Abstract
Flow diverter (FD) deployment combined with coil therapy is effective and considered superior to FD deployment alone for treating large, complex anterior circulation aneurysms. However, the optimal strategy for coil usage in posterior circulation aneurysms, particularly intracranial vertebral artery dissection aneurysms (IVADAs), remains unclear. This study used patient-specific aneurysm models and finite element analysis to determine the ideal packing density (PD) of coils following FD placement in IVADAs. We prospectively analyzed 22 patients with 24 aneurysms, all treated with FD at our hospital. Hemodynamic parameters were analyzed before treatment, after FD alone, and at three different coiling rates (5%, 15%, and 25%) using software simulation. All 22 patients underwent FD procedures to treat IVADAs. FD deployment and additional coil use both reduced the inflow rate at the aneurysm neck, the inflow concentration index, and the mean velocity in the aneurysm. However, compared with FD treatment alone, coils provided a smaller reduction in these parameters. No significant difference in the reduction ratio was observed when the coiling PD increased from 5 to 15% and then to 25%. Further coil addition beyond a 5% PD produced no notable hemodynamic benefits. Adjunct coiling improves the post-FD hemodynamic environment of treated IVADAs. However, dense packing is unnecessary because the intra-aneurysmal hemodynamics tend to stabilize once the PD reaches approximately 5%.
Keywords: Hemodynamic, Flow diverter, Vertebral artery, Dissection aneurysms, Coil
Introduction
Intracranial vertebral artery dissection aneurysm (IVADA) is a very rare subtype of intracranial aneurysm that, when ruptured, is associated with high morbidity and mortality rates [1–4]. The optimal treatment method for IVADA has not yet been clearly established. Endovascular therapy has become a first-line treatment for IVADA and includes techniques such as deconstructive therapy (e.g. vessel sacrifice), overlapping stent placement and stent-assisted coiling [5–7]. With advancements in endovascular devices, flow diverters (FDs) have been used in treating these cases. For large and giant aneurysms, FDs offer significant advantages over conventional coil embolization [8, 9]. FD deployment combined with coiling may be more effective in achieving aneurysm occlusion than FD deployment alone [10, 11]. Although FDs were originally designed as a stand-alone treatment, many practitioners prefer using FDs in combination with coil embolization. Compared with loose packing, dense packing did not enhance the treatment effectiveness but increased the treatment cost [12]. Loose packing is often performed to minimize manipulation within the aneurysm sac because excessive manipulation increases the risk of intraoperative rupture. However, the above studies are rather old and the topic is still debated.
Treating ruptured aneurysms with FDs is more challenging than treating unruptured aneurysms. In ruptured aneurysms, coiling is advised if possible as FD alone traditionally requires time to reconstruct the vessel, during which a ruptured aneurysms has a high risk of re-rupture. Although using more coils may reduce the risk of re-rupture, it can also increase the risk of thromboembolic complications. Therefore, an appropriate packing density (PD) is needed to balance these risks. One study showed that intra-aneurysmal hemodynamics tend to stabilize when the PD reaches an average of 7.06% [13]. However, this study focused on individual cases of internal carotid artery aneurysms; the PD for IVADAs has not yet been reported. Based on hemodynamic simulation studies, adding coils should reduce cavity flow velocity and wall shear stress, promoting thrombosis formation and aneurysm occlusion. This approach should increase the rate of aneurysm occlusion and decrease the recurrence rate compared with using an FD alone.
In this study, we explored the hemodynamic changes induced by coil packing after FD deployment to identify a balanced point for loose packing in these cases. Important parameters associated with aneurysm rupture include the inflow rate at the aneurysm neck (Qinflow) [14–16], the concentration of the flow stream entering the aneurysm sac (inflow concentration index [ICI]) [17, 18], and the mean velocity within the aneurysm (Va) [19]. We hypothesized that coils provide no further hemodynamic benefit once the PD reaches a certain threshold. To investigate this, we studied the coil embolization strategy following FD deployment using patient-specific aneurysm models from 22 patients treated at our center. We then applied finite element analysis to create a more accurate post-treatment computational fluid dynamics (CFD) model, simulating the real therapeutic process involving the FD and all coils [20]. The hemodynamic effects of different coil densities were analyzed, assuming the FD had already been implanted.
Materials and methods
Patient population
This study was approved by the Ethics Committee on Scientific Research of Qilu Hospital, Shandong University. Informed consent was obtained from each patient or their relatives, and all data were collected anonymously. We prospectively included 22 representative patients with 24 aneurysms who were consecutively treated with FDs at our hospital from March 2020 to March 2022. Of these, 2 were ruptured aneurysms and 22 were unruptured. All patients were diagnosed with IVADA through digital subtraction angiography (DSA), and each aneurysm was treated with a single FD. The decision to combine FD treatment with coil embolization was made based on the individual circumstances of each case.
The inclusion criteria were either absence of symptoms or symptoms of intracranial hypertension with subarachnoid hemorrhage; a preliminary diagnosis of IVADA via computed tomography angiography or magnetic resonance angiography, confirmed by DSA; and IVADA involving the intracranial segment of the vertebral artery (V4 segment). The exclusion criteria were subarachnoid hemorrhage caused by trauma or other cerebrovascular diseases, IVADA involving the extracranial vertebral artery, and prolonged dilation of the vertebrobasilar artery due to tortuosity.
FD deployment and apposition were successful in all patients. The FDs used in this study were the Tubridge (MicroPort NeuroTech, Shanghai, China) and Pipeline (Medtronic, Minneapolis, MN, USA).
Model reconstruction
Patient-specific aneurysm and vessel models were reconstructed from three-dimensional DSA images in DICOM format, which were then exported as initial STL models. The untreated STL models were imported into Geomagic Wrap 2015 (Geomagic, Research Triangle Park, NC, USA) for trimming and smoothing of the aneurysm and artery models.
Finite element simulation
Based on the reconstructed aneurysm models, we performed virtual implantation of the stents and coils following the method established by Leng et al. [20]. First, the FDs were modeled using NX 12.0 (Siemens PLM Software, Plano, TX, USA), while the coils were modeled using MATLAB (MathWorks, Natick, MA, USA). Virtual implantation of the FD and coils was carried out in ABAQUS version 6.14 (SIMULIA, Providence, RI, USA). The FD was compressed and delivered to the landing zone via a microcatheter, and deployment was achieved by withdrawing the catheter. Coil implantation was simulated in two steps: the coil was first pulled into the microcatheter, then pushed into the aneurysm sac. All models were saved in STL format for subsequent analysis.
CFD simulation
Aneurysm, FD, and coil models were used to simulate the hemodynamic environment. Each aneurysm was virtually treated with an FD alone and with FDs combined with 5%, 15%, and 25% coil PD. The PD was defined as the ratio of coil volume to aneurysm volume. For simulation purposes, all models were imported into ANSYS ICEM CFD version 16.2 (ANSYS Inc., Canonsburg, PA, USA) to generate a mesh. The global mesh size was set to 0.16 mm, with the FD mesh size set to 1/6 of the wire circumference and the coil mesh size set to 0.1 mm [21]. The generated mesh was then imported into ANSYS CFX version 2019 (ANSYS Inc.) to perform calculations based on the Navier–Stokes equations. Blood was modeled as an incompressible, laminar, Newtonian fluid with a density of 1056 kg/m3 and viscosity of 0.0035 kg/m·s. The vessel wall was assumed to be rigid with no-slip conditions. The inflow rate was set to 4.6 mL/s, and the outlet flow rate was calculated according to Murray’s law of flow distribution [22].
The Qinflow, ICI, and Va were calculated. To evaluate the effects of the FDs and coils, the reduction ratio was also determined following the methodology outlined by Zhang et al. [22] The reduction ratio for each parameter (X) was calculated using the following equations:
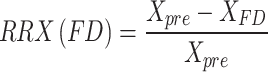 |
 |
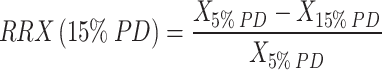 |
 |
Statistical analysis
Statistical analysis was performed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism version 7.5.0 (GraphPad Software, La Jolla, CA, USA). The normality of each continuous variable was confirmed. The reduction ratios for the different treatments were analyzed using one-way analysis of variance followed by multiple comparisons. Regression models were used to assess the relationship of PD with Qinflow, ICI, and Va. A p-value of < 0.05 was considered statistically significant.
Results
Patient characteristics
In total, 22 patients with 24 IVADAs were enrolled. The patients comprised 9 women and 13 men, and 21 of the 24 aneurysms were unruptured. The mean age of the patients was 57.21 years (range, 38–74 years). Among the 22 patients, 6 experienced headaches, 4 experienced dizziness, and 2 experienced unilateral limb weakness; the remaining patients were asymptomatic. Four of the 24 aneurysms were treated with a combination of coils and an FD, while the rest were treated with an FD alone. Specifically, 5 were treated with the Tubridge and 15 were treated with the Pipeline. The basic information for all aneurysms is presented in Table 1.
Table 1.
Clinical characteristics and demographics of study patients
| Case NO. | Age (Y) | Gender | Location | SAH | LD (mm) | Volume (mm3 ) | FD type | Size of FD (cm×mm) | PD (%) | mRS | Radiographic outcomes | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 70s | F | R-VA | N | 6.8 | 157.838 | PEP | 3.5 × 35 | - | 2 | - | 33 |
| 2 | 40s | M | R-VA | Y | 7.3 | 710.682 | TUB | 3.0 × 15 | - | 1 | CO | 3 |
| 3 | 60s | M | R-VA | N | - | 20.239 | TUB | 4.5 × 25 | 24 | 2 | ISS | 8 |
| 4 | 60s | M | R-VA | N | - | 44.026 | TUB | 4.5 × 25 | 15 | 2 | ISS | 8 |
| 5 | 40s | M | L-VA | N | 10.8 | 52.788 | TUB | 3.0 × 25 | - | 1 | CO | 7 |
| 6 | 50s | F | L-VA | N | 12.5 | 385.706 | PEP | 4.0 × 20 | - | 1 | CO | 6 |
| 7 | 40s | M | R-VA | N | 23 | 490.594 | PEP | 4.0 × 30 | - | 0 | CO | 7 |
| 8 | 70s | F | L-VA | N | 6.2 | 85.697 | PEP | 3.5 × 25 | - | 1 | - | 24 |
| 9 | 40s | M | L-VA | N | 8.6 | 29.306 | PEP | 4.0 × 20 | - | 1 | CO | 6 |
| 10 | 70s | M | R-VA | N | 9.2 | 263.030 | PEP | 3.5 × 25 | - | 1 | CO | 6 |
| 11 | 70s | F | L-VA | N | 7.6 | 113.192 | PEP | 3.0 × 25 | - | 1 | CO | 12 |
| 12 | 60s | M | R-VA | N | 9.4 | 41.201 | PEP | 4.5 × 30 | - | 1 | - | 6 |
| 13 | 60s | F | L-VA | N | 4.4 | 47.367 | PEP | 4.25 × 20 | - | 1 | CO | 9 |
| 14 | 30s | M | L-VA | N | 4.3 | 31.167 | TUB | 3.5 × 25 | - | 5 | CO | 6 |
| 15 | 50s | M | L-VA | N | 13.1 | 166.633 | PEP | 4.25 × 20 | - | 1 | CO | 6 |
| 16 | 60s | F | L-VA | Y | 4 | 19.625 | PEP | 2.75 × 20 | - | 5 | CO | 7 |
| 17 | 50s | M | L-VA | N | 11.4 | 123.203 | PEP | 4.5 × 30 | - | 1 | CO | 6 |
| 18 | 50s | F | L-VA | N | 14.88 | 545.495 | PEP | 4.5 × 30 | 15 | 1 | CO | 4 |
| 19 | 50s | M | L-VA | N | 5.5 | 51.669 | TUB | 3.5 × 15 | - | 1 | - | 17 |
| 20 | 60s | F | L-VA | N | 25.8 | 79.886 | PEP | 4.0 × 35 | - | 2 | - | 14 |
| 21 | 40s | M | L-VA | N | 5 | 8.882 | TUB | 3.5 × 20 | - | 1 | CO | 6 |
| 22 | 50s | M | R-VA | N | 17.31 | 656.217 | PEP | 4.75 × 30 | - | 1 | CO | 12 |
| 23 | 50s | F | L-VA | N | 4.81 | 20.227 | PEP | 3.5 × 35 | - | 1 | CO | 6 |
| 24 | 50s | F | L-VA | N | 5.81 | 19.896 | PEP | 3.5 × 35 | - | 1 | CO | 6 |
F, female; M, male; R, right; L, left; VA, vertebral artery; Y, yes; N, no; LD, largest/longest diameter; MC, metal coverage; mRS, modified Rankin scale; PD, packing density; FD, flow diverter; CO, complete occlusion; PEP, Pipeline; TUB, Tubridge; ISS, in-stent stenosis; SAH, subarachnoid hemorrhage
Clinical and radiological follow-up
All patients in this study underwent endovasclue treatment without acute complications, and there were no deaths. Clinical follow-up was conducted for all survivors over a period of 3 to 33 months (median, 6 months). Two cases developed cerebral infarction, but their symptoms completely disappeared during follow up. Angiographic follow-up data were available for 17 (77.27%) of the 22 patients. One (5.88%) patient had remnants of the aneurysm, and one (5.88%) patient developed asymptomatic in-stent restenosis. The overall complete occlusion rate was 94.12%.
Hemodynamics of FD
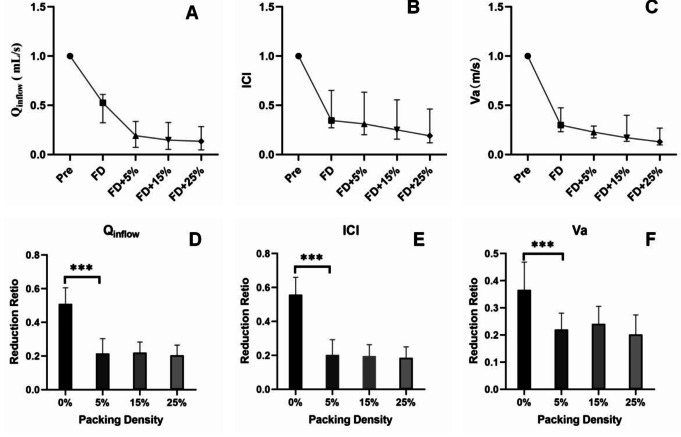
Following FD implantation, both blood flow and flow velocity into the aneurysm decreased. The mean values for Qinflow, ICI, and Va before implantation were 0.479 (0.257–1.077) mL/s, 1.046 (0.373–2.095), and 0.030 (0.018–0.109) m/s, respectively. After FD implantation, these parameters decreased to 0.218 (0.113–0.426) mL/s, 0.363 (0.155–0.761), and 0.014 (0.005–0.033) m/s, respectively (Fig. 1a–c). The reduction ratios of Qinflow, ICI, and Va after FD deployment were 46.33%, 62.36%, and 67.20%, respectively. A representative case illustrating the changes in flow impingement and velocity within the aneurysm after FD and coil implantation, at varying coiling PD levels, is shown in Fig. 2.
Fig. 1.
(A–C) Mean values of Qinflow, ICI, and Va before and after FD and coil implantation. (D–F) Analysis of covariance between the two groups for Qinflow, ICI, and Va, showing no significant reduction after FD with 5% coil PD. A p-value of < 0.05 was considered statistically significant. ***p < 0.001. FD, flow diverter; PD, packing density
Fig. 2.
Two representative cases showing hemodynamic changes in a vertebral artery aneurysm after FD and coil implantation at different coiling PDs. (A) DSA image before treatment. (B) Aneurysm model before virtual implantation. (C) DSA image after treatment. (D) DSA image showing the deployed FD. (E) Aneurysm model after virtual implantation of FD. (Fa) Flow impingement before treatment. (Fb) Flow impingement after FD deployment. (Fc–e) Flow impingement after FD and 5%, 15%, and 25% coils in PD. (Ga) Velocity distribution before treatment. (Gb) Velocity distribution after FD deployment. (Gc–e) Velocity distribution after FD and 5%, 15%, and 25% coils in PD. FD, flow diverter; PD, packing density; DSA, digital subtraction angiography
Hemodynamics of coils after FD deployment
After FD deployment, the addition of coils further reduced Qinflow, ICI, and Va, although the reductions were smaller than with the FD alone (Fig. 1a–c). The mean Qinflow after the implantation of 5%, 15%, and 25% coiling PDs was 0.198 (0.096–0.353) mL/s, 0.157 (0.076–0.353) mL/s, and 0.138 (0.070–0.317) mL/s, respectively. The mean ICI values were 0.322 (0.126–0.709), 0.279 (0.100–0.656), and 0.231 (0.089–0.605), respectively. The mean Va values were 0.013 (0.004–0.028) m/s, 0.011 (0.003–0.025) m/s, and 0.009 (0.002–0.023) m/s, respectively.
The effects of FD and coils were compared using one-way analysis of variance and multiple comparisons. The reduction ratio with FD alone was significantly greater than that observed with 5% coiling PD (p < 0.001) for all parameters (Qinflow, ICI, and Va), as shown in columns labeled 0% and 5% in Fig. 2d to f. However, there was no significant difference in the reduction ratio when the coiling PD increased from 5 to 15% and subsequently to 25%.
Discussion
This study aimed to explore the strategy for coil embolization following FD deployment in patients with IVADA from a hemodynamic perspective. We believe the results reflect the hemodynamic changes and may help determine the optimal PD when using an FD. Using high-fidelity finite element simulations of both FDs and coils, we analyzed the hemodynamic changes in a cohort of patients. The focus was on the necessity of coil usage and how to perform coil embolization after FD deployment in patients with IVADA. We found that although coil use contributed to intra-aneurysmal flow reduction, further packing beyond an average PD of 5% may be unnecessary.
One study indicated that hemodynamics can be used to estimate clinical outcomes following FD deployment [23]. It is valuable to analyze and discuss aneurysm outcomes after FD treatment. Several clinical issues, including aneurysm occlusion and delayed hemorrhage post-FD placement, have been reported using hemodynamic methods [24]. Li et al. [25] showed that unstable flow patterns and higher energy loss after Pipeline FD placement in intracranial aneurysm treatment could be key hemodynamic risk factors for delayed aneurysm rupture. Hirato et al. [26] and Rouchaud et al. [27] stated that increase in aneurysmal pressure and autolysis of the aneurysmal wall due to inflammation induced by thrombus formation in the aneurysm may be the risks of delayed aneurysm rupture in large aneurysms treated with FD. Other studies by Chen et al. [28] and Darsaut et al. [29] highlighted that persistent high inflow jet impingement, elevated wall shear stress areas, and abnormal pressure increases on the aneurysm wall might lead to postoperative rupture and bleeding, while additional coils may accelerate thrombus formation, obstructing inflow. Cebral et al. [30] suggested that higher pressure and continued inflow post-FD placement might contribute to delayed rupture. Therefore, supplementary loose coil packing is sometimes considered a safe and effective treatment for aneurysms at high risk of rupture. However, no cases of delayed hemorrhage occurred in our study, which may have been due to the small sample size. Further research with larger cohorts is warranted. However, in clinical practice, dense embolization is still recommended for patients with ruptured aneurysms because of the need of anti aggregation therapy after FD.
In our study, 21 patients had aneurysms smaller than 15 mm; only 1 patient had an aneurysm larger than 25 mm. One patient with concurrent aneurysms treated with FD and coiling developed postoperative cerebral infarction. Fortunately, the symptoms resolved with dual antiplatelet therapy, although follow-up at 8 months revealed 60% in-stent restenosis. The space-occupying effect of the coil may compress perforator vessels, causing ischemic infarction, while excessive coil extrusion into the stent or parent artery may increase intimal hyperplasia, increasing the risk of restenosis. The PDs for the two above-mentioned aneurysms were 24% and 15%, far exceeding the 5% PD identified as optimal in this study. By contrast, all other patients had complete aneurysm resolution and excellent parent artery patency. Based on our results, small and medium-sized IVADAs seem to require FD treatment alone, but large IVADAs present challenges with treatment strategies such as FD alone, FD with coil embolization, or stent-assisted coiling. Practitioners often prefer FD combined with loose packing, but there are no guidelines regarding the optimal PD for IVADA treatment with an FD.
Many studies have shown that an FD combined with coils is more effective than an FD alone and reduces the risk of delayed aneurysm rupture [31, 32]. One study revealed that an FD combined with coiling significantly improved the occlusion rate for medium aneurysms without increasing complications [33], similar to our findings. A multicenter retrospective study also showed that the occlusion rate with an FD and coiling (85.9%) was significantly higher than with an FD alone (77.1%) (p < 0.001) [34]. Lin et al. [35] found that an FD with coils achieved a higher occlusion rate than an FD alone (93.1% vs. 74.7%, p = 0.03). However, coiling could also increase the total complication risk, prolong procedure time, and increase the total cost. Loose packing is commonly used, but there is no standard for the degree of embolization. In this study, one patient treated with an FD alone showed a residual aneurysm at 7 months, with a small amount of contrast retention and subtotal occlusion. This raises the question of whether appropriate coil packing might increase the rate of complete aneurysm occlusion. Damiano et al. [36] found that coils provided no additional hemodynamic benefit until the PD exceeded 11%, although their model was idealistic. Chen et al. [37] used CFD methods to study aneurysm outcomes and showed that in patients treated with an FD and coils (mean PD of 9.84%), the intra-aneurysmal velocity decreased by 76.3%, compared with 55.8% for an FD alone. However, these studies focused on anterior circulation, and no hemodynamic studies have examined the necessary number of coils after FD placement in patients with IVADA.
Modeled after Zhang et al.’s [13] research, we also used a series of simulations based on clinical therapy to create idealized models for patients with IVADA. Our study shows that coil packing can further reduce intra-aneurysmal velocity, ICI, and Qinflow. Based on these results, the use of coils may improve the post-FD hemodynamic effect in treated aneurysms. As PD increases, the intra-aneurysmal blood flow velocity gradually decreases, indicating that the impact of additional coils becomes more significant. However, for unruptured aneurysms treated with an FD, excessive packing of the aneurysm sac may be unnecessary and could even increase the risk of rupture [38]. Excessive packing in aneurysms with a higher PD could lead to adverse outcomes because of the excellent blood flow diversion capability of the FD.
The goal of this study was to identify the critical PD value for effective hemodynamic benefits during coil packing. Through high-fidelity finite element simulations, we aimed to avoid unnecessary packing after the optimal PD is reached. Our results showed that intra-aneurysmal hemodynamics tended to stabilize as PD approached an average of 5% with FD in place. This finding differs from Zhang et al.’s [13] study, which identified a threshold of 7.06%. One explanation for this discrepancy is the difference between the internal carotid artery and the vertebral artery. The internal carotid artery has a larger diameter and faster blood flow than the vertebral artery, and it therefore may require a higher PD to block the flow into the aneurysm cavity under the same metal coverage and mesh density. Additionally, Zhang et al.’s [13] study was based on actual clinical coil packing, while our study involved simulated coil packing.
In our simulation, intra-aneurysmal hemodynamics stabilized around a 5% PD, but in our actual cohort of 24 cases, only 3 aneurysms were embolized with coils. The PDs in these cases were 15%, 24%, and 15%, respectively, far exceeding the 5% threshold found in our simulation. Therefore, more real-world cases are needed to verify if there is a statistically significant difference between a 5% PD and higher PDs. Nevertheless, our findings may assist physicians in determining how many coils are needed to achieve the best hemodynamic results when treating IVADA with an FD.
Based on our preliminary findings, we propose a more meaningful future investigation. This study would focus on unruptured IVADAs, with grouping criteria based on aneurysm neck and depth. The control group would receive FD treatment alone, while the observation group would undergo FD treatment combined with coil embolization. We would aim to measure changes in hemodynamic parameters before and after coil embolization to compare the effects of different levels of embolization on treatment outcomes. The goal would be to determine the necessity of coil embolization in unruptured IVADAs and, if required, to identify the optimal degree of embolization for aneurysms of various sizes.
Limitations of the study
This study had several limitations. First, the small sample size may have affected the accuracy of the results. Larger prospective studies with more comprehensive assessments are necessary in the future. Second, we used a uniformly pulsatile inflow boundary condition, which could have influenced the hemodynamic results. Third, because this was a single-center study, it was subject to inherent biases in patient selection. Furthermore, aneurysm sizes varied among the patients, resulting in differences in PD. Finally, the biological process of thrombosis formation is too complex to simulate and was therefore omitted. This idealized simulation requires validation with real cases at different embolization rates and long-term follow-up results.
Conclusion
From a hemodynamic perspective, adjunct coiling can enhance the post-FD hemodynamic environment of treated IVADA. However, dense packing is unnecessary, because the intra-aneurysmal hemodynamics tend to stabilize once the PD reaches an average of 5%.
Acknowledgements
We thank Angela Morben, DVM, ELS, from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
Author contributions
TZ and WZ: article drafting and writing. DZ and YX: Data collection and statistics. JZ and ML: collection of original data. SW, DW, and WY reviewed the article. All authors contributed to the article and approved the submitted version.YW is the author acting as a guarantor.
Funding
This study was sponsored by International Scientific Exchange Foundation of China (No. Z2018LSD001),and the National Natural Science Foundation of Shandong Province (ZR2022MH263, ZR2022MH131).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval
This retrospective study was approved by the Ethics Committee of Qilu Hospital of Shandong University.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kim BM, Shin YS, Baik MW et al (2016) Pipeline Embolization device for Large/Giant or Fusiform aneurysms: an initial Multi-center experience in Korea[J]. Neurointervention 11(1):10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zang YZ, Wang ZG, Wang CW et al (2016) [Clinical analysis of endovascular strategies in the treatment of vertebrobasilar dissecting aneurysms][J]. Zhonghua Yi Xue Za Zhi 96(41):3329–3332 [DOI] [PubMed] [Google Scholar]
- 3.Santos-Franco JA, Zenteno M, Lee A (2008) Dissecting aneurysms of the vertebrobasilar system. A comprehensive review on natural history and treatment options[J]. Neurosurg Rev 31(2):131–140 [DOI] [PubMed] [Google Scholar]
- 4.Ro A, Kageyama N, Abe N et al (2009) Intracranial vertebral artery dissection resulting in fatal subarachnoid hemorrhage: clinical and histopathological investigations from a medicolegal perspective[J]. J Neurosurg 110(5):948–954 [DOI] [PubMed] [Google Scholar]
- 5.Urasyanandana K, Withayasuk P, Songsaeng D et al (2017) Ruptured intracranial vertebral artery dissecting aneurysms: an evaluation of prognostic factors of treatment outcome[J]. Interv Neuroradiol 23(3):240–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madaelil TP, Wallace AN, Chatterjee AN et al (2016) Endovascular parent vessel sacrifice in ruptured dissecting vertebral and posterior inferior cerebellar artery aneurysms: clinical outcomes and review of the literature[J]. J Neurointerv Surg 8(8):796–801 [DOI] [PubMed] [Google Scholar]
- 7.Zhao KJ, Fang YB, Huang QH et al (2013) Reconstructive treatment of ruptured Intracranial spontaneous vertebral artery dissection aneurysms: long-term results and predictors of unfavorable Outcomes[J]. PLoS ONE 8(6):e67169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalouhi N, Tjoumakaris S, Starke RM et al (2013) Comparison of flow diversion and coiling in large unruptured intracranial saccular aneurysms[J]. Stroke 44(8):2150–2154 [DOI] [PubMed] [Google Scholar]
- 9.Walcott BP, Stapleton CJ, Choudhri O et al (2016) Flow Diversion for the treatment of intracranial Aneurysms[J]. JAMA Neurol 73(8):1002–1008 [DOI] [PubMed] [Google Scholar]
- 10.Mcauliffe W, Wycoco V, Rice H et al (2012) Immediate and midterm results following treatment of unruptured intracranial aneurysms with the pipeline embolization device[J]. AJNR Am J Neuroradiol 33(1):164–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson PK, Lylyk P, Szikora I et al (2011) The pipeline embolization device for the intracranial treatment of aneurysms trial[J]. Ajnr Am J Neuroradiol 32(1):34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong X, Han M, Xue X et al (2023) Coiling embolization strategy for medium-to-giant-sized intracranial aneurysms treated with pipeline embolization device: a propensity score-weighted study[J]. Eur Radiol 33(11):7967–7977 [DOI] [PubMed] [Google Scholar]
- 13.Zhang M, Tian Z, Zhang Y et al (2023) How to perform intra-aneurysmal coil embolization after Pipeline deployment: a study from a hemodynamic viewpoint[J]. J Neurointerv Surg 15(2):157–162 [DOI] [PubMed] [Google Scholar]
- 14.Futami K, Misaki K, Uno T et al (2020) Effect of Neck size on the inflow magnitude evaluated on 4D Flow MRI in Unruptured Internal Carotid Artery Aneurysms[J]. J Stroke Cerebrovasc Dis 29(10):105116 [DOI] [PubMed] [Google Scholar]
- 15.Su T, Reymond P, Brina O et al (2020) Large Neck and strong ostium inflow as the potential causes for delayed occlusion of Unruptured Sidewall Intracranial aneurysms treated by Flow Diverter[J]. Ajnr Am J Neuroradiol 41(3):488–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang HH, Sayre J, Dinh H et al (2023) Image-derived Metrics quantifying hemodynamic instability predicted growth of Unruptured Intracranial Aneurysms[J]. Stroke Vasc Interv Neurol 3(1) [DOI] [PMC free article] [PubMed]
- 17.Kojima M, Irie K, Fukuda T et al (2012) The study of flow diversion effects on aneurysm using multiple enterprise stents and two flow diverters[J]. Asian J Neurosurg 7(4):159–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbour MC, Chassagne F, Chivukula VK et al (2021) The effect of Dean, Reynolds and Womersley numbers on the flow in a spherical cavity on a curved round pipe. Part 2. The haemodynamics of intracranial aneurysms treated with flow-diverting stents[J]. J Fluid Mech 915 [DOI] [PMC free article] [PubMed]
- 19.Stahl J, Marsh L, Thormann M et al (2023) Assessment of the flow-diverter efficacy for intracranial aneurysm treatment considering pre- and post-interventional hemodynamics[J]. Comput Biol Med 156:106720 [DOI] [PubMed] [Google Scholar]
- 20.Leng X, Wang Y, Xu J et al (2018) Numerical simulation of patient-specific endovascular stenting and coiling for intracranial aneurysm surgical planning[J]. J Transl Med 16(1):208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang Y, Lu G, Ge L et al (2022) Hemodynamic Comparison of Treatment Strategies for intracranial vertebral artery fusiform Aneurysms[J]. Front Neurol 13:927135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiang J, Natarajan SK, Tremmel M et al (2011) Hemodynamic-morphologic discriminants for intracranial aneurysm rupture[J]. Stroke 42(1):144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujimura S, Brehm A, Takao H et al (2022) Hemodynamic characteristics and clinical outcome for intracranial aneurysms treated with the Derivo Embolization Device, a Novel Second-Generation Flow Diverter[J]. World Neurosurg 159:e252–e259 [DOI] [PubMed] [Google Scholar]
- 24.Kang H, Zhou Y, Luo B et al (2021) Pipeline Embolization device for intracranial aneurysms in a large Chinese cohort: complication risk factor Analysis[J]. Neurotherapeutics 18(2):1198–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li W, Tian Z, Zhu W et al (2019) Hemodynamic Analysis of Postoperative Rupture of Unruptured Intracranial Aneurysms after Placement of Flow-Diverting stents: a matched case-control Study[J]. Ajnr Am J Neuroradiol 40(11):1916–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirato M, Tsumoto T, Kobayashi Y et al (2023) Delayed rupture of a large intracranial internal carotid artery aneurysm after flow diverter placement[J]. Surg Neurol Int 14:446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rouchaud A, Brinjikji W, Lanzino G et al (2016) Delayed hemorrhagic complications after flow diversion for intracranial aneurysms: a literature overview[J]. Neuroradiology 58(2):171–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen S, Bai B, LV N et al (2021) Hemodynamic analysis and implantation strategies of delayed intracranial aneurysm rupture after flow diverter treatment[J]. Ann Transl Med 9(23):1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darsaut TE, Rayner-Hartley E, Makoyeva A et al (2013) Aneurysm rupture after endovascular flow diversion: the possible role of persistent flows through the transition zone associated with device deformation[J]. Interv Neuroradiol 19(2):180–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cebral JR, Mut F, Raschi M et al (2011) Aneurysm rupture following treatment with flow-diverting stents: computational hemodynamics analysis of treatment[J]. AJNR Am J Neuroradiol 32(1):27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sweid A, Atallah E, Herial N et al (2018) Pipeline-assisted coiling versus pipeline in flow diversion treatment of intracranial aneurysms[J]. J Clin Neurosci 58:20–24 [DOI] [PubMed] [Google Scholar]
- 32.Park MS, Nanaszko M, Sanborn MR et al (2016) Re-treatment rates after treatment with the Pipeline Embolization device alone versus Pipeline and coil embolization of cerebral aneurysms: a single-center experience[J]. J Neurosurg 125(1):137–144 [DOI] [PubMed] [Google Scholar]
- 33.Wang C, Luo B, LI T et al (2022) Comparison of the Pipeline embolisation device alone or combined with coiling for treatment of different sizes of intracranial aneurysms[J]. Stroke Vasc Neurol 7(4):345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo B, Kang H, Zhang H et al (2020) Pipeline Embolization device for intracranial aneurysms in a large Chinese cohort: factors related to aneurysm occlusion[J]. Ther Adv Neurol Disord 13:1279189396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin N, Brouillard AM, Krishna C et al (2015) Use of coils in conjunction with the pipeline embolization device for treatment of intracranial aneurysms[J]. Neurosurgery 76(2):142–149 [DOI] [PubMed] [Google Scholar]
- 36.Damiano RJ, Ma D, Xiang J et al (2015) Finite element modeling of endovascular coiling and flow diversion enables hemodynamic prediction of complex treatment strategies for intracranial aneurysm[J]. J Biomech 48(12):3332–3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J, Zhang Y, Tian Z et al (2019) Relationship between haemodynamic changes and outcomes of intracranial aneurysms after implantation of the pipeline embolisation device: a single centre study[J]. Interv Neuroradiol 25(6):671–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Son W, Kang DH (2020) Risk factor analysis of delayed Intracerebral Hemorrhage after Coil Embolization of Unruptured Cerebral Aneurysms[J]. Front Neurol 11:584596 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.