Abstract
Zebrafish has become a favorite model organism not only in genetics and developmental biology, but also for the study of cancer, neuroscience and metabolism. However, strategies for reverse genetics in zebrafish are mostly limited to the use of antisense oligonucleotides, and therefore the development of other targeting methods is highly desirable. Here, we report an approach to gene targeting in this system in which single-stranded oligonucleotides and zebrafish Rad52 protein are employed. It has been proposed that a single-stranded oligonucleotide containing a mutation can be incorporated into the genome by annealing to the single-stranded region of the lagging strand of the replication fork. Rad52 is expected to accelerate the annealing step. In vitro experiments using purified truncated Rad52 proteins and replication protein A (RPA) showed that annealing of oligonucleotides is accelerated by Rad52 in the presence of RPA. We developed a simple and sensitive PCR-based method to detect point mutations in the genome. In exploratory experiments, we found that microinjection of single-stranded oligonucleotide targeted to a specific gene together with truncated Rad52 into zebrafish embryos resulted in a low level of recombinant copies in 3 of the 80 embryos tested under these conditions.
INTRODUCTION
The concept that single-stranded oligonucleotides can be used to introduce mutations into genomes was first explored by Sherman's laboratory (1–3). A different study showed that M13 single-stranded DNA can also be a substrate for homologous recombination (4). Within the past decade, the potential of both unmodified and modified oligonucleotides for gene targeting has been extensively studied with the goal of using this approach for directed mutagenesis and gene therapy of genetic diseases (5–7). Despite their apparent similarities, the methods described seem to involve at least two different recombination pathways. Recombination mediated by unmodified single-stranded oligonucleotides 40–70 residues in length exhibited strand specificity determined by the direction of replication through the site, in that the efficiency of single-stranded oligonucleotides that correspond to Okazaki fragments was 2- to 50-fold higher than those representing the complementary strand (8,9). High recombination efficiencies (up to 6%) were achieved by overexpressing the β protein of bacteriophage λ, a single-strand DNA-annealing protein, while introducing targeting single-stranded oligonucleotides into bacteria (8). On the other hand, studies of modified oligonucleotides (both chimeric RNA/DNA oligonucleotides and single-stranded oligonucleotides with phosphorothioate or 2′-O-methyl RNA modifications at the ends) have shown that recombination efficiency correlates with the level of transcription, and is at least 5- to 50-fold higher when the oligonucleotides anneal to the non-template strand (10–12). The efficiency could be also enhanced by genetic re-engineering of RAD51 in yeast (13). One study has been published so far that reports recombination of single-stranded corrective DNA of 236 nt with a vector containing a mutated EGFP gene after coinjection with RecA protein into zebrafish embryos (14).
Zebrafish has many advantages as a vertebrate model organism. Its transparent body makes it easier to observe the morphology of organs and cells, and of reporter gene expression. Forward genetic screens using chemical mutagens have been successful in discovering many developmental mutations (15), but strategies for reverse genetics have mostly been limited to the use of antisense oligonucleotides that are effective for a limited period of time only (16,17). Recently, methods have been developed for effectively selecting random mutations in a gene of interest by resequencing or enzyme digestion assays (TILLING) after chemical mutagenesis (18,19). As ES cell-based gene targeting technology is not likely to be available in zebrafish for some years (20–23), other methods that allow introduction of targeted modifications into a gene of interest are highly desirable.
Early zebrafish embryos undergo cell division every 15 min, and hence DNA replication proceeds rapidly (24). Further, microinjection of DNA, mRNA or proteins can easily introduce desired reagents into the embryo. These properties of zebrafish make it attractive to pursue the potential of unmodified single-stranded oligonucleotides as site-specific mutagens in this organism. Here, we report the characterization of components of the recombination machinery in zebrafish that are involved in targeted mutagenesis, and we provide preliminary evidence for the ability of an unmodified single-stranded oligonucleotide to introduce a site-specific base substitution into the zebrafish genome.
MATERIALS AND METHODS
Zebrafish Rad52 expression constructs
The nuclear localization signal from the zebrafish Rad52 C-terminus was generated by annealing of two complementary synthetic oligonucleotides, BamHI–Rad52NLS–BclI: 5′-gatccAGACACGGCCAGATGATGAAGAAACGAAGATTGGACACGT-3′ and BclI–SLN25daR–BamHI: 5′-gaTCACGTGTCCAATCTTCGTTTCTTCATCATCTGGCCGTGTCTg-3′. This annealed fragment was subcloned into the BamHI site of the pET-15b vector (Novagen) to give ‘NLSpET15b’; 15 amino acids (GSRHGQMMKKRRLDT) will be added to a protein-coding sequence that is inserted into the BamHI site of this construct. Rad52 1–204Q fragment was PCR amplified with primer pairs, XhoNde–Rad52F1: 5′-CCGctcgagCATatggattatagcagcgggaggcaag-3′ and BamHI–Rad52R612: 5′-CGggatccTTGGGCCAGGCTGTCAAAC-3′. Rad52 1–317Q fragment was amplified with XhoNde–Rad52F1 and BamHI–Rad52R949 (5′-CGggatccctGCTGGTCTTTCTTCTCTTGCAG-3′). These PCR fragments were cut by NdeI and BamHI, and inserted into the NdeI and BamHI sites of the NLSpET15b to generate ‘Rad52 1–204Q NLS pET15b’ and ‘Rad52 1–317Q NLS pET15b’.
Expression and purification of zebrafish truncated Rad52 proteins
Expression constructs in BL21-CodonPlus (DE3)-RIL cells (Stratagene) were cultured in Luria–Bertani medium supplemented with ampicillin and chloramphenicol at 22°C to A600 of 0.6, and induced with 1 mM Isopropyl-β-d-thiogalactopyranoside (IPTG) for 3 h. The bacterial pellet was resuspended in 40 ml of BD TALON xTractor buffer (Clontech) (pH was adjusted to 8.0 and supplemented with additional 200 mM NaCl, 5 mM imidazole and Complete protease inhibitor, EDTA-free, from Roche), treated with 8 mg of lysozyme and 1 kU of benzonase nuclease (Novagen) for 10 min. The lysate was centrifuged at 10 000 g for 30 min. The supernatant was filtered through a 0.45 μm pore filter and incubated with 4 ml bed volume of TALON beads (Clontech) for 1 h. The beads were pelleted by centrifugation (700 g for 10 min), resuspended in 20 ml of wash buffer (20 mM Tris–HCl, pH 8.0, 500 mM NaCl, 10% glycerol, 0.02% Triton X-100, 40 mM imidazole and Complete protease inhibitor), and transferred to a disposable gravity column. The column was washed with 30 ml of wash buffer and 90 ml of wash buffer without protease inhibitor. Rad52 proteins were eluted by elution buffer (20 mM Tris–HCl, pH 8.0, 500 mM NaCl, 10% glycerol, 0.02% Triton X-100 and 500 mM imidazole), and dialyzed four times against 750 ml of buffer (20 mM Tris–HCl, pH 8.0, 500 mM NaCl, 10% glycerol and 0.02% Triton X-100). Protein concentration was measured with Micro BCA Protein Assay Reagent Kit (PIERCE).
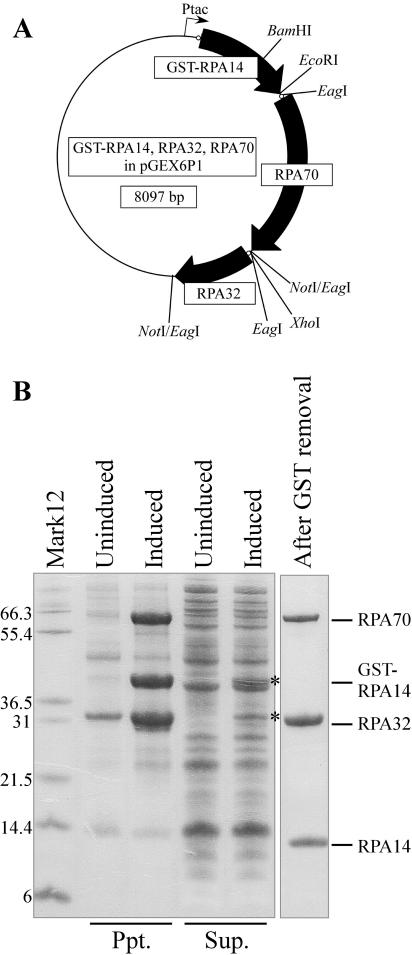
Construction of a zebrafish RPA triple expression construct
The open reading frame of RPA14 (GenBank BM860452) was PCR amplified with primers (BamHI–RPA14F1: 5′-cgGGATCCatgacgggcgtttatgagtc-3′ and EcoRI–RPA14R370: 5′-gGAATTCttgtttaaccactcattgacacttc-3′), cut by BamHI and EcoRI, and inserted into the BamHI and EcoRI sites of pGEX6P1 vector to generate ‘RPA14pGEX6P1’. The open reading frame of RPA32 (AI877547) was PCR amplified with primers (RBS-RPA32F1: 5′-TTGTTTAACTTTAAGAAGGAGATATACCatgtggaaccaaggtaaatacg-3′ and XN-RPA32R833: 5′-aaaaactcgagGCGGCCGCggctcttgtggatgttaggc-3′). This PCR product was used as template with primers (EagI-RBS: 5′-CCCAAcggccgAATAATTTTGTTTAACTTTAAGAAGGAGATATACC-3′ and XN-RPA32R833) to produce a product that has EagI and NotI sites at the ends. This PCR product was digested with EagI and inserted into the NotI site of RPA14pGEX6P1 in the correct orientation to produce ‘RPA14 and 32pGEX6P1’. The open reading frame of RPA70 (AW133988) was PCR amplified with primers (EagI-R-RPA70F1: 5′-CCCAAcggccgAATAATTTTGTTTAACTTTAAGAAGGAGATATACCatgactgttagaccgtcggaag-3′ and XN-RPA70R1822: 5′-aaaaactcgagGCGGCCGCgaccacggcagtccttctac-3′), subcloned into pGEM-T Easy vector (Promega), digested with EcoRI and XhoI, and inserted into the EcoRI and XhoI sites of the RPA14 and 32pGEX6P1 to generate ‘GST-RPA14, RPA32, RPA70 in pGEX6P1’.
Expression and purification of zebrafish RPA proteins
The RPA triple expression construct in BL21-CodonPlus (DE3)-RIL cells was cultured in LB medium supplemented with ampicillin and chloramphenicol at 37°C to A600 of 0.6, and induced with 1 mM IPTG for 2 h. The bacterial pellet was resuspended in 25 ml of P-BER reagent (PIERCE) and inclusion bodies were purified according to the manufacturer's instructions. Inclusion bodies (150 mg) were resuspended in 15 ml of solubilization buffer (50 mM CAPS, pH 11, 0.3% N-lauroylsarcosine, 5 mM DTT and 10 μM ZnCl2), and dialyzed twice for 2 h against buffer (20 mM Tris–HCl, pH 8.5, 5 mM DTT and 10 μM ZnCl2), and once overnight against the same buffer but at pH 7.5. NaCl was added to 150 mM and the sample was incubated with 1 ml bed volume of glutathione beads (Amersham Biosciences) for 30 min at 4°C. The beads were washed 5 times with 50 ml of buffer (20 mM Tris–HCl, pH 7.5, 150 mM NaCl, 5 mM, 10 μM ZnCl2 and 0.02% Triton X-100). The beads were incubated at 4°C overnight with 1 ml of PreScission protease (Amersham) mix (160 U enzyme, 50 mM Tris–HCl, 150 mM NaCl, 5 mM DTT and 10 μM ZnCl2). The eluted RPA proteins were collected and used for in vitro experiments.
Annealing assays
Annealing assays were done with two complementary oligonucleotides (oligo-25: 5′-GCAATTAAGCTCTAAGCCATCCGCAAAAATGACCTCTTATCAAAAGGA-3′ and oligo-26: 5′-TCCTTTTGATAAGAGGTCATTTTTGCGGATGGCTTAGAGCTTAATTGC-3′). Oligo-26 was labeled with [γ-32P]ATP (Amersham) using T4 polynucleotide kinase (Roche). Specific conditions of annealing experiments are given in the legend to Figure 4.
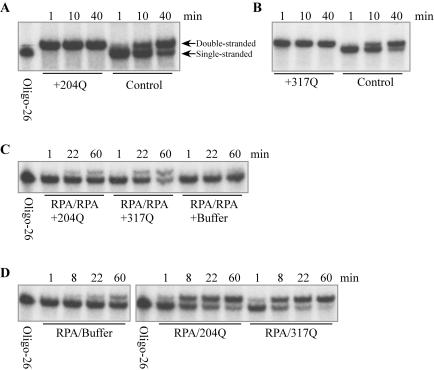
Figure 4.
Annealing assays in vitro. (A and B) Annealing of oligonucleotides is accelerated by truncated Rad52 proteins. Labeled oligo-26 (125 fmol) and 100 pmol truncated Rad52 protein were mixed in 50 μl RPA buffer (20 mM Tris–HCl, pH 7.5, 150 mM NaCl, 10% glycerol, 1 mM DTT and 0.02% Triton X-100) and incubated at 22°C for 20 min. Then, 125 fmol unlabeled oligo-25 was added to initiate annealing. At various times, 10 μl was removed and 50 pmol of unlabeled oligo-26 was added to stop annealing. Samples were treated with 0.5% SDS and 0.1 mg/ml proteinase K for 15 min at 37°C, and analyzed by 10% PAGE. The type of truncated Rad52 that was used is indicated; controls contained buffer in place of Rad52. (C) Rad52 1–317Q NLS mediates annealing when both strands are complexed by RPA. Labeled oligo-26 and unlabeled oligo-25 (188 fmol each) were preincubated separately with 260 pmol RPA in 75 μl RPA buffer for 20 min at 22°C, and then the samples were mixed. Aliquots (48 μl) of this mixture were incubated with 140 pmol of either Rad52 1–204Q NLS or Rad52 1–317Q NLS proteins. At various times, the reaction was stopped and analyzed by PAGE as described above. (D) Both Rad52 1–204Q NLS and Rad52 1–317Q NLS mediate annealing when RPA is bound to one strand. Unlabeled oligo-25 (375 fmol) was preincubated with 510 pmol RPA in 150 μl RPA buffer for 20 min at 22°C, while 125 fmol labeled oligo-26 was preincubated separately with 1.8 nmol Rad52 1–204Q NLS or Rad52 1–317Q NLS in 50 μl RPA buffer. Fifty microliters of the oligo-25+RPA sample was mixed with one of the oligo-26+Rad52 samples, incubated at 22°C and analyzed as described above.
Targeting oligonucleotides
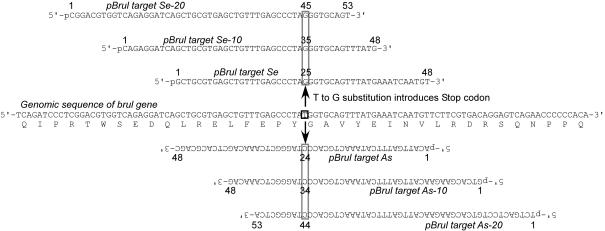
Sequences of the targeting oligonucleotides are shown in Figure 5. Oligonucleotides were synthesized using cyanoethyl phosphoramidite chemistry, and then purified by PAGE. These targeting oligonucleotides have 5′ phosphate modifications at the 5′ ends (Midland Certified Reagent Company, Inc.).
Figure 5.
Targeting oligonucleotides. The sense strand of the brul gene and the deduced amino acid sequence in the region of targeting are shown in the middle. The targeting oligonucleotides are homologous to the genomic sequence except for a single nucleotide substitution (indicated by open boxes) that introduces a Stop codon. pBrul target Se, pBrul target Se-10 and pBrul target Se-20 oligonucleotides correspond to the sense strand, while pBrul target As, pBrul target As-10 and pBrul target Se-20 oligonucleotides correspond to the antisense strand (shown upside down). All of the targeting oligonucleotides carried a phosphate at the 5′ end.
Microinjection of targeting oligonucleotides/Rad52 mixtures and DNA extraction from injected embryos
Injection needles were siliconized with AquaSil (Pierce) reagent. One of the targeting oligonucleotides (10 fmol/nl final) and one of the Rad52 deletion proteins (80 or 660 fmol/nl final) were preincubated for 20 min at room temperature. One-cell stage embryos were microinjected with 1–2 nl of the mixture, and cultured until shield to 80% epiboly stages. To extract genomic DNA, individual embryos were placed into 25 μl of ice-cold DNA extraction buffer (10 mM Tris–HCl, pH 8.0, 2 mM EDTA, 0.2% Triton X-100, 200 μg/ml Proteinase K) and incubated at 50°C for 16 h, then at 95°C for 10 min. To remove remaining targeting oligonucleotide, 25 μl of Exonuclease I mixture [0.04 U/μl Exonuclease I (New England Biolabs), 134 mM Glycine-KOH, pH 9.5, 13.4 mM MgCl2, 20 mM 2-mercaptoethanol] was added, and incubated at 37°C for 1 h and at 80°C for 20 min.
PCR-based mutation detection system
Mutant template was detected with a pair of PCR primers (BrulATRFO: 5′-AAGCTGAACAGTCAGAGTTGGTC-3′ (Tm = 55°C) and BrulMutMcAsR: 5′-TGATTTCATAAACTGCACCC-3′ (Tm = 48°C). BrulATRFO primer matches a site at ∼250 bases upstream of the targeted site. BrulMutMcAsR primer matches the mutant sequence, but has a mismatch with the wild-type sequence at the 3′ end. Each 20 μl reaction contained 200 μM of dNTP, 300 nM of each primer, one-twentieth of the genomic DNA from a single embryo and 0.7 U FastStart Taq DNA polymerase (Roche Applied Science) in 1× PCR buffer with 2.0 mM MgCl2. The thermal cycler (MJ Research, DNA Engine Tetrad, PTC-225) was programmed as follows: 4°C for 1 min, 94°C for 4.5 min (94°C for 15 s, 63°C for 30 s and 72°C for 30 s) × 38 cycles or more, 72°C for 7 min and 4°C until collected. Note that the program was started at 4°C and that the annealing temperature was significantly higher than the Tm of the primers. Tight contact between tubes and the heat block was essential for suppressing false positives. We used 8-strip PCR tubes instead of 96-well plates.
RESULTS
With the aim to test the applicability of single-stranded oligonucleotide-dependent gene targeting in zebrafish embryos, we first carried out in vitro control experiments. As gene targeting in bacteria is accelerated by β protein which is believed to facilitate annealing of the introduced single-stranded oligonucleotide to the cognate sequence in the genome, we tested the zebrafish homolog of β, Rad52, for its annealing-promoting ability in vitro. RPA is known to associate with single-stranded DNA in the replication fork and prevent annealing. Our in vitro experiments therefore tested whether Rad52 could displace zebrafish RPA from the DNA and facilitate annealing. Having found that this did happen in vitro, we tested the ability of single-stranded oligonucleotide co-injected with Rad52 to introduce a targeted mutation into a gene within the zebrafish genome in vivo.
Cloning of zebrafish Rad52
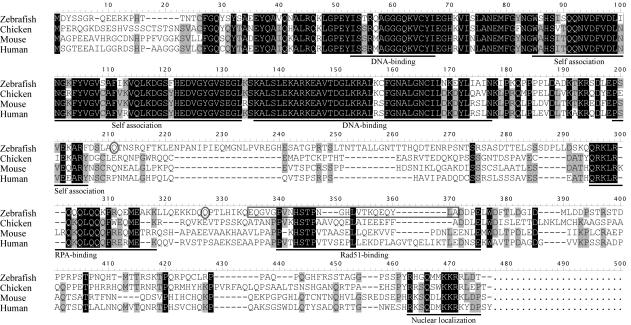
The coding region of Rad52 was PCR-amplified from a cDNA library of 24 h stage zebrafish embryos. We could not amplify the Rad52 coding region with primers designed from GeneScan prediction data (Sanger Center genome database), but we were able to find end sequences of zebrafish Rad52 in the genome database by using very short amino acid sequences from chicken (19-CFGQY-23, numbering is based on zebrafish sequence) and medaka (427-MKKRRLDT*-434) as BLAST queries. There is an in-frame stop codon (TAG) upstream of the ATG initiation codon in the sequence we derived (Figure 1), but we cannot exclude the possible existence of additional upstream exons. We found two splice variants, a short isoform with an internal deletion within the Rad51-binding domain, and a longer isoform that is similar to Rad52 of other species (Figure 1). Zebrafish Rad52 showed high sequence homology to other species in the N-terminal half that has DNA-binding and self-association domains, as documented before (25,26). The sequence of the C-terminal half is rather diverged except in the RPA-binding region, a small stretch in the Rad51-binding domain, and a nuclear localization signal. GenBank accession numbers for longer and shorter isoforms are DQ021477 and DQ021478, respectively.
Figure 1.
Multiple alignment of Rad52 from zebrafish and other species. Multiple alignments were made using the DNASIS software (Hitachi Software Engineering). The longer splice isoform of zebrafish Rad52 is shown. Amino acid residues conserved in three or four species are highlighted in gray or black, respectively. Known domains/regions are underlined. DNA-binding—regions that are involved in single-strand DNA binding. The amino acid sequence that is deleted in the short isoform is indicated by an open gray box. Amino acids Q204 and Q317 are indicated by open gray ovals.
Expression and purification of Rad52
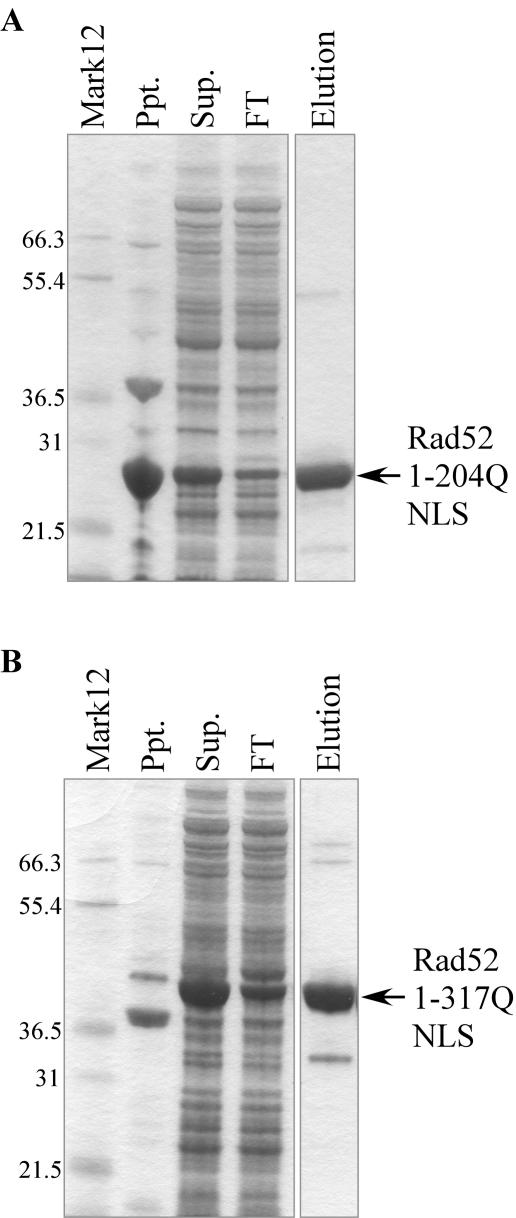
Histidine-tagged full-length zebrafish Rad52 was expressed as a major band of the predicted size of 50.7 kDa and several apparent proteolytic fragments (data not shown). Although intact Rad52 could be isolated from this preparation, we reasoned that the region relevant for our purpose was probably represented by the N-terminal DNA-binding domain, and possibly by the RPA-binding domain (27). Therefore, several Rad52 constructs truncated at the C-terminus were made, each containing a nuclear localization signal (see Materials and Methods). The deletion constructs ending in 327G, 342T, 355W, 362I and 376P showed multiple proteolytic fragments, whereas 204Q, 205T, 206N, 317Q and 369S were isolated mostly intact (Figure 2 and data not shown). Rad52 1–204Q NLS and Rad52 1–317Q NLS were chosen for further analysis, although some tests were also done with full-length Rad52.
Figure 2.
Expression and purification of truncated zebrafish Rad52 proteins. (A) SDS–PAGE analysis of bacterially expressed Rad52 1–204Q NLS protein. Ppt. and Sup. are the pellet and supernatant derived from the initial lysate, FT and Elution are the flow-through and eluted fractions of the column fractionations. Rad52 1–204Q NLS showed the predicted molecular weight (MW) of 26.6 kDa. (B) Rad52 1–317Q NLS protein. Lane designations as in (A). The protein migrates as expected for its predicted MW of 39.4 kDa. Mark12; molecular weight marker.
Expression and purification of RPA proteins
We could not obtain soluble RPA proteins by expressing them individually as GST-fusion proteins (data not shown). Previous studies with yeast and human RPA showed that these proteins could be expressed in soluble form when all three subunits were expressed simultaneously (28,29). Therefore, we generated a triple expression construct in which GST-RPA14, RPA32 and RPA70 were expressed from a tricistronic mRNA (Figure 3A). Using this construct, a fraction of GST-RPA14 and RPA32 were expressed in soluble form, but RPA70 was insoluble (Figure 3B). Therefore, we tried to refold the RPA proteins. Inclusion bodies were purified and solubilized in a high-pH buffer supplemented with N-laurylsarcosine and DTT. After dialysis, most of the protein remained soluble. It has been reported that RPA70, 32 and 14 form a tight heterotrimeric complex, which presumably accounts for the fact that RPA32 and 70 were co-purified with GST-RPA14 on glutathione beads (Figure 3B, right panel). This heterotrimeric complex of zebrafish RPA proteins could bind strongly to poly(dT)50 and poly(dC)50, but weakly to poly(dA)50 and poly(dG)50, as previously reported in other species (29,30) (data not shown).
Figure 3.
Expression and purification of RPA proteins. (A) Schematic drawing of tricistronic expression construct as described in Materials and Methods. Ribosome binding sites inserted upstream of each open reading frame are depicted as small open circles. Restriction enzyme sites that were used for construction are shown. (B) SDS–PAGE analysis of RPA proteins from induced and uninduced cultures fractionated into precipitate (Ppt.) and supernatant (Sup.). Most RPA proteins were found in the precipitate, with small fractions of GST-RPA14 and RPA32 also found in the supernatant (asterisks). The final preparation of RPA after refolding and GST removal is shown in the right lane. Mark12; molecular weight marker.
Rad52 facilitates annealing of oligonucleotides in the presence of RPA
Annealing of the two complementary oligonucleotides studied occurred slowly at 22°C when no protein was added (Figure 4A and B, control). This annealing was significantly accelerated by both truncated Rad52 proteins (Figure 4A and B), as expected from the fact that these deletion proteins retain the N-terminal DNA-binding domain. It has been reported that incubation of both strands with yeast RPA inhibits annealing of complementary oligonucleotides, and that Rad52 can displace RPA and mediate annealing if both proteins are from the same species (31,32). Zebrafish RPA likewise inhibited annealing of oligonucleotides (Figure 4C, right). Addition of Rad52 1–317Q NLS to RPA-bound oligonucleotides resulted in annealing of the oligonucleotides (Figure 4C, middle), as expected because this Rad52 derivative retains the RPA-binding domain. Addition of Rad52 1–204Q NLS protein, in which the RPA-binding domain was deleted, facilitated annealing at a lower rate (Figure 4C, left).
We next asked whether truncated Rad52 proteins can accelerate annealing when only one of the complementary strands is bound by RPA. This mimics the situation that should happen at the replication fork in vivo in single-stranded oligonucleotide-mediated mutagenesis. Without Rad52 proteins, annealing occurred slowly (Figure 4D, left), and both Rad52 1–204Q NLS and Rad52 1–317Q NLS proteins strongly accelerated annealing under these conditions (Figure 4D, middle and right). Similar results were obtained when full-length zebrafish Rad52 or β protein of bacteriophage λ were used (data not shown).
Microinjection of unmodified single-stranded oligonucleotide and Rad52 1–317Q NLS can induce a targeted mutation into the zebrafish genome
Having tested the reagents in vitro, we investigated whether single-stranded oligonucleotides and Rad52 proteins can be used for site-specific base substitution of the zebrafish genome. We chose the bruno-like gene (brul) as a target. This gene is expressed maternally and the mRNA is localized to the vegetal pole of early embryos (33). The genomic sequence of the brul gene was obtained from the Sanger Center genome database, and the sequence around the targeted site was verified by resequencing. The targeting oligonucleotides are homologous to the exon sequence except for a single-nucleotide substitution, which was chosen so that it will introduce an in-frame stop codon into the brul ORF (Figure 5).
During the process of Okazaki fragment maturation in mammals, the first ∼30 nt are synthesized by DNA polymerase α that has no proof-reading activity (34), and are subsequently displaced by DNA polymerase δ from the upstream direction (34,35). This is in contrast to the situation in yeast where only 2–3 nt are displaced by DNA polymerase δ (36). As DNA replication in zebrafish is likely to follow the mammalian pattern, we designed targeting oligonucleotides that have a longer distance between the mutation site and the 5′ end, in addition to targeting oligonucleotides that have the mutation site at their center (Figure 5). As we do not know the direction of DNA replication at the site, we designed both sense and antisense oligonucleotides.
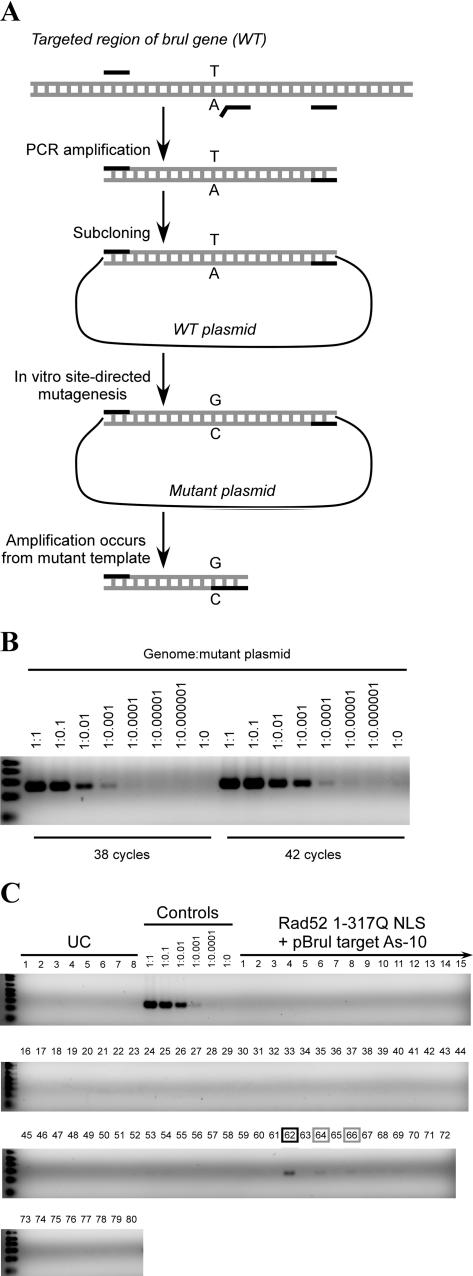
We developed a PCR-based mutation detection system in which one of two PCR primers matches only the mutant template, but is mismatched to the wild-type template at its 3′ end (Figure 6A). Previously, this strategy has been hampered by the emergence of false-positive bands caused by mispriming and/or ‘mis-elongation’ (37). We tested different conditions for amplification using a dilution series of mutant plasmid DNA in the presence of a level of genomic DNA that was higher than that encountered in the actual experiments. The conditions chosen (see Materials and Methods) allow us to detect one mutant copy in 1000 or 10 000 wild-type copies of the template using 38 or 42 cycles of amplification, respectively (Figure 6B); faint false positive bands appeared with 46 cycles (data not shown). This sensitivity matches the recently developed LigAmp method (38). The method can be used reliably for large numbers of samples as long as the conditions are carefully controlled.
Figure 6.
In vivo experiments. (A) A schematic of the PCR-based mutation detection system. PCR primers are shown as black lines, template DNAs and amplified fragments are shown as gray lines. A mutant plasmid was made by PCR amplification of the targeted region of the brul gene with flanking primers, followed by subcloning and in vitro site-directed mutagenesis. (B) Sensitivity of the mutation detection system. Serial dilutions of the mutant plasmid, as indicated, were mixed with a fixed amount of zebrafish genomic DNA isolated from a group of embryos. Each 20 μl reaction contained 0.39 μg genomic DNA that corresponded to 4.3 × 105 copies of the wild-type template. One mutant template in 1000 or 10 000 wild-type templates can be detected with 38 or 42 cycles, respectively. An aliquot (40%) of each reaction was electrophoresed on 3% agarose gels, and stained with ethidium bromide. (C) In vivo targeting experiments. Lanes labeled UC contain DNA from individual-uninjected embryos (1/20th embryo equivalent per lane). Control lanes contained DNA from uninjected embryos (1/20th embryo equivalent per lane) with mutant plasmid DNA added in the proportion indicated. Lanes labeled ‘Rad52 1–317Q NLS protein and pBrul target As-10’ contained DNA from individual embryos (1/20th embryo equivalent per lane) injected with these reagents. Amplification was carried out for 38 cycles. Injected embryos number 62 (black box) and to a lesser extent 64 and 66 (gray boxes) tested positive for the targeted mutation.
Exploratory in vivo targeting experiments were carried out using the reagents and conditions generated through our in vitro tests. In these experiments, we calibrated the assay by diluting mutant plasmid DNA into genomic DNA extracted from embryos at the same stage as the injected embryos, and we applied the same level of total DNA (equivalent to 1/20th of one embryo) on each lane. Under these conditions, the sensitivity of the assay was again ∼1 mutant copy in 1000 wild-type copies (Figure 6C, Controls). To test for targeted recombination, the oligonucleotide/Rad52 mixture was injected into one-cell stage embryos (see Materials and Methods, and Figure 6 legend), and the embryos were allowed to develop to the shield-to-80% epiboly stage. Injection of Rad52 did not affect embryonic development, but the oligonucleotides are toxic. The concentration used was chosen to give a 50% survival by mid-gastrulation; embryos that survived to this stage continued normal development for several days, but we did not attempt to raise them to adulthood. Genomic DNA was extracted from individual-injected embryos and tested in the PCR-based mutation detection assay. We tested 80 embryos for each condition, i.e. for each of the oligonucleotides shown in Figure 5 at two protein concentrations (see Materials and Methods). When using pBrul target As-10 oligonucleotide and Rad52 1–317Q NLS protein at a level of 660 fmol/nl, we found three apparent recombinants among 80 embryos (Figure 6C). No recombinants were detected using an 8-fold lower level of Rad52 1–317Q NLS protein, or with any of the other oligonucleotides tested. In the experiment in Figure 6C, the intensities of the bands suggest that 2–3 mutant copies are present for 1000 wild-type copies in sample 62 and ∼1 per 1000 in samples 64 and 66. These results were reproduced in an independent PCR experiment with the same template DNAs.
DISCUSSION
Pioneering work in Escherichia coli (8) prompted us to pursue the possibility that unmodified single-stranded oligonucleotides and annealing enzymes might be capable of mediating site-directed base substitution in the zebrafish genome. In this paper, we report the biochemical basis for this approach. It is known that when both strands of two complementary oligonucleotides are bound by RPA, Rad52 can displace the RPA and mediate annealing. For this activity, species-specific interactions between RPA and Rad52 are required (31,32). It was not known whether annealing of oligonucleotides can be accelerated by Rad52 when only one strand is bound by RPA, and whether species-specific interactions of RPA and Rad52 are involved. These questions are relevant to events that should happen at the replication fork in unmodified single-stranded oligonucleotide-mediated mutagenesis. Crystal structures of both Rad52 and RPA suggest that the phosphate backbone of single-stranded DNA is bound by both proteins while the bases remain exposed to the solvent, making them accessible from the outside (25,26,39). But it is still difficult to predict the outcome of an annealing event, because annealing of the oligonucleotides would require gross conformational changes of both proteins if they remain bound through the process. Our in vitro results showed that annealing of oligonucleotides was accelerated by truncated Rad52 and by β protein when only one strand was bound by RPA, and that species-specific interactions between RPA and Rad52 do not appear to be necessary. This suggests that truncated Rad52 proteins that retain only the annealing domain and even β protein of bacteriophage λ might be utilized in unmodified single-stranded oligonucleotide-mediated mutagenesis in animal cells.
In our exploratory in vivo experiments, we found a low frequency of recombinants when we microinjected pBrul target As-10 oligonucleotide with Rad52 1-317Q NLS protein. It is reasonable that we found recombinants by using an oligonucleotide whose 5′ homology region is longer than 30, but which still carries a sufficient region of homology 3′ of the targeted site. Further, the highest concentration of protein that was tested appeared to be the most effective. We found apparent recombinants in 3 of the 80 embryos tested with this particular combination of protein concentration and targeting oligonucleotide. The intensity of the PCR product suggests that only a small number of targeted copies existed in the genomes of these embryos, presumably because recombination took place in one of several hundred cells at the blastula stage. But optimal design principles of targeting oligonucleotides and the optimal domain architecture of Rad52 proteins remain to be determined in future studies. Various ways of delivery, such as expression of annealing enzymes from transgenes, may also be tested. We believe that, if this method is to be practical for mutagenesis, the overall frequency needs to be increased greatly.
Finally, we developed a simple and sensitive PCR-based mutation detection system. As this system does not require any special equipment or reagents other than commercially available hot start polymerases, it can be used as a cost-effective point mutation detection system.
Acknowledgments
We thank Dr Sergey Gaidamakov of the LMG, NICHD, NIH for help in ion exchange chromatography. Funding to pay the Open Access publication charges for this article was provided by the Intramural Research Program of the NIH, NICHD.
Conflict of interest statement. None declared.
REFERENCES
- 1.Moerschell R.P., Tsunasawa S., Sherman F. Transformation of yeast with synthetic oligonucleotides. Proc. Natl Acad. Sci. USA. 1988;85:524–528. doi: 10.1073/pnas.85.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamamoto T., Moerschell R.P., Wakem L.P., Ferguson D., Sherman F. Parameters affecting the frequencies of transformation and co-transformation with synthetic oligonucleotides in yeast. Yeast. 1992;8:935–948. doi: 10.1002/yea.320081104. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto T., Moerschell R.P., Wakem L.P., Komar-Panicucci S., Sherman F. Strand-specificity in the transformation of yeast with synthetic oligonucleotides. Genetics. 1992;131:811–819. doi: 10.1093/genetics/131.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon J.R., Moore P.D. Homologous recombination between single-stranded DNA and chromosomal genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 1987;7:2329–2334. doi: 10.1128/mcb.7.7.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Igoucheva O., Alexeev V., Yoon K. Oligonucleotide-directed mutagenesis and targeted gene correction: a mechanistic point of view. Curr. Mol. Med. 2004;4:445–463. doi: 10.2174/1566524043360465. [DOI] [PubMed] [Google Scholar]
- 6.Andersen M.S., Sorensen C.B., Bolund L., Jensen T.G. Mechanisms underlying targeted gene correction using chimeric RNA/DNA and single-stranded DNA oligonucleotides. J. Mol. Med. 2002;80:770–781. doi: 10.1007/s00109-002-0393-8. [DOI] [PubMed] [Google Scholar]
- 7.Parekh-Olmedo H., Czymmek K., Kmiec E.B. Targeted gene repair in mammalian cells using chimeric RNA/DNA oligonucleotides and modified single-stranded vectors. Sci. STKE. 2001;2001:PL1. doi: 10.1126/stke.2001.73.pl1. [DOI] [PubMed] [Google Scholar]
- 8.Ellis H.M., Yu D., DiTizio T., Court D.L. High efficiency mutagenesis, repair, and engineering of chromosomal DNA using single-stranded oligonucleotides. Proc. Natl Acad. Sci. USA. 2001;98:6742–6746. doi: 10.1073/pnas.121164898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X.T., Costantino N., Lu L.Y., Liu D.P., Watt R.M., Cheah K.S., Court D.L., Huang J.D. Identification of factors influencing strand bias in oligonucleotide-mediated recombination in Escherichia coli. Nucleic Acids Res. 2003;31:6674–6687. doi: 10.1093/nar/gkg844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu L., Rice M.C., Kmiec E.B. In vivo gene repair of point and frameshift mutations directed by chimeric RNA/DNA oligonucleotides and modified single-stranded oligonucleotides. Nucleic Acids Res. 2001;29:4238–4250. doi: 10.1093/nar/29.20.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu L., Rice M.C., Drury M., Cheng S., Gamper H., Kmiec E.B. Strand bias in targeted gene repair is influenced by transcriptional activity. Mol. Cell. Biol. 2002;22:3852–3863. doi: 10.1128/MCB.22.11.3852-3863.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Igoucheva O., Alexeev V., Yoon K. Targeted gene correction by small single-stranded oligonucleotides in mammalian cells. Gene. Ther. 2001;8:391–399. doi: 10.1038/sj.gt.3301414. [DOI] [PubMed] [Google Scholar]
- 13.Liu L., Maguire K.K., Kmiec E.B. Genetic re-engineering of Saccharomyces cerevisiae RAD51 leads to a significant increase in the frequency of gene repair in vivo. Nucleic Acids Res. 2004;32:2093–2101. doi: 10.1093/nar/gkh506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui Z., Yang Y., Kaufman C.D., Agalliu D., Hackett P.B. RecA-mediated, targeted mutagenesis in zebrafish. Mar. Biotechnol. 2003;5:174–184. doi: 10.1007/s10126-002-0059-0. [DOI] [PubMed] [Google Scholar]
- 15.Haffter P., Granato M., Brand M., Mullins M.C., Hammerschmidt M., Kane D.A., Odenthal J., van Eeden F.J., Jiang Y.J., Heisenberg C.P., et al. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development. 1996;123:1–36. doi: 10.1242/dev.123.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Ekker S.C. Nonconventional antisense in zebrafish for functional genomics applications. Methods Cell Biol. 2004;77:121–136. doi: 10.1016/s0091-679x(04)77007-7. [DOI] [PubMed] [Google Scholar]
- 17.Wickstrom E., Urtishak K.A., Choob M., Tian X., Sternheim N., Cross L.M., Rubinstein A., Farber S.A. Downregulation of gene expression with negatively charged peptide nucleic acids (PNAs) in zebrafish embryos. Methods Cell Biol. 2004;77:137–158. doi: 10.1016/s0091-679x(04)77008-9. [DOI] [PubMed] [Google Scholar]
- 18.Wienholds E., Plasterk R.H. Target-selected gene inactivation in zebrafish. Methods Cell Biol. 2004;77:69–90. doi: 10.1016/s0091-679x(04)77004-1. [DOI] [PubMed] [Google Scholar]
- 19.Draper B.W., McCallum C.M., Stout J.L., Slade A.J., Moens C.B. A high-throughput method for identifying N-ethyl-N-nitrosourea (ENU)-induced point mutations in zebrafish. Methods Cell Biol. 2004;77:91–112. doi: 10.1016/s0091-679x(04)77005-3. [DOI] [PubMed] [Google Scholar]
- 20.Fan L., Crodian J., Collodi P. Culture of embryonic stem cell lines from zebrafish. Methods Cell Biol. 2004;76:151–160. doi: 10.1016/s0091-679x(04)76009-4. [DOI] [PubMed] [Google Scholar]
- 21.Fan L., Crodian J., Collodi P. Production of zebrafish germline chimeras by using cultured embryonic stem (ES) cells. Methods Cell Biol. 2004;77:113–119. doi: 10.1016/s0091-679x(04)77006-5. [DOI] [PubMed] [Google Scholar]
- 22.Ju B., Huang H., Lee K.Y., Lin S. Cloning zebrafish by nuclear transfer. Methods Cell Biol. 2004;77:403–411. doi: 10.1016/s0091-679x(04)77022-3. [DOI] [PubMed] [Google Scholar]
- 23.Dodd A., Chambers S.P., Nielsen P.E., Love D.R. Modeling human disease by gene targeting. Methods Cell Biol. 2004;76:593–612. doi: 10.1016/s0091-679x(04)76027-6. [DOI] [PubMed] [Google Scholar]
- 24.Kimmel C.B., Ballard W.W., Kimmel S.R., Ullmann B., Schilling T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 25.Kagawa W., Kurumizaka H., Ishitani R., Fukai S., Nureki O., Shibata T., Yokoyama S. Crystal structure of the homologous-pairing domain from the human Rad52 recombinase in the undecameric form. Mol. Cell. 2002;10:359–371. doi: 10.1016/s1097-2765(02)00587-7. [DOI] [PubMed] [Google Scholar]
- 26.Singleton M.R., Wentzell L.M., Liu Y., West S.C., Wigley D.B. Structure of the single-strand annealing domain of human RAD52 protein. Proc. Natl Acad. Sci. USA. 2002;99:13492–13497. doi: 10.1073/pnas.212449899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mer G., Bochkarev A., Gupta R., Bochkareva E., Frappier L., Ingles C.J., Edwards A.M., Chazin W.J. Structural basis for the recognition of DNA repair proteins UNG2, XPA, and Rad52 by replication factor RPA. Cell. 2000;103:449–456. doi: 10.1016/s0092-8674(00)00136-7. [DOI] [PubMed] [Google Scholar]
- 28.Alani E., Thresher R., Griffith J.D., Kolodner R.D. Characterization of DNA-binding and strand-exchange stimulation properties of y-RPA, a yeast single-strand-DNA-binding protein. J. Mol. Biol. 1992;227:54–71. doi: 10.1016/0022-2836(92)90681-9. [DOI] [PubMed] [Google Scholar]
- 29.Henricksen L.A., Umbricht C.B., Wold M.S. Recombinant replication protein A: expression, complex formation, and functional characterization. J. Biol. Chem. 1994;269:11121–11132. [PubMed] [Google Scholar]
- 30.Kim C., Snyder R.O., Wold M.S. Binding properties of replication protein A from human and yeast cells. Mol. Cell. Biol. 1992;12:3050–3059. doi: 10.1128/mcb.12.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugiyama T., New J.H., Kowalczykowski S.C. DNA annealing by RAD52 protein is stimulated by specific interaction with the complex of replication protein A and single-stranded DNA. Proc. Natl Acad. Sci. USA. 1998;95:6049–6054. doi: 10.1073/pnas.95.11.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shinohara A., Shinohara M., Ohta T., Matsuda S., Ogawa T. Rad52 forms ring structures and co-operates with RPA in single-strand DNA annealing. Genes Cells. 1998;3:145–156. doi: 10.1046/j.1365-2443.1998.00176.x. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki H., Maegawa S., Nishibu T., Sugiyama T., Yasuda K., Inoue K. Vegetal localization of the maternal mRNA encoding an EDEN-BP/Bruno-like protein in zebrafish. Mech. Dev. 2000;93:205–209. doi: 10.1016/s0925-4773(00)00270-7. [DOI] [PubMed] [Google Scholar]
- 34.Waga S., Stillman B. The DNA replication fork in eukaryotic cells. Annu. Rev. Biochem. 1998;67:721–751. doi: 10.1146/annurev.biochem.67.1.721. [DOI] [PubMed] [Google Scholar]
- 35.Maga G., Villani G., Tillement V., Stucki M., Locatelli G.A., Frouin I., Spadari S., Hubscher U. Okazaki fragment processing: modulation of the strand displacement activity of DNA polymerase delta by the concerted action of replication protein A, proliferating cell nuclear antigen, and flap endonuclease-1. Proc. Natl Acad. Sci. USA. 2001;98:14298–14303. doi: 10.1073/pnas.251193198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garg P., Stith C.M., Sabouri N., Johansson E., Burgers P.M. Idling by DNA polymerase delta maintains a ligatable nick during lagging-strand DNA replication. Genes Dev. 2004;18:2764–2773. doi: 10.1101/gad.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwok S., Kellogg D.E., McKinney N., Spasic D., Goda L., Levenson C., Sninsky J.J. Effects of primer-template mismatches on the polymerase chain reaction: human immunodeficiency virus type 1 model studies. Nucleic Acids Res. 1990;18:999–1005. doi: 10.1093/nar/18.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi C., Eshleman S.H., Jones D., Fukushima N., Hua L., Parker A.R., Yeo C.J., Hruban R.H., Goggins M.G., Eshleman J.R. LigAmp for sensitive detection of single-nucleotide differences. Nat. Methods. 2004;1:141–147. doi: 10.1038/nmeth713. [DOI] [PubMed] [Google Scholar]
- 39.Bochkarev A., Pfuetzner R.A., Edwards A.M., Frappier L. Structure of the single-stranded-DNA-binding domain of replication protein A bound to DNA. Nature. 1997;385:176–181. doi: 10.1038/385176a0. [DOI] [PubMed] [Google Scholar]