ABSTRACT
Root perforation may happen pathologically or iatrogenically at any stage of endodontic treatment. Repairing a Perforation can be challenging and can negatively affect the prognosis of the treatment. The prognosis depends on various factors, including the size and site of the perforation, the presence of microorganisms, and the time lapse between injury and repair. One crucial factor is using a suitable material with favorable biocompatibility, moisture tolerance, and sealing abilities. Thus, choosing the suitable perforation sealing material can significantly affect the outcomes. Many materials have been suggested for perforation repair. However, searching for an ideal material continues, indicating the complicated nature of root perforations. In this study, three patients, two male and one female, received treatment for root perforation, each varying in the prognosis determinant factors. All cases were managed non‐surgically with cold ceramic (CC) as the repairing material. After follow‐up, they were clinically and radiographically examined, and all three cases revealed relatively complete healing of the tissues and no signs or symptoms of inflammation. The results obtained from the presented cases indicated CC's sealing ability, biocompatibility, moisture tolerance, and bone and periodontium regeneration, which are essential for successful perforation repair. The favorable healing of the perforation and the elimination of inflammation in every case, as well as the existing literature, support the use of CC as a suitable material for sealing perforations. However, additional clinical research is recommended to further understand CC's qualities and potential.
Keywords: case report, cold ceramic, MTA, retreatment, root canal therapy, root filling material
Summary.
Cold ceramic (CC) has proven to be effective for repairing root perforations.
This case series, with three patients showing healing, highlights CC's biocompatibility, sealing ability, and moisture tolerance.
Further clinical research is recommended to fully understand CC's potential.
1. Introduction
Root perforation may happen at any point during endodontic treatments, resulting in communication between the root canal and the surrounding tissues. It can have a pathological origin. However, they are mostly iatrogenic, potentially contributing to up to 10% of unsuccessful endodontic procedures. Perforation negatively influences the prognosis of the tooth. The prognosis depends on the extent of the initial damage to the periodontal tissues, the location of the perforation to the gingival sulcus, the size, the duration of time between the occurrence of the injury and the subsequent treatment, the efficiency of the perforation seal, the presence of microorganisms and contamination at the perforation site, the repairing material, and the final restoration [1, 2, 3]. Hence, choosing the most suitable material is crucial [4].
The requirements of an ideal repair material have been described in the literature, including favorable sealing properties, biocompatibility, lack of toxicity, non‐carcinogenicity, radiopacity, moisture tolerance, permanence, insolubility, and inexpensiveness. Moreover, it should be easy to manipulate and capable of inducing cementogenesis and osteogenesis [1, 5]. Cold ceramic (CC), a mineral trioxide aggregate (MTA)‐like compound, can serve various purposes, such as root‐end filling material, an apical barrier in teeth with open apices, root perforation repair, pulp capping, and pulpotomy [6]. The characteristics of CC include exquisite sealing ability, biocompatibility, nontoxicity, noncarcinogenicity, radiopacity, easy handling, and minimal adverse effects on tissues. Therefore, CC can be used as the root filling and the perforation repair material [4, 7].
Modaresi et al. demonstrated periodontal ligament formation at the root end of teeth after apexification with CC, indicating the potential for cementum formation on the CC surface. Tissue regeneration around the root confirms the positive effects of CC on bone repair and periapical lesion healing [8]. An animal‐based research study showed that the presence of CC leads to the development of new bone, cementum, and periodontal ligament in over 50% of the samples [7].
The potential of CC material for endodontic applications has been successfully demonstrated. However, existing research on the clinical usage of this material still needs to be investigated. Thus, further studies should be conducted to boost the current knowledge and address the existing gaps [9, 10]. This study aims to investigate the efficiency and clinical outcomes of using CC to repair root perforations further by presenting three different cases. The current study does not focus on the superiority of CC over MTA but aims to demonstrate and explain the procedures for managing complex iatrogenic perforations in endodontics.
2. Case Report
Three patients were referred to the endodontic department of the faculty of dentistry at Mashhad University of Medical Sciences. All cases were managed by nonsurgical endodontic treatment using CC to repair the perforations. Written informed consent was obtained from patients in every case after explaining the diagnosis, treatment plan, and potential risks to the patients. The procedures conducted for all patients are described below, with detailed radiographic and photographic figures.
3. Case 1
3.1. Case History
A 42‐year‐old male patient was referred for the right mandibular first molar (tooth #30) with a chief complaint of abscess and swelling. The patient's medical history revealed no significant systemic conditions that would contraindicate endodontic treatment.
3.2. Examinations
Clinical examinations revealed a pocket depth of 10 mm and a coronal abscess of tooth #30 (Figure 1–1). It was noted that tooth #30 had been recently endodontically treated, and the periapical tissues were not sensitive to percussion. Radiographic examinations showed a former root canal filling of tooth #30. Additionally, two distinct perforations with relatively extensive lesions were diagnosed: a strip perforation located in the coronal half of the mesiobuccal canal and an apical perforation of the mesiolingual canal (Figure 1–2).
FIGURE 1‐1.

Clinical examinations revealing abscess and deep pocket.
FIGURE 1‐2.

A periapical radiographic image showing the lesions around the mesial root.
3.3. Diagnosis
Based on the clinical and radiographic findings, the pulpal diagnosis was previous root canal treatment with signs of infection. The periradicular diagnosis was chronic apical periodontitis. An acute periodontal abscess with a 10 mm pocket depth indicated a primary endodontic–secondary periodontal lesion.
3.4. Treatment
Following the administration of local anesthetics (2% lidocaine and epinephrine 1:100,000) (Daroupakhsh, Tehran, Iran) and rubber dam isolation, the access was prepared using a high‐speed diamond round bur No. 2 (Jota AG, Rüthi, Switzerland) under magnification (Carl Zeiss, Meditec Inc., Dublin, CA, USA) (Figures 1–3 and 1–4). After locating the canals' orifices, Canal‐filling materials were removed using size one Gates–Glidden (MANI gates drills, MANI, Japan) and H‐files (MANI H‐files, MANI, Japan). In addition to radiographic images, both perforations were confirmed using an apex locator (Dempex, DEM Ltd., Barnstaple, Devon, England) (Figure 1–5). The working length was also assessed with an apex locator (Dempex, DEM Ltd., Barnstaple, Devon, England) and radiographic image (Figure 1–6).
FIGURE 1‐3.

After removing the filling material, massive bleeding was visible.
FIGURE 1‐4.
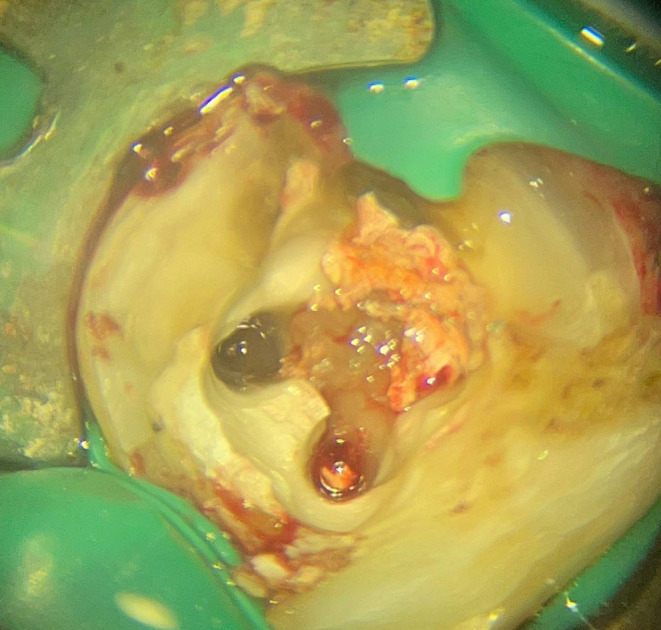
Clinical view of the perforations.
FIGURE 1‐5.

A periapical radiographic image showing the strip perforation.
FIGURE 1‐6.

Working length assessment and the apical perforation.
Cleaning and shaping were completed by crown‐down technique with M3 rotary files (UDG, Changzhou, China) up to size 25/04 under repeated irrigation with 5.25% sodium hypochlorite and sterile saline. The extensive coronal perforation was covered with PTFE tape before using sodium hypochlorite. Root canals were dried with sterile paper points (META, Chugbuk, South Korea). The distal canals were obturated with gutta‐percha (META, Chugbuk, South Korea) and AH plus sealer (Dentsply DeTrey, Konstanz, Germany) using the warm vertical technique by FastFill warm obturator (Fast Fill Obturation System, Eighteeth, China).
Following the manufacturer's instructions, the strip perforation was sealed with CC (SJM, Iran). CC (SJM, Iran) was carried into the perforation site using MAP One carrier (Maillefer, Dentsply, Switzerland), under a dental operating microscope (Carl Zeiss, Meditec Inc., Dublin, CA, USA), and a size 20 finger plugger (Maillefer, Dentsply, Switzerland) was used to compact CC (SJM, Iran) (Figure 1–7). A #25 K‐file (Mani Inc., Utsunomiya, Japan) was placed in the mesiobeccal canal with strip perforation as a space maintainer (Figure 1–8) [1]. The file was carefully and slowly loosened after cc's initial setting so that it can be easily removed when it sets completely. In order to prevent occlusal interference, the handle of the K‐file was cut and covered with Cavit (Cavisol, Tehran, Iran).
FIGURE 1‐7.

Repair of the strip perforation with CC.
FIGURE 1‐8.

A periapical radiographic image showing the #25 K‐file as the space maintainer.
At the 1‐week follow‐up appointment, the coronal and apical abscesses were still detectable (Figure 1–9). The apical perforation of the mesiolingual canal was sealed with CC (SJM, Iran), and the mesial canal obturation was completed (Figures 1–10 and 1–11). The mesial canal's orifices were covered with Glass‐ionomer (GC Fuji I, Tokyo, Japan) to keep the sealing material intact (Figure 1–12), and the pulp chamber was covered with Cavit (Cavisol, Tehran, Iran).
FIGURE 1‐9.

A periapical radiographic image showing the 10‐mm deep pocket of tooth #30.
FIGURE 1‐10.

A periapical radiographic image showing the negotiation of the perforated canal to the estimated working length.
FIGURE 1‐11.

Repair of the perforations with CC.
FIGURE 1‐12.

Covering CC with Glass‐ionomer.
3.5. Postoperative Care
Postoperative instructions were given to the patient, including oral hygiene instructions and analgesic medication (Ibuprofen 400 mg) for postoperative symptoms. Five weeks later, the radiographic examination showed an acceptable root canal filling and complete perforation seal with CC (SJM, Iran) (Figure 1‐13). After confirming the lack of clinical signs and symptoms, the patient was referred for a permanent restoration, and the tooth was restored with an Amalgam filling.
FIGURE 1‐13.

Postoperative radiograph.
3.6. Follow‐Up
The 12‐month follow‐up exhibited no clinical signs or symptoms. The reduction of periapical radiolucency and the probing depth within the normal range indicated healing of the tissues (Figures 1‐14 and 1‐15).
FIGURE 1‐14.

Twelve‐month follow‐up examinations exhibiting tissue healing with no signs of inflammation.
FIGURE 1‐15.

Twelve‐month postoperative radiograph showing favorable healing of the lesions.
The final restoration was later replaced with full cusp coverage porcelain fused to metal (PFM) crown due to being poorly adapted with a distal overhang, which could be seen in the 12‐month follow‐up radiographic examinations (Figures 1‐14 and 1‐16). In the 15‐month follow‐up, tooth #30 showed no signs of inflammation, and the pocket depth was within the normal range (Figure 1‐17). Both clinical and radiographic examinations confirmed the favorable periradicular healing and tissue regeneration, indicating sufficient coronal and apical seal and the treatment success (Figures 1‐16, 1‐17, 1‐18).
FIGURE 1‐16.

Clinical view of tooth #30 in the 15‐month follow‐up.
FIGURE 1‐17.

Fifteen‐month follow‐up examinations of tooth #30 with full cusp coverage PFM crown as the permanent restoration, healthy periodontium, negative BOP, and normal probing depth.
FIGURE 1‐18.

Periapical follow‐up radiographic image after 15 months of treatment, exhibiting complete tissue healing bone formation.
4. Case 2
4.1. Case History
A 26‐year‐old male patient was referred for incomplete endodontic treatment of the left mandibular first molar (tooth #19) due to perforation (Figure 2‐1). The patient's medical history showed no systemic disorders and contraindications to endodontic treatment.
FIGURE 2‐1.

A periapical radiographic image showing the misposition of the file due to strip perforation.
4.2. Examinations
Clinical examination revealed swelling and abscess in the tissues surrounding the tooth (Figure 2–2). The pulp was nonresponsive to both pulp testing and periapical testing. Concurrent furcal and strip perforation with lesions were noted in the radiological examination. Calcification of the canals was presumed to be the probable cause of the perforations (Figure 2–3).
FIGURE 2‐2.

Clinical examination of tooth #19 exhibiting swelling and abscess.
FIGURE 2‐3.

A periapical radiographic image demonstrateing furcal and coronal lesions.
4.3. Diagnosis
Based on the clinical and radiographic findings, the pulpal diagnosis was previously initiated root canal treatment, and periradicular was diagnosed as normal periapical tissue with acute periodontal abscess due to iatrogenic perforation.
4.4. Treatment
The treatment was started by administering local anesthetics (2% lidocaine and epinephrine 1:100,000) (Daroupakhsh, Tehran, Iran) and placing a rubber dam. Under magnification (Carl Zeiss, Meditec Inc., Dublin, CA, USA), the temporary restoration was removed (Figure 2–4). After locating all the orifices (Figure 2–5) and estimating the working length with apex locator (Dempex, DEM Ltd., Barnstaple, Devon, England) and radiographic image (Figure 2–6), chemo‐mechanical debridement was completed by crown‐down technique with M3 rotary files (UDG, Changzhou, China) up to size 25/04 and frequent irrigation with 5.25% sodium hypochlorite and sterile saline.
FIGURE 2‐4.

Clinical view after removing the temporary filling material.
FIGURE 2‐5.

Clinical view after locating the actual orifices.
FIGURE 2‐6.

Negotiation and working length assessment.
Following drying the canals with paper points (META, Chugbuk, South Korea), the distal canal and the apical two‐thirds of mesial canals were obturated with gutta‐percha (META, Chugbuk, South Korea), and AH plus sealer (Dentsply DeTrey, Konstanz, Germany) using the warm vertical technique by FastFill warm obturator (Fast Fill Obturation System, Eighteeth, China). The coronal third of the mesial canals were filled with CC (SJM, Iran) to seal the strip perforation. The furcal perforation was also sealed using CC (SJM, Iran) following the manufacturers' order using MAP One carrier (Maillefer, Dentsply, Switzerland) and condensed by a #20 finger plugger (Maillefer, Dentsply, Switzerland) (Figure 2–7). Cavit (Cavisol, Tehran, Iran) was placed as the temporary restoration (Figures 2–8 and 2–9).
FIGURE 2‐7.

Repair of the perforations with CC.
FIGURE 2‐8.

Postoperative radiograph.
FIGURE 2‐9.

Postoperative angled radiograph showing the furcal and strip perforations.
4.5. Postoperative Care
Postoperative guidance was given, including oral hygiene instructions and analgesic medication (Ibuprofen 400 mg) for postoperative symptoms. After confirming the lack of clinical signs and symptoms in the 2‐week follow‐up, the patient was referred for permanent restoration. The tooth was restored with a full‐coverage PFM crown as the final restoration.
4.6. Follow‐Up
In the 3‐month follow‐up, the patient did not complain of any symptoms, and the radiographic examination exhibited favorable signs of healing and regeneration in the perforation areas (Figures 2‐10 and 2‐11). In the 11‐month follow‐up, there was no sign of inflammation. Both clinical and radiographic examinations exhibited significant healing of the lesions, tissue regeneration, and the tooth (Figures 2‐12, 2‐13, 2‐14).
FIGURE 2‐10.

Clinical examinations of 3‐month follow‐up showing tissue healing with no signs of inflammation.
FIGURE 2‐11.

Three‐month follow‐up exhibiting favorable healing of the lesions.
FIGURE 2‐12.

Clinical examinations in the 11‐month follow‐up showing tooth #19 with full cusp coverage PFM crown as the permanent restoration and healthy periodontium.
FIGURE 2‐13.

Clinical examinations in the 11‐month follow‐up showing tooth #19 fully functional in the occlusion.
FIGURE 2‐14.

11‐month follow‐up exhibiting significant healing and tissue regeneration with no signs of inflammation.
5. Case 3
5.1. Case History
A 31‐year‐old female patient was referred with a complaint of left maxillary second premolar (tooth #13). A problematic endodontic treatment was done 3 years ago, and recently, the patient has been experiencing severe pain and was forced to use analgesic medicine. A review of her medical history indicated a good health condition and no systemic disorder.
5.2. Examinations
Clinical examination indicated spontaneous pain accompanied by swelling of soft tissues, excessive bleeding on probing (BOP), and an 8 mm probing depth (Figure 3‐1). The tooth had been previously endodontically treated, and the periapical tissues were sensitive to both percussion and palpation. Radiographic examinations exhibited coronal and apical lesions and mesial perforation. Further examinations revealed that the palatal canal was missed, and an iatrogenic canal extending into the bone was made and obturated due to perforation (Figure 3‐2).
FIGURE 3‐1.

Clinical examination revealing a deep pocket in the mesial of tooth #13.
FIGURE 3‐2.

A periapical radiograph image showing coronal and apical lesions due to mesial perforation and missed canal.
5.3. Diagnosis
Based on the clinical and radiographic findings, the pulpal diagnosis was previous root canal treatment with signs of infection, and the periradicular diagnosis was secondary acute apical periodontitis with primary endodontic–secondary periodontal lesion.
5.4. Treatment
After anesthesia (2% lidocaine and epinephrine 1:100,000) (Daroupakhsh, Tehran, Iran) and rubber dam isolation, the access was prepared using a high‐speed diamond round bur No. 2 (Jota AG, Rüthi, Switzerland) under magnification (Carl Zeiss, Meditec Inc., Dublin, CA, USA) (Figure 3–3). The retreatment was done using size one Gates‐Glidden (MANI gates drills, MANI, Japan) for canals' obturation material and H‐files (MANI H‐files, MANI, Japan) for perforation's obturation material. Upon locating the canals' orifices, including the missed canal, the working length was determined with the apex locator (Dempex, DEM Ltd., Barnstaple, Devon, England) and radiographic image (Figure 3–4).
FIGURE 3‐3.

Clinical view of the mesial perforation.
FIGURE 3‐4.

Working length assessment.
After chemo‐mechanical debridement of the tooth by crown‐down technique with M3 rotary files (UDG, Changzhou, China) up to size 25/04 with 5.25% sodium hypochlorite and sterile saline solution, the canals were obturated with gutta‐percha (META, Chugbuk, South Korea) and AH plus sealer (Dentsply DeTrey, Konstanz, Germany) using the warm vertical technique by FastFill warm obturator (Fast Fill Obturation System, Eighteeth, China). The perforations were sealed with CC (SJM, Iran) following the manufacturer's instructions using MAP One carrier (Maillefer, Dentsply, Switzerland) and a #20 finger plugger (Maillefer, Dentsply, Switzerland) (Figure 3–5). The Cavit (Cavisol, Tehran, Iran) was used as the temporary restoration (Figure 3–6).
FIGURE 3‐5.

Repair of the mesial perforation with CC.
FIGURE 3‐6.
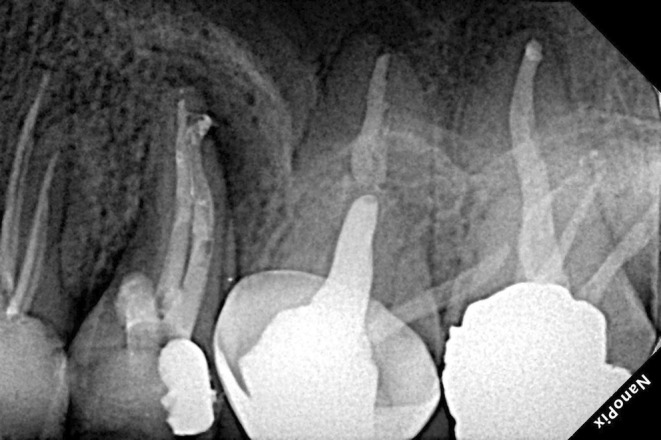
Postoperative radiograph.
5.5. Postoperative Care
The patient received postoperative guidance, including oral hygiene instructions and analgesic medication (Ibuprofen 400 mg) for postoperative symptoms. After confirming the lack of signs or symptoms, the patient was referred for the final restoration, and the tooth was restored with a ceramic onlay restoration.
5.6. Follow‐Up
In the 8‐month follow‐up, the patient did not complain of any signs or symptoms. The examinations displayed elimination of BOP, reduced probing depth to 3 mm, and significant dwindle of the lesions, indicating tissue regeneration and healing (Figure 3–7). Furthermore, the control radiograph showed favorable healing of the bone and lesions (Figure 3–8).
FIGURE 3‐7.

Clinical examination showing probing depth within normal limit, no bleeding on probing, and no signs of inflammation.
FIGURE 3‐8.

Eight‐month follow‐up shows nearly complete healing and tissue regeneration with no signs of inflammation.
6. Conclusion
Successful perforation repair requires an effective perforation seal, bone and periodontium regeneration, and the eradication of any infection, all of which were successfully achieved in the presented cases. Considering the obtained results and the complexities associated with root perforations, it can be concluded that CC is an appropriate repairing material due to its favorable properties. Therefore, CC can be considered a material of choice for treating root perforations.
7. Discussion
Numerous factors may contribute to root perforation, including pulp stones, calcifications, resorptions, tooth malposition, extra‐coronal restorations, or intracanal posts. Perforation may lead to an inflammatory response associated with degeneration of periodontal tissues and alveolar bone. Depending on the extent of the injury and potential risk of bacterial infection and inflammation, it may lead to the formation of granulation tissue, swelling, suppuration, periodontal pocket, alveolar bone resorption, and, in severe cases, extraction of the involved tooth [1, 2, 11]. Solid coronal seal, bone regeneration, and eradication of infection are necessities for a successful perforation repair [4]. The aim of repairing a root perforation is to preserve the integrity of the adjacent periodontium to prevent inflammation, attachment loss, and tissue regeneration in the event of periodontal breakdown [11].
Perforation management is challenging, and its prognosis depends on numerous factors, including the repairing material. Many materials have been used for perforation repair. However, many have proven inadequate due to insufficient sealing ability or biocompatibility. Consequently, new bioactive materials, such as MTA, Biodentine, and other bio‐ceramic materials, have been suggested for perforation treatment because of their superior properties and favorable clinical outcomes [5, 11, 12].
MTA has been the most widely used material for repairing root perforations [1, 2, 13]. Clinical studies have demonstrated that MTA provides a biocompatible and durable root perforation seal with a high success rate. MTA promotes cementum regeneration due to its excellent biocompatibility and osteoconduction properties, thereby facilitating the regeneration of the periodontium [1]. With increasing research on bioceramic materials, the selection of perforation‐repairing materials is expanding [14, 15].
Cold ceramic (CC) was first introduced in 2000 by Modaresi from Yazd University, Iran [9]. CC is an MTA‐like material that can serve multiple purposes. The primary elemental components of CC are calcium oxide, silicon oxide, barium oxide, and sulfur trioxide, comprising up to 93% of its components. It is reported that CC is prepared by mixing its white powder with its special liquid in an appropriate powder‐liquid ratio [9]. To date, CC has shown favorable properties. Like other bioactive materials, CC is biocompatible and nontoxic, and multiple studies have approved its biocompatibility [16, 17, 18].
Calcium hydroxide is the main component of CC, and it is biocompatible. Modaresi et al. confirmed the optimal biocompatibility of both MTA and CC by conducting a comparative analysis of the tissue responses to CC and MTA in rats. The results exhibited that MTA induced less inflammatory responses in a short observation period. However, CC might be more biocompatible for slightly extended periods. Nevertheless, both MTA and CC were confirmed to be biocompatible [17]. Khedmat et al. assessed the biocompatibility of MTA and CC in an in vitro study using periodontal ligament fibroblasts. The results showed that both materials were highly biocompatible, and fibroblasts favorably attached to the surface of both materials [10]. Additionally, it has been revealed that CC can trigger cementum deposition, bone formation, and periodontal ligament regeneration upon contact with tissues [8].
The sealing ability and marginal adaptation of CC have been assessed through various methods [9, 19, 20]. An in vitro study compared the sealing properties of MTA and CC in different environments using a dye penetration test. The results indicated that the sealing property of CC is better than MTA in blood‐contaminated conditions and similar to MTA in dry and saliva‐contaminated conditions [20]. Mokhtari et al. compared the marginal adaptation of CC with MTA using scanning electron microscopy. They concluded that both materials exhibited equivalent marginal adaptation, although there was a tendency toward higher interfacial adaptation in CC [19].
The bond strength of most materials is significantly reduced by moisture contamination from the tissue. In contrast, MTA sets in the presence of moisture and blood [1]. Consequently, set MTA can reach optimal strength and produce an excellent seal in the presence of tissue fluids, which do not affect its sealing ability. This characteristic is similar to CC, a calcium hydroxide‐based material, and its powder contains fine hydrophilic particles that set in the presence of moisture [9]. In the present case series, a completely dried‐out site was not attainable due to perforations. Nevertheless, the favorable outcomes observed during follow‐up indicate the excellent performance of CC in the presence of moisture and blood.
Several studies have assessed the marginal adaptation, microleakage, and other properties of MTA and CC in different conditions [21]. The optimum condition for MTA and CC is that their powder should be mixed with the suggested liquid by the manufacturer without any contamination, including blood [9, 21, 22]. However, Mokhtari et al. investigated the effect of blood contamination on the marginal adaptation of CC and MTA using scanning electron microscopes. The sealing properties of both materials were assessed by mixing their powder with blood instead of the recommended liquid. This in vitro study indicated that CC showed significantly superior marginal adaptation following complete blood contamination of powder than MTA [21].
According to the literature, MTA is the repairing material of choice in most cases. Yet, in some cases, MTA presents certain limitations, therefore necessitating the consideration of alternative materials. The main disadvantage of some available MTA formulations is the extended setting time [23]. In such clinical situations, preference should be given to other bioactive materials with shorter setting times [1]. It is reported that the primary setting time of CC is about 15 min, which is significantly shorter than MTA, which has been reported to be around 165 min. Furthermore, the complete setting of CC occurs within 24 h [20, 24].
Perforation size negatively affects the treatment prognosis. This was one of the challenges of the presented cases. The results showed that they were successfully treated with CC. Another challenging factor is the duration between injury and repair. In the third case, 3 years had passed since the perforation was created, and based on the literature, the prognosis was unfavorable. The perforation was sealed using CC, and the 8‐month follow‐up exhibited complete healing of the perforation's signs and symptoms.
Perforations are associated with the loss of excess tooth structure, and if they remain untreated, they can also lead to the destruction of the adjacent tissues. Subsequent treatments and the disruption of the structural integrity are likely to lead to an increased risk of fracture [25, 26]. This challenge was present in all the cases and was considered throughout the treatment. In all cases, the cleaning and shaping were done with minimum instrumentation and maximum irrigation to conserve the tooth structure as much as possible. In all cases, the final restoration was full cusp coverage to enhance the stress distribution and minimize the fracture risk. Ultimately, in the long‐term follow‐up, the teeth were fully functional in all three cases with no signs of fracture or inflammation.
The aim of repairing a perforation is to achieve mechanical and biological sealing of the connection between the periradicular tissues and the root canals using a biocompatible material that facilitates a favorable tissue healing response [27]. The postoperative radiograph showed that this had been achieved in all presented cases. Additionally, some material was extruded into the tissues, indicating that similar to MTA, CC exhibits minimal adverse effects on healing responses and periradicular tissues [1, 4], which was confirmed in the presented cases. Nevertheless, further studies are recommended in this field.
In addition to the current case series, CC has been successfully used in another study for strip perforation treatment [4]. In this study and previous studies, no clinical signs or symptoms in the postoperative follow‐up were noted, and the control radiograph has consistently exhibited remarkable tissue healing and regeneration [4]. Moreover, several clinical studies have demonstrated the efficiency of CC in various treatments, including root canal therapy and periapical lesions treatment, thereby further confirming its reliability [6, 7].
Evidence confirms that perforations can be successfully repaired with meticulous cleaning and shaping, sufficient disinfection, proficient handling of the repair material, and sufficient filling, followed by appropriate coronal restoration [28, 29]. Nonetheless, additional studies are recommended to investigate the clinical outcomes of CC in long‐term follow‐ups and to further compare CC with other bio‐ceramic materials in clinical practice.
Author Contributions
Ali Chamani: conceptualization, investigation, resources, writing – review and editing. Maryam Forghani: conceptualization, supervision, validation, writing – review and editing. Ghazal Asadi: visualization, writing – original draft, writing – review and editing.
Consent
Informed written consent was obtained from all patients prior to the initiation of treatment procedures.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding: The authors received no specific funding for this work.
Data Availability Statement
The clinical pictures and radiographs data that support the findings of this study are included in the article.
References
- 1. Clauder T., “Repair of Pulp Chamber and Root Perforations,” in Endodontic Advances and Evidence‐Based Clinical Guidelines (Wiley, 2022), 475–510. [Google Scholar]
- 2. Estrela C., Decurcio D. A., Rossi‐Fedele G., Silva J. A., Guedes O. A., and Borges Á. H., “Root Perforations: A Review of Diagnosis, Prognosis and Materials,” Brazilian Oral Research 32, no. 1 (2018): e73, 10.1590/1807-3107bor-2018.vol32.0073. [DOI] [PubMed] [Google Scholar]
- 3. Thamilselvan A. and Ramesh S., “Clinical Practice Guidelines for the Management of Endodontic Perforation,” Journal of Pharmacological Research 12 (2020): 4046–4053, 10.31838/ijpr/2020.SP2.501. [DOI] [Google Scholar]
- 4. Modaresi J., Parashos P., Mousavi R., Mirzaeeian A., and Almodaresi Z., “Treatment of Strip Perforation Using Cold Ceramic,” Dental Research Journal 20 (2023): 31. [PMC free article] [PubMed] [Google Scholar]
- 5. Kakani A. K., Veeramachaneni C., Majeti C., Tummala M., and Khiyani L., “A Review on Perforation Repair Materials,” Journal of Clinical and Diagnostic Research 9, no. 9 (2015): ZE09, 10.7860/jcdr/2015/13854.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Modaresi J., Yazdani Rostam A., Mahini F., and Nasr N., “Cold Ceramic as a Root Canal Filling Material: A Case Series,” Journal of Dental School, Shahid Beheshti University of Medical Sciences 42, no. 1 (2024): 49–55, 10.22037/jds.v42i1.44184. [DOI] [Google Scholar]
- 7. Modaresi J. and Nasr N., “Nonsurgical Endodontic Management of Large Periapical Lesion With Cold Ceramic: A Literature Review and Case Series,” Iranian Endodontic Journal 18, no. 2 (2023): 113–121, 10.22037/iej.v18i2.40184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Modaresi J., Almodaresi z., Mousavi R., and Mirzaeeian A., “Successful Root Canal Treatment With Cold Ceramic: A Case Report,” Journal of Mashhad Dental School 45, no. 3 (2021): 309–313, 10.22038/jmds.2021.52768.1956. [DOI] [Google Scholar]
- 9. Modaresi J. and Hemati H. R., “The Cold Ceramic Material,” Dental Research Journal 15, no. 2 (2018): 85–88. [PMC free article] [PubMed] [Google Scholar]
- 10. Khedmat S., Sarraf P., Seyedjafari E., Sanaei‐Rad P., and Noori F., “Comparative Evaluation of the Effect of Cold Ceramic and MTA‐Angelus on Cell Viability, Attachment and Differentiation of Dental Pulp Stem Cells and Periodontal Ligament Fibroblasts: An In Vitro Study,” BMC Oral Health 21, no. 1 (2021): 628, 10.1186/s12903-021-01979-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Siew K., Lee A. H., and Cheung G. S., “Treatment Outcome of Repaired Root Perforation: A Systematic Review and Meta‐Analysis,” Journal of Endodontics 41, no. 11 (2015): 1795–1804, 10.1016/j.joen.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 12. Puri T., “Non‐Surgical Management of Perforation,” in Pitt Ford's Problem‐Based Learning in Endodontology (Wiley, 2024), 271–278. [Google Scholar]
- 13. Silva R. A. B., Borges A. T. N., Hernandéz‐Gatón P., et al., “Histopathological, Histoenzymological, Immunohistochemical and Immunofluorescence Analysis of Tissue Response to Sealing Materials After Furcation Perforation,” International Endodontic Journal 52, no. 10 (2019): 1489–1500, 10.1111/iej.13145. [DOI] [PubMed] [Google Scholar]
- 14. Wang Z., “Bioceramic Materials in Endodontics,” Endodontic Topics 32, no. 1 (2015): 3–30, 10.1111/etp.12075. [DOI] [Google Scholar]
- 15. Modaresi J., Fakhari N., and Shokani M., “Comparison of the Sealing Ability of the CC Sealer and Endoseal MTA Sealer by the Dye Penetration Method: A Lab‐Based Experimental Study,” 2024.
- 16. Zare Jahromi M. and Razavi S. M., “The Comparative Effect of Cold Ceramic and PROROOT on the Inflammation of Periodontal Tissues After Sealing Furcal Perforation in Dog Teeth (A Histologic Study),” Journal of Dental School, Shahid Beheshti University of Medical Sciences 24, no. 4 (2007): 439–446. [Google Scholar]
- 17. Modaresi J., Yavari S. A., Dianat S. O., and Shahrabi S., “A Comparison of Tissue Reaction to MTA and an Experimental Root‐End Restorative Material in Rats,” Australian Endodontic Journal 31, no. 2 (2005): 69–72, 10.1111/j.1747-4477.2005.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 18. Mozayeni M. A., Salem Milani A., Alim Marvasti L., Mashadi Abbas F., and Modaresi S. J., “Cytotoxicity of Cold Ceramic Compared With MTA and IRM,” Iranian Endodontic Journal 4, no. 3 (2009): 106–111. [PMC free article] [PubMed] [Google Scholar]
- 19. Mokhtari F., Modaresi J., Javadi G., Davoudi A., and Badrian H., “Comparing the Marginal Adaptation of Cold Ceramic and Mineral Trioxide Aggregate by Means of Scanning Electron Microscope: An In Vitro Study,” Journal of International Oral Health 7, no. 9 (2015): 7–10. [PMC free article] [PubMed] [Google Scholar]
- 20. Hasheminia S. M., Nejad S. L., Dianat O., Modaresi J., and Mahjour F., “Comparing the Sealing Properties of Mineral Trioxide Aggregate and an Experimental Ceramic Based Root End Filling Material in Different Environments,” Indian Journal of Dental Research 24, no. 4 (2013): 474–477, 10.4103/0970-9290.118399. [DOI] [PubMed] [Google Scholar]
- 21. Mokhtari F., Mohammadhoseini M., Fakhari N., and Rostam A. Y., “Evaluation and Comparing the Tooth Discoloration Induced by Cold Ceramic and MTA Angelus as Endodontic Cements,” Journal of Dental Medicine 35 (2023): 22. [Google Scholar]
- 22. Torabinejad M., Parirokh M., and Dummer P. M. H., “Mineral Trioxide Aggregate and Other Bioactive Endodontic Cements: An Updated Overview—Part II: Other Clinical Applications and Complications,” International Endodontic Journal 51, no. 3 (2017): 284–317, 10.1111/iej.12843. [DOI] [PubMed] [Google Scholar]
- 23. Clauder T., “Present Status and Future Directions—Managing Perforations,” International Endodontic Journal 55, no. S4 (2022): 872–891, 10.1111/iej.13748. [DOI] [PubMed] [Google Scholar]
- 24. Parirokh M. and Torabinejad M., “Mineral Trioxide Aggregate: A Comprehensive Literature Review—Part I: Chemical, Physical, and Antibacterial Properties,” Journal of Endodontics 36, no. 1 (2010): 16–27, 10.1016/j.joen.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 25. Askerbeyli Örs S., Aksel H., Küçükkaya Eren S., and Serper A., “Effect of Perforation Size and Furcal Lesion on Stress Distribution in Mandibular Molars: A Finite Element Analysis,” International Endodontic Journal 52, no. 3 (2019): 377–384, 10.1111/iej.13013. [DOI] [PubMed] [Google Scholar]
- 26. Elwazan G. I., Roshdy N. N., and Abdelaziz S., “Effect of Mid‐Root Perforation and Its Repair on Stress Distribution and Fracture Resistance: A 3D Finite Element Analysis and In Vitro Study,” BMC Oral Health 24, no. 1 (2024): 1340, 10.1186/s12903-024-05066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pace R., Giuliani V., and Pagavino G., “Mineral Trioxide Aggregate as Repair Material for Furcal Perforation: Case Series,” Journal of Endodontics 34, no. 9 (2008): 1130–1133, 10.1016/j.joen.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 28. Pontius V., Pontius O., Braun A., Frankenberger R., and Roggendorf M. J., “Retrospective Evaluation of Perforation Repairs in 6 Private Practices,” Journal of Endodontics 39, no. 11 (2013): 1346–1358, 10.1016/j.joen.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 29. Morocho‐Monteros D., Masson‐Palacios M. J., and Parise‐Vasco J. M., “Management of Maxillary Incisors With Middle‐Third Root Perforation: A Case Report,” Case Reports in Dentistry 2024, no. 1 (2024): 5957016, 10.1155/2024/5957016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The clinical pictures and radiographs data that support the findings of this study are included in the article.


