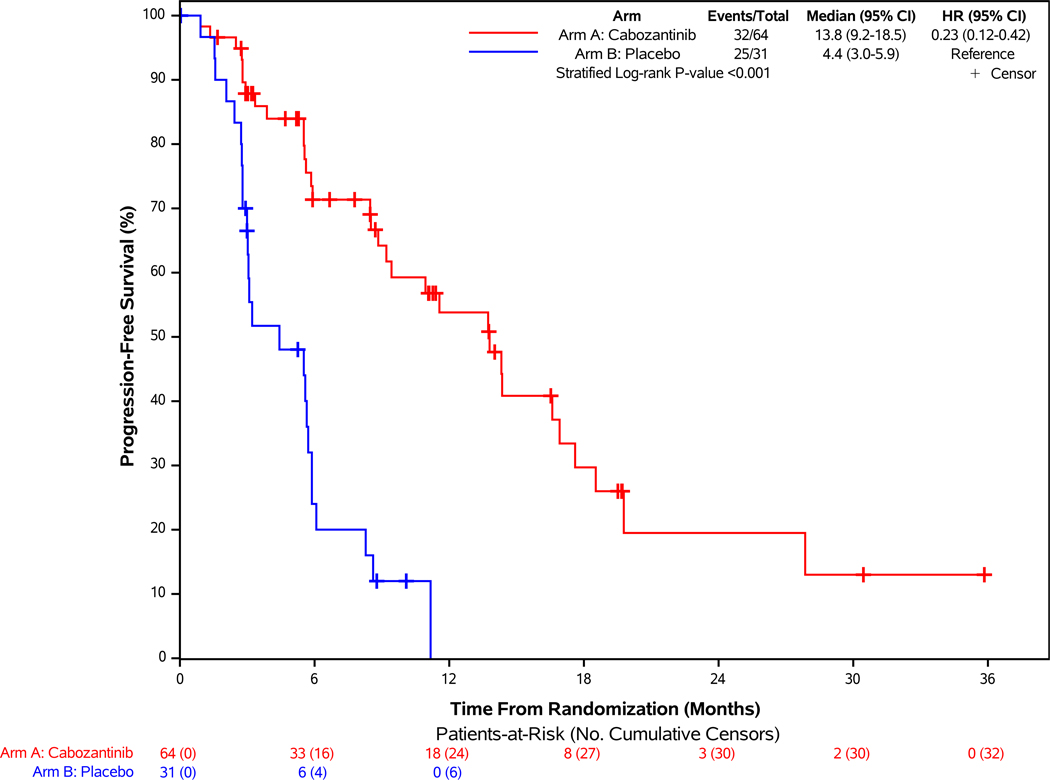
Of the 95 patients in this cohort, 57 progression events by BICR were reported with median follow-up of 13.8 months (95% CI: 10.1 – 19.7 months). At the data cutoff date, 32 participants (50%) in the cabozantinib group and 25 (81%) in the placebo group had disease progression or had died. Cabozantinib was associated with a 77% lower risk of disease progression or death on average compared with placebo (stratified HR = 0.23, 95% CI: 0.12–0.42; p<0.001; Figure 2A). Median progression-free survival was 13.8 months (95% CI: 9.2–18.5 months) with cabozantinib and 4.4 months (95% CI: 3.0–5.9 months) with placebo. Subgroup analyses are shown in Figure 2C. Sensitivity analyses, including progression-free survival by investigator assessment and analyses accounting for misallocation of patients into the incorrect disease cohort, were consistent with the primary progression-free survival analysis (Supplementary Figures 2B and 3B).
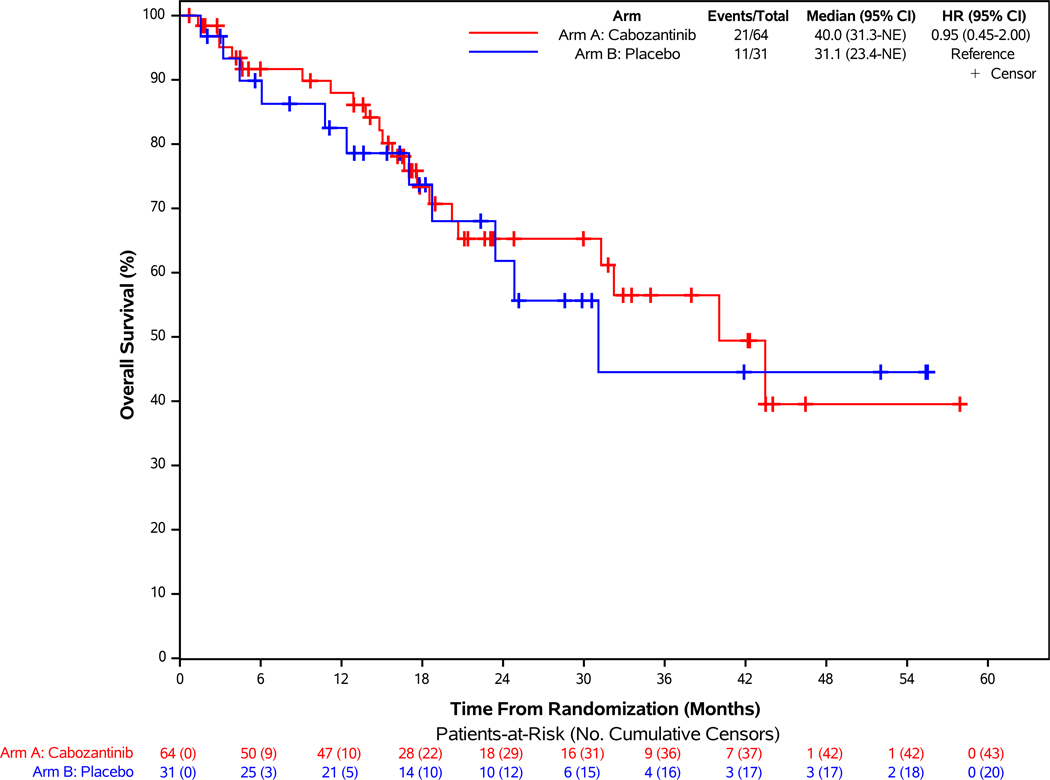
Figure 2: Progression-free Survival According to Blinded Independent Central Review and Overall Survival of Patients Enrolled in the Pancreatic NET Cohort.
Panel A demonstrates the results of progression-free survival (PFS), as assessed retrospectively per RECIST 1.1 criteria by blinded independent central review (BICR), for patients enrolled in the pNET cohort. For the comparison between treatment groups, two-sided stratified log-rank P-value <0.001. Panel B demonstrates the results of overall survival of patients enrolled in the pNET cohort. Panel C demonstrates the results of PFS according to stratification factors and selected clinical subgroups of patients enrolled in the pNET cohort. For the Histologic Differentiation subgroup analysis, “Moderately differentiated” and “Not specified” subgroups are not displayed in the figure due to having zero PFS events in at least one treatment arm.
Cabozantinib resulted in a higher confirmed response rate than placebo. Partial responses were observed in 19% of patients in the cabozantinib group (95% CI: 10%−30%) compared with 0% (95% CI: 0%−11%) in the placebo group (p=0.01). Best response of stable disease occurred in 61% with cabozantinib versus 55% with placebo, and of progressive disease in 8% with cabozantinib versus 39% with placebo (Table 2). Any reduction in target lesions was observed for 80% and 24% of the cabozantinib and placebo groups, respectively (Supplementary Figure 4B).
Median survival follow-up was 23.1 months, with 21 (33%) and 11 (35%) deaths reported in the cabozantinib and placebo groups, respectively. Median overall survival was 40 months with cabozantinib versus 31.1 months with placebo (HR= 0.95; 95% CI: 0.45 to 2.00; Figure 2B). Subsequent anticancer therapy was received by 51% of patients in the cabozantinib group and 62% of patients in the placebo group, including 12 (41%) who crossed over to open-label cabozantinib on protocol (Supplementary Table 5). Similar to the epNET cohort, overall HRQL remained stable over time and similar in both arms (Supplementary Figure 5B).
Exposure and Safety
Extra-pancreatic NET Cohort
The safety population in this cohort consisted of 132 patients treated with cabozantinib and 67 with placebo; median duration of treatment was 5.5 months (range, 0.2 to 32.4 months) and 2.8 months (range, 0.6 to 21.4 months), respectively. Dose reductions occurred in 66% of patients treated with cabozantinib and 10% treated with placebo. The median average daily dose was 38.4 mg for cabozantinib and 59.0 mg for placebo. The rate of treatment discontinuation due to adverse events was 31% (n = 34) and 15% (n = 9) in the cabozantinib and placebo groups, respectively.
The incidence of adverse events (any grade) attributed to study treatment was 98% with cabozantinib and 82% with placebo, with incidence of grade 3 or higher adverse events of 62% with cabozantinib and 27% with placebo (Table 3A). The most common treatment-related grade 3 or 4 adverse events with cabozantinib were hypertension (21%), fatigue (13%), and diarrhea (11%). Grade 5 events, primarily attributed to underlying disease, occurred in nine patients (7%) in the cabozantinib group and four patients (6%) in the placebo group. Four patients in the cabozantinib group experienced a grade 5 event deemed at least possibly related to treatment [(gastric hemorrhage (n=1), cardiac arrest (n=1), cause of death not specified (n=2)].
Table 3A:
Treatment-related Adverse Events in the Extra-pancreatic Neuroendocrine Tumor Cohort
| Cabozantinib (N=132) | Placebo (N=67) | |||
|---|---|---|---|---|
| Any Grade | Grades 3–5* | Any Grade | Grades 3–5 | |
| Number of patients (%) | ||||
| Any adverse event at least possibly related to treatment | 130 (98) | 82 (62) | 55 (82) | 18 (27) |
| Common treatment-related adverse events (>20% any grade and/or >10% grade 3–5) | ||||
| Fatigue | 82 (62) | 17 (13) | 28 (42) | 5 (8) |
| Diarrhea | 74 (56) | 14 (11) | 20 (30) | 3 (5) |
| AST increase | 86 (65) | 4 (3) | 12 (18) | 0 |
| Hypertension | 70 (53) | 28 (21) | 13 (19) | 2 (3) |
| ALT increase | 77 (58) | 1 (1) | 9 (13) | 0 |
| Thrombocytopenia | 62 (47) | 1 (1) | 5 (8) | 1 (2) |
| Nausea | 46 (35) | 2 (2) | 10 (15) | 0 |
| Mucositis oral | 48 (36) | 5 (4) | 6 (9) | 0 |
| Palmar-plantar erythrodysesthesia | 48 (36) | 4 (3) | 5 (8) | 0 |
| Anorexia | 40 (30) | 2 (2) | 6 (9) | 0 |
| Leukopenia | 46 (35) | 4 (3) | 2 (3) | 0 |
| Dysgeusia | 45 (34) | 0 | 1 (2) | 0 |
| Neutropenia | 40 (30) | 4 (3) | 2 (3) | 0 |
| Anemia | 28 (21) | 2 (2) | 8 (11) | 0 |
| Lymphopenia | 31 (23) | 5 (4) | 6 (9) | 0 |
| Hypothyroidism | 36 (27) | 0 | 1 (2) | 0 |
| Maculopapular rash | 30 (23) | 0 | 2 (3) | 0 |
| Weight loss | 28 (21) | 3 (2) | 2 (3) | 0 |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase
The safety population included all patients who underwent randomization and received at least one dose of study treatment. Shown are all adverse events with an attribution of being at least possibly related to study treatment that were reported in 20% or more of the patients in the cabozantinib group across all grades and/or common grade 3 or higher treatment-related adverse events that were reported in 10% or more of the patients in the cabozantinib group. The presence or absence of the following adverse events was solicited for each treatment cycle: neutropenia, thrombocytopenia, alanine or aspartate aminotransferase increase, palmar-plantar erythrodysesthesia syndrome, maculopapular rash, hyperglycemia, hypothyroidism, hypertension, fatigue, diarrhea, mucositis oral.
Grade 5 events in cabozantinib arm possibly related to study therapy: n=1 gastrointestinal hemorrhage; n=1 cardiac arrest; n=2 death not otherwise specified
Pancreatic NET cohort
The safety population for this cohort consisted of 63 patients treated with cabozantinib and 31 treated with placebo; median duration of treatment was 8.3 months (range, 0.5 to 39.6 months) and 2.9 months (range, 0.5 to 11.2 months), respectively. Dose reductions occurred in 68% of patients treated with cabozantinib and 19% treated with placebo. The median average daily dose was 37.9 mg for cabozantinib and 56.9 mg for placebo. The rate of treatment discontinuation due to adverse events was 20% (n = 10) and 0% in the cabozantinib and placebo groups, respectively.
The incidence of adverse events (any grade) attributed to study treatment was 98% with cabozantinib and 84% with placebo, with incidence of grade 3–4 treatment-related adverse events of 65% with cabozantinib and 23% with placebo (Table 3B). The most common treatment-related grade 3–4 adverse events with cabozantinib were hypertension (22%), fatigue (11%), and thromboembolic events (11%). No grade 5 events were noted in the pNET cohort.
Table 3B:
Treatment-related Adverse Events in the Pancreatic Neuroendocrine Tumor Cohort
| Cabozantinib (N=63) | Placebo (N=31) | |||
|---|---|---|---|---|
| Adverse Event | Any Grade | Grade 3 or 4 | Any Grade | Grade 3 or 4 |
| Number of patients (%) | ||||
| Any adverse event at least possibly related to treatment | 62 (98) | 41 (65) | 26 (84) | 7 (23) |
| Common treatment-related adverse events (>20% any grade and/or >10% grade 3–5) | ||||
| Fatigue | 47 (75) | 7 (11) | 10 (32) | 1 (3) |
| AST increase | 40 (63) | 1 (2) | 9 (29) | 0 |
| ALT increase | 39 (62) | 1 (2) | 9 (29) | 0 |
| Hypertension | 36 (57) | 14 (22) | 7 (23) | 3 (10) |
| Diarrhea | 37 (59) | 4 (6) | 4 (13) | 0 |
| Nausea | 24 (38) | 5 (8) | 7 (23) | 1 (3) |
| Palmar-plantar erythrodysesthesia | 28 (44) | 6 (10) | 4 (13) | 0 |
| Mucositis oral | 30 (48) | 5 (8) | 1 (3) | 0 |
| Thrombocytopenia | 21 (33) | 0 | 3 (10) | 0 |
| Dysgeusia | 19 (30) | 0 | 3 (10) | 0 |
| Neutropenia | 17 (27) | 1 (2) | 2 (7) | 0 |
| Alkaline phosphatase increase | 13 (21) | 0 | 3 (10) | 0 |
| Anorexia | 13 (21) | 1(2) | 3 (10) | 0 |
| Vomiting | 13 (21) | 4 (6) | 3 (10) | 0 |
| Hypophosphatemia | 13 (21) | 0 | 2 (7) | 0 |
| Thromboembolic event | 11 (18) | 7 (11) | 0 | 0 |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase
The safety population included all patients who underwent randomization and received at least one dose of study treatment. Shown are all adverse events with an attribution of being at least possibly related to study treatment that were reported in 20% or more of the patients in the cabozantinib group across all grades and/or common grade 3 or higher treatment-related adverse events that were reported in 10% or more of the patients in the cabozantinib group. The presence or absence of the following adverse events was solicited for each treatment cycle: neutropenia, thrombocytopenia, alanine or aspartate aminotransferase increase, palmar-plantar erythrodysesthesia syndrome, maculopapular rash, hyperglycemia, hypothyroidism, hypertension, fatigue, diarrhea, mucositis oral.
Discussion
Cabozantinib significantly improved progression-free survival compared to placebo in patients with previously treated, progressive epNET or pNET. No overall survival difference between the treatment groups has been observed to date; however, overall survival data were not mature at the time of analyses and may have been impacted by crossover and the high rate of treatment with subsequent anticancer therapies. Adverse events observed in this trial were similar to the known safety profile of cabozantinib and consistent with what has been observed with cabozantinib as a single agent in other disease settings.19–21 A majority of patients treated with cabozantinib required dose modifications or reductions to manage adverse events.
The treatment landscape in NET has evolved over the last fifteen years with the addition of targeted agents, Lu-177 dotatate for gastrointestinal NET and pNET, and alkylating agent chemotherapy with temozolomide-based regimens for patients with pNET. Use of these agents in clinical practice can be extended to NET arising in other locations. Because disease ultimately will progress on these agents, additional therapies are needed. The CABINET trial is a randomized study designed to evaluate efficacy of therapy following treatment with FDA-approved therapies such as Lu-177 dotatate and/or targeted therapy. The results support the use of cabozantinib as a new treatment option for patients with advanced extra-pancreatic NET or pancreatic NET whose disease has progressed after or who have experienced intolerance of at least one other line of therapy for their disease, not including somatostatin analogs. Choice of therapy should be individualized and based on patient and tumor characteristics. Future clinical trials evaluating optimal sequencing of therapy are needed.
Although our results show significant efficacy of cabozantinib, several limitations deserve comment. The early termination of the trial based on interim analysis results could potentially lead to overestimation of treatment effect. However, results for both cohorts represent relatively mature analyses of progression-free survival. In the epNET cohort, 111 of the planned 164 events had been observed, and in the pNET cohort, 57 of the 95 enrolled patients experienced a progression event by BICR. Second, placebo rather than an active comparator was used. Notably, placebo was selected because the efficacy of therapy for patients with advanced NET whose disease has progressed after treatment with Lu-177 dotatate and/or targeted agents has not been well established. Although everolimus, sunitinib, and Lu-177 dotatate are approved agents, the phase 3 trials evaluating these agents were conducted primarily in patients who had not yet received molecularly targeted therapy or peptide receptor radionuclide therapy. Furthermore, placebo was chosen as a control because patients could have received all available therapies before enrollment on the trial.
Antiangiogenic agents and tyrosine kinase inhibitors targeting the VEGF receptor have established efficacy in the treatment of NET, and sunitinib is approved for treatment of pNET based on the results of a phase 3 trial.7 Other VEGF receptor targeting tyrosine kinase inhibitors, including axitinib, lenvatinib, pazopanib, and surufatinib, have been evaluated in epNET and/or pNET, but are not approved for treatment in the United States or Europe.22–26 The anti-VEGF monoclonal antibody bevacizumab has also been evaluated in randomized trials in NET.27,28 The antitumor activity of cabozantinib may be related to its receptor targets, including MET. Previous studies have demonstrated that MET activation can stimulate growth of NET and contribute to increased tumor invasion and metastasis that can be overcome by combining anti-VEGF therapy with a MET inhibitor or treatment with cabozantinib.12,29,30 Inhibition of MET and AXL also can impede resistance to VEGF inhibition and may contribute to superior efficacy with cabozantinib compared to sunitinib in patients with renal cell carcinoma.21,31
In conclusion, cabozantinib increases progression-free survival in patients with epNET or pNET that has progressed after prior therapy with Lu-177 dotatate or targeted agents including everolimus or sunitinib. Adverse events, which were managed with dose reduction in a majority of patients, are consistent with cabozantinib’s known safety profile.
Supplementary Material
Acknowledgements
We thank the participating patients and their families and caregivers; investigators, research nurses and study coordinators at participating sites; collaborating radiologists at Imaging and Radiation Oncology Core (IROC), including Volkan Beylergil, M.D., Lawrence Schwartz, M.D., Chad Wright, M.D.; operations staff at the Alliance for Clinical Trials in Oncology and the Alliance Statistics and Data Management Center, in particular Adam Eggert.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The first draft of the manuscript was written by Jennifer Chan, M.D., M.P.H.
(Funded by National Cancer Institute and other; Trial registration number: NCT03375320.)
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology); https://acknowledgments.alliancefound.org, UG1CA189856, UG1CA232760, UG1CA233180, UG1CA233290, UG1CA233329, UG1CA233331; U10CA180820 (ECOG-ACRIN Cancer Research Group); U10CA180868 (NRG Oncology); and U10CA180888 (SWOG Cancer Research Network). Also supported in part by Exelixis.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Dasari A, Shen C, Halperin D, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol 2017;3(10):1335–1342. DOI: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caplin ME, Pavel M, Ćwikła JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014;371(3):224–33. (In eng). DOI: 10.1056/NEJMoa1316158. [DOI] [PubMed] [Google Scholar]
- 3.Rinke A, Müller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 2009;27(28):4656–63. (In eng). DOI: 10.1200/jco.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 4.Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med 2017;376(2):125–135. (In eng). DOI: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brabander T, van der Zwan WA, Teunissen JJM, et al. Long-Term Efficacy, Survival, and Safety of [(177)Lu-DOTA(0),Tyr(3)]octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin Cancer Res 2017;23(16):4617–4624. (In eng). DOI: 10.1158/1078-0432.Ccr-16-2743. [DOI] [PubMed] [Google Scholar]
- 6.Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 2016;387(10022):968–977. (In eng). DOI: 10.1016/s0140-6736(15)00817-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364(6):501–13. (In eng). DOI: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 8.Kunz PL, Graham NT, Catalano PJ, et al. Randomized Study of Temozolomide or Temozolomide and Capecitabine in Patients With Advanced Pancreatic Neuroendocrine Tumors (ECOG-ACRIN E2211). J Clin Oncol 2023;41(7):1359–1369. (In eng). DOI: 10.1200/jco.22.01013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moertel CG, Lefkopoulo M, Lipsitz S, Hahn RG, Klaassen D. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med 1992;326(8):519–23. (In eng). DOI: 10.1056/nejm199202203260804. [DOI] [PubMed] [Google Scholar]
- 10.Bowen KA, Silva SR, Johnson JN, et al. An analysis of trends and growth factor receptor expression of GI carcinoid tumors. J Gastrointest Surg 2009;13(10):1773–80. (In eng). DOI: 10.1007/s11605-009-0958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Jia Z, Li Q, et al. Elevated expression of vascular endothelial growth factor correlates with increased angiogenesis and decreased progression-free survival among patients with low-grade neuroendocrine tumors. Cancer 2007;109(8):1478–86. (In eng). DOI: 10.1002/cncr.22554. [DOI] [PubMed] [Google Scholar]
- 12.You WK, Sennino B, Williamson CW, et al. VEGF and c-Met blockade amplify angiogenesis inhibition in pancreatic islet cancer. Cancer Res 2011;71(14):4758–68. (In eng). DOI: 10.1158/0008-5472.Can-10-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan JA, Faris JE, Murphy JE, et al. Phase II trial of cabozantinib in patients with carcinoid and pancreatic neuroendocrine tumors (pNET). J Clin Oncol 2017;35 (suppl 4S; abstract 228). [Google Scholar]
- 14.Kulke MH, Siu LL, Tepper JE, et al. Future directions in the treatment of neuroendocrine tumors: consensus report of the National Cancer Institute Neuroendocrine Tumor clinical trials planning meeting. J Clin Oncol 2011;29(7):934–43. (In eng). DOI: 10.1200/jco.2010.33.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh S, Hope TA, Bergsland EB, et al. Consensus report of the 2021 National Cancer Institute neuroendocrine tumor clinical trials planning meeting. J Natl Cancer Inst 2023;115(9):1001–1010. (In eng). DOI: 10.1093/jnci/djad096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85(5):365–76. (In eng). DOI: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 17.Yadegarfar G, Friend L, Jones L, et al. Validation of the EORTC QLQ-GINET21 questionnaire for assessing quality of life of patients with gastrointestinal neuroendocrine tumours. Br J Cancer 2013;108(2):301–10. (In eng). DOI: 10.1038/bjc.2012.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giesinger JM, Kieffer JM, Fayers PM, et al. Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J Clin Epidemiol 2016;69:79–88. (In eng). DOI: 10.1016/j.jclinepi.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med 2018;379(1):54–63. (In eng). DOI: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373(19):1814–23. (In eng). DOI: 10.1056/NEJMoa1510016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial. J Clin Oncol 2017;35(6):591–597. (In eng). DOI: 10.1200/jco.2016.70.7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Carbonero R, Benavent B, Jiménez Fonseca P, et al. A phase II/III randomized double-blind study of octreotide acetate with axitinib versus octreotide acetate with placebo in patients with advanced G1-G2 NETs of non-pancreatic origin (AXINET trial-GETNE-1107). J Clin Oncol 2021;39 (suppl 3; abstr 360). [Google Scholar]
- 23.Capdevila J, Fazio N, Lopez C, et al. Lenvatinib in Patients With Advanced Grade 1/2 Pancreatic and Gastrointestinal Neuroendocrine Tumors: Results of the Phase II TALENT Trial (GETNE1509). J Clin Oncol 2021;39(20):2304–2312. (In eng). DOI: 10.1200/jco.20.03368. [DOI] [PubMed] [Google Scholar]
- 24.Bergsland EK, Mahoney MR, Asmis TR, et al. Prospective randomized phase II trial of pazopanib versus placebo in patients with progressive carcinoid tumors (CARC)(Alliance A021202). J Clin Oncol 2019;37 (suppl; abstr 4005). [Google Scholar]
- 25.Xu J, Shen L, Bai C, et al. Surufatinib in advanced pancreatic neuroendocrine tumours (SANET-p): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 2020;21(11):1489–1499. (In eng). DOI: 10.1016/s1470-2045(20)30493-9. [DOI] [PubMed] [Google Scholar]
- 26.Xu J, Shen L, Zhou Z, et al. Surufatinib in advanced extrapancreatic neuroendocrine tumours (SANET-ep): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 2020;21(11):1500–1512. (In eng). DOI: 10.1016/s1470-2045(20)30496-4. [DOI] [PubMed] [Google Scholar]
- 27.Kulke MH, Ou FS, Niedzwiecki D, et al. Everolimus with or without bevacizumab in advanced pNET: CALGB 80701 (Alliance). Endocr Relat Cancer 2022;29(6):335–344. (In eng). DOI: 10.1530/erc-21-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao JC, Guthrie KA, Moran C, et al. Phase III Prospective Randomized Comparison Trial of Depot Octreotide Plus Interferon Alfa-2b Versus Depot Octreotide Plus Bevacizumab in Patients With Advanced Carcinoid Tumors: SWOG S0518. J Clin Oncol 2017;35(15):1695–1703. (In eng). DOI: 10.1200/jco.2016.70.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krampitz GW, George BM, Willingham SB, et al. Identification of tumorigenic cells and therapeutic targets in pancreatic neuroendocrine tumors. Proc Natl Acad Sci U S A 2016;113(16):4464–9. (In eng). DOI: 10.1073/pnas.1600007113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sennino B, Ishiguro-Oonuma T, Wei Y, et al. Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov 2012;2(3):270–87. (In eng). DOI: 10.1158/2159-8290.Cd-11-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou L, Liu XD, Sun M, et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene 2016;35(21):2687–97. (In eng). DOI: 10.1038/onc.2015.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.