Abstract
To improve serodiagnostic methods for the diagnosis of acute toxoplasmosis during pregnancy, a new test system has been developed and evaluated based on the use of recombinant antigens. Five recombinant Toxoplasma gondii antigens (ROP1, MAG1, SAG1, GRA7, and GRA8) were cloned in Escherichia coli, purified, and applied directly onto nitrocellulose membranes in a line assay (recomLine Toxoplasma). A panel of 102 sera from 25 pregnant women with supposed recent toxoplasmosis and from two symptomatic children was compared to a panel of 71 sera from individuals with past infection. Both panels were analyzed using a recombinant line assay for immunoglobulin G (IgG), IgM, and IgA antibodies and a reference enzyme-linked immunosorbent assay. Within the IgM-positive samples, antibodies against ROP1 were predominant regardless of the infection state. In IgG analysis a characteristic antibody pattern was found for very recent infections. This pattern changed to a different one during the time course of infection: antibodies against GRA7 and GRA8 were characteristic for very early IgG, whereas antibodies against SAG1 and MAG1 appeared significantly later. These results were further confirmed by determination of the IgG antibody avidity for every single recombinant antigen. In the time course of infection, IgG antibodies against the early recognized antigens matured significantly earlier than those directed against the later antigens did. The IgA patterns did not give reliable information about the infection time points. The data revealed that the recombinant line assay provides valuable information on the actual state of infection, especially during the early infection time points.
Toxoplasmosis is caused by the parasite Toxoplasma gondii. Cats are the primary host of this pathogen. Sexual reproduction takes place only in the primary host, leading to excretion of infectious oocytes that can be incorporated by humans while gardening or by the consumption of insufficiently washed fruits or vegetables. In an alternative pathway, humans can be infected as a result of consumption of insufficiently heated infected meat, since Toxoplasma gondii persists in pigs, goats, and other mammals. A further source of infection is diaplacental transmission of Toxoplasma gondii from an acutely infected mother to her unborn child (congenital toxoplasmosis). Congenital toxoplasmosis may cause abortion and serious damage to the fetus, with severe neurological disorders (reviewed in references 27, 29, and 32). Therefore, an accurate diagnosis of toxoplasmosis during pregnancy and early treatment is crucial.
Since the course of a toxoplasma infection is generally asymptomatic, the diagnosis is generally based on serological methods. The methods used are immunofluorescence, enzyme immunoassay, complement fixation, and immunosorbent agglutination assay (11, 15, 16). There are numerous test systems commercially available. In nearly all these test kits, various preparations of tachyzoite antigen that might be contaminated by nonparasitic material and that vary due to different antigen preparation methods are utilized.
Thus, recombinantly produced Toxoplasma gondii antigens were considered to replace tachyzoite material in toxoplasmosis serology. In the past, a large number of different recombinant antigens were produced in Escherichia coli and studied for their potential to serve as diagnostic markers of Toxoplasma gondii infections; these included dense granule proteins GRA1 (p24 [1, 2, 5]), GRA2 (p28 [1, 25]), GRA4 (p41 [1, 20]), GRA6 (p32 [1, 26]), GRA7 (p29 [1, 2, 10, 12]), and GRA8 (p35 [1, 2, 12, 13, 31]); surface antigens SAG1 (p30 [1, 2, 4, 7]) and SAG2 (p22 [1, 22]); rhoptry antigens ROP1 (p66 [1, 12]) and ROP2 (p54 [1, 33]); matrix protein MAG1 (p65, p68 [1, 12, 23]); microneme proteins MIC3 and MIC5 (2); and other recombinant antigens of Toxoplasma gondii. In most studies the recombinant antigens were coated alone or in some cases in various combinations on enzyme-linked immunosorbent assay (ELISA) plates. A combination of GRA7, GRA8, and ROP1 was previously suggested for the detection of Toxoplasma gondii-specific immunoglobulin M (IgM) antibodies (1). A combination of GRA7, GRA8, and SAG1 (1) or of GRA7, GRA8, SAG2, and H4 (14) was proposed for the detection of IgG antibodies. The latter combination was supposed to be reactive in patients with acute profiles but not in patients with chronic profiles. So far, combinations of different recombinantly produced antigens have not been applied separately on nitrocellulose membranes. Such a technique would allow the detection of human anti-Toxoplasma gondii antibodies directed against every single recombinant antigen as well as the determination of the avidities of the individual IgG antibodies.
The main objective of all diagnostic efforts in toxoplasmosis serology (mostly as preventive measures during pregnancy) is clarification of whether or not the pregnant woman has been acutely infected or whether infection occurred before conception. Due to the fact that low IgM titers in most cases persist long beyond the acute phases of infection, confirmation of IgM antibodies in serum is an inadequate criterion for diagnosing an acute toxoplasmosis (17). Therefore, determination of the avidities of the IgG serum antibodies is a very important step in diagnostics (8, 18). However, it has been shown that the IgG antibodies raised against the individual Toxoplasma antigen differ in their maturation characteristics (19, 34). In particular, there were antigens found that did not induce the synthesis of high-avidity IgG antibodies at all (19, 34). Thus, using the complete mixture of tachyzoite antigens as it is used in conventional avidity assays, the determination of avidity is compromised by these differences. The use of recombinant antigens in avidity determination might overcome these limitations. This should be especially effective if the avidities of the IgG antibodies directed against the individual recombinant antigens are determined independently from one another. Applying this approach, an improved estimation of the probable time point of infection should be possible.
The aim of this study was to investigate the changes in seroreactivity to and avidity for individual recombinant antigens during the time course of infection and to examine whether differences might be exploited as diagnostic tools in toxoplasmosis serology.
MATERIALS AND METHODS
Cloning and expression of recombinant proteins.
Genomic DNA of Toxoplasma gondii strain RH was isolated using a QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany). Toxoplasma gondii tachyzoites (RH strain) were provided by R. Disko (Klinikum rechts der Isar, Munich, Germany). Transformation of Escherichia coli and the production of competent cells were carried out according to the method of Hanahan (6). Restriction endonucleases and T4 DNA ligase (Roche Diagnostics, Mannheim, Germany) were used as recommended by the manufacturer. Five immunodominant Toxoplasma gondii antigens, ROP1 (p66 [12]), MAG1 (p65 [12]), SAG1 (p30 [7]), GRA7 (p29 [10]), and GRA8 (p35 [12, 13]), GenBank accession numbers M71274, U09029, X14080, Y13863, and AF310261, respectively, were expressed as full-length proteins in Escherichia coli. The genes were amplified from Toxoplasma gondii by PCR with specific primers based on the sequence information obtained from the GenBank database. Useful restriction enzyme sites were incorporated into these primers. All genes were expressed without the sequences coding for the signal peptides. The PCR was carried out using a commercially available PCR kit (Roche Diagnostics). Samples were denatured at 94°C for 2 min, annealed at 45°C for 2 min, and extended at 72°C for 4 min. The total number of cycles was 30. The reaction products were analyzed by electrophoresis on 1.0% agarose gels containing ethidium bromide (0.5 mg/ml). DNA was extracted with a gel extraction kit (QIAGEN, Hilden, Germany). Subsequently, the DNA was cleaved with suitable restriction enzymes. Fragments were ligated into pUC8 or pDS1 plasmid vectors. Escherichia coli JM109, M15, or OmpT− strains were transformed. The cloned sequences were verified by sequence analysis. The recombinant antigens were expressed after induction with 2 mM IPTG (isopropyl-β-d-thiogalactopyranoside) at 37°C. The bacteria were harvested 3 h after induction. Bacterial lysis and purification of recombinant antigens were performed following the method of Soutschek et al. (30).
Production and processing of nitrocellulose strips.
Individual dilutions of the purified recombinant antigens were applied directly onto nitrocellulose membranes in different lines using a specialized device (Biodot). The appropriate line conditions for all recombinant antigens were determined empirically with standard serum samples. Membranes were blocked with 1% skim milk solution in phosphate-buffered saline, air dried, and cut into individual test strips. Strips were stored at 4°C. Processing of nitrocellulose test strips was performed according to the instruction manual for recomLine Toxoplasma (MIKROGEN) using the reagents supplied in the kit. Details are as follows. Serum samples were applied at 1:100 dilutions and incubated together with the nitrocellulose test strips for 1 h at room temperature. Following three washing steps of 5 min each, a second incubation of 45 min with peroxidase-labeled secondary antibody (anti-human IgG, IgM, or IgA) was performed. Strips were stained for about 8 min using tetramethylbenzidine after three additional washing steps of 5 min each. The reactivities of serum antibodies against the recombinant antigens were evaluated by comparison to a cutoff band. The cutoff band gives a slight and strictly reproducible reactivity with every single serum sample and can therefore be used as a reference band. Reactivities stronger than or equivalent to that of the reference band were regarded as positive, while weaker reactivities or the absence of reaction were regarded as negative. For avidity determination, strips were washed once after serum incubation and then incubated with avidity reagent for exactly 3 minutes. The protocol was continued with three washing steps. Avidity was estimated by comparison of two individual test strips, one of which was incubated with the avidity reagent. Avidity was regarded to be low if the band intensity loss significantly exceeded 50 percent. Avidity was regarded to be intermediate if the band intensity loss was about 50 percent. Avidity was regarded to be high if band intensity loss was significantly less than 50 percent.
Sera.
All serum samples were analyzed and characterized by Cobas Core IgG (Roche) and by ETI-TOXOK-M reverse PLUS and partly by ETI-TOXOK-A reverse PLUS (DiaSorin). In addition, IgG avidities were determined using VIDAS TOXO IgG AVIDITY (BioMerieux). According to the manufacturer, high-avidity test results are present only in individuals who have been infected for at least 4 months. All test systems were applied according to the manufacturers' instructions. Serum samples were collected from patients suspected of having acute toxoplasmosis (panel A: IgM positive, IgG positive or IgG seroconversion on second sample, and/or IgG low avidity) and from individuals with chronic toxoplasmosis (panel B: IgG positive and IgM negative, with IgG avidity not tested). Panel A consisted of 102 sera from 27 patients (25 pregnant woman and two children) collected at two to seven samplings ranging from 4 weeks to 6 months from the first to the last sample tested. This panel was divided into three subgroups. Subgroup A1 included all serum samples of panel A collected initially in the time course of infection. Subgroup A2 comprised the subsequent sera taken until 3 months after the first serum sample. Subgroup A3 comprised sera taken subsequently between 3 and 6 months after the first serum sample. Panel B consisted of 71 sera of 71 patients.
RESULTS
We cloned and expressed five recombinant antigens, ROP1 (p66), MAG1 (p65), SAG1 (p30), GRA7 (p29), and GRA8 (p35), of Toxoplasma gondii in Escherichia coli. The antigens were selected from a larger panel of antigens investigated (including in addition GRA4, GRA6, ROP2, and SAG4) and proved to be most suitable for serodiagnosis (data not shown). The recombinant antigens were purified by standard chromatographic methods and applied directly onto nitrocellulose for a line assay. These test strips (Fig. 1 and 2) were used to investigate different serum panels for IgG, IgM, and IgA antibody patterns and IgG avidity.
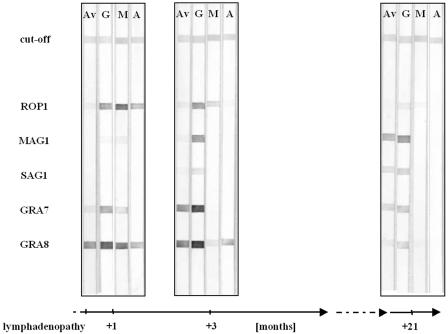
FIG. 1.
Reactivities of recombinant antigens to IgG (G), IgM (M), and IgA (A) antibodies and avidities of IgG antibodies (Av) in infection time course of case 1.
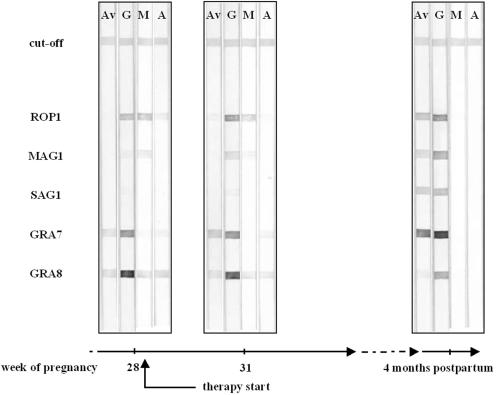
FIG. 2.
Reactivities of recombinant antigens to IgG (G), IgM (M), and IgA (A) antibodies and avidities of IgG antibodies (Av) in infection time course of case 2.
Comparison of antibody patterns in acute and past infections.
Antibody results with the recomLine Toxoplasma assay are presented in Tables 1 and 2. IgM and IgA activities with the recombinants showed essentially no distinctive features over time. However, IgG reactivities with MAG1, SAG1, and GRA8 showed different levels over times of infection. Examples of antibody reactivity are shown in Fig. 1 and 2. In initial specimens from six patients, the recombinant line assay detected Toxoplama-specific IgG antibodies which were not detected by the Cobas Core IgG.
TABLE 1.
Reactivities of individual recombinant antigens to Toxoplasma gondii-specific IgG antibodies of the IgG-positive sera of subgroups A1, A2, and A3 and to all sera of panel B
| Recombinant antigen | % (no.) IgG-positive serum samples from:
|
|||
|---|---|---|---|---|
| Subgroup A1 (n = 22) | Subgroup A2 (n = 51) | Subgroup A3 (n = 10) | Panel B (n = 71) | |
| ROP1 | 50.0 (11) | 52.9 (27) | 40.0 (4) | 38.0 (27) |
| MAG1 | 31.8 (7) | 56.9 (29) | 70.0 (7) | 62.0 (44) |
| SAG1 | 9.1 (2) | 29.4 (15) | 60.0 (6) | 91.5 (65) |
| GRA7 | 90.9 (20) | 94.1 (48) | 90.0 (9) | 88.7 (63) |
| GRA8 | 95.5 (21) | 94.1 (48) | 100.0 (10) | 46.5 (33) |
TABLE 2.
Reactivities of individual recombinant antigens to Toxoplasma gondii-specific IgM and IgA antibodies of panel A and panel B
| Recombinant antigen | % (no.) serum samples from:
|
|||
|---|---|---|---|---|
| Panel A (n = 102) positive for:
|
Panel B (n = 71) positive for:
|
|||
| IgM (n = 68) | IgA (n = 34) | IgM | IgA | |
| ROP1 | 92.6 (63) | 32.3 (11) | 7.0 (5)a | 0.0 (0) |
| MAG1 | 11.8 (8) | 8.8 (3) | 0.0 (0) | 0.0 (0) |
| SAG1 | 7.4 (5) | 2.9 (1) | 0.0 (0) | 0.0 (0) |
| GRA7 | 20.6 (14) | 47.1 (16) | 0.0 (0) | 0.0 (0) |
| GRA8 | 64.7 (44) | 67.6 (23) | 0.0 (0) | 1.4 (1) |
All these samples had very high levels of IgG (ELISA value > 1.5; cutoff value, 0.58) and strongly reacted to IgG with all five recombinant antigens.
Development of the immune response in individual time courses of infection.
To further demonstrate the typical features of the immune response development over time directed against the individual recombinant antigens, two representative cases from panel A will be discussed in detail.
The serum samples of case 1 were derived from a lymphadenopathy patient who received no therapeutic treatment. In contrast, all pregnant women included in this study received therapeutic treatment either by spiramycin or by a combination of pyrimethamine and sulfadiazine. Case 1 represents a 13-year-old child suffering from lymphadenopathy. Serum samples were collected 1, 3, and 21 months after the first onset of symptoms. The results of the recombinant line assay are shown in Fig. 1. In the first serum sample, IgG antibodies were reactive to GRA7, GRA8, and ROP1, while in the second and third samples, MAG1 and SAG1 reactions were also seen. In the first sample, IgG antibodies against GRA8 were of high avidity, but those directed against GRA7 and ROP1 were of low avidity. IgG antibodies against GRA7 had already matured by the time that sample 2 was taken, whereas antibodies directed against MAG1 and SAG1 exhibited high avidities in the last sample only. In contrast, ROP1 and GRA8 showed low-avidity IgG antibodies in the last sample. ROP1 and GRA8 were strongly reactive to IgM antibodies in the first sample. IgM antibody reaction diminished in the second sample and was negative in the last sample. The IgA response was restricted to ROP1 and GRA8 in the first sample and to GRA8 in the second sample.
In case 2, three serum samples were collected from a 35-year-old woman pregnant in the last trimester (Fig. 2). Therapy started 3 days after the collection of the first serum sample. Analyzing the first sample in the recombinant line assay, low- to intermediate-avidity antibodies to GRA7, GRA8, and ROP1 were detected, but no antibodies directed against MAG1 or SAG1 were detected. IgM (ROP1, MAG1, GRA8) and IgA antibody reactions (GRA8) were positive. Three weeks later, IgG avidity to GRA7 had already increased, while IgG antibodies against MAG1 appeared. IgM antibody reactions were unchanged (only the reaction with MAG1 was weaker), and IgA antibody reactions decreased. In the last serum sample, collected 7 months after the first one, IgG antibodies of high avidity for ROP1, MAG1, SAG1, and GRA7 were accompanied by antibodies of low avidity for GRA8. Neither IgM nor IgA antibodies against the recombinant antigens were found.
DISCUSSION
So far, many different recombinant antigens have been produced and characterized for their abilities to detect Toxoplasma gondii-specific antibodies. In general, only the reactivities of single recombinant antigens were investigated, or cocktails of recombinant antigens were produced and analyzed in combination with the ELISA technique (1, 14, 24). Up to the time of this study, differentiation between individual reactivities of a combined panel of recombinant antigens was not examined. These differentiations as well as the avidities of human Toxoplasma gondii-specific IgG antibodies directed against single antigens have been evaluated so far using only Toxoplasma gondii lysates analyzed by Western blotting (19, 34).
In this work, we evaluated the individual reactivities and IgG avidities of five different Toxoplasma gondii antigens cloned and expressed in Escherichia coli, which were combined for a line assay. We tested the IgG, IgM, and IgA reactivities to and IgG avidities of Toxoplasma gondii-specific human sera for these recombinant antigens, using two different panels of human sera which were characteristic for presumed acute and chronic infections.
For patients with presumed acute toxoplasmosis, positive IgM results in the recombinant line assay were provoked mainly by IgM antibodies directed against ROP1 and to a lesser extent GRA8. The importance of these antigens in IgM serology relative to toxoplasmosis has already been described (1, 12). No information on the actual state of infection could be concluded from the IgM antibody pattern. ROP1 was responsible for the long-term persistence of the IgM in the recombinant line assay in most cases. This might explain why ROP1 reacted with IgM of some strongly IgG-positive sera in the group of samples that were IgM negative by ELISA. Nevertheless, unspecific reactivity of ROP1 in those cases cannot be excluded.
The IgA responses in the recombinant line assay varied greatly, an observation that is consistent with the results of earlier studies (9). Quite often, response was lacking completely. The most prominent antigens reactive with human IgA antibodies were GRA8 and GRA7. The IgA pattern itself gave no substantial information on the actual state of infection.
In contrast to findings with IgM as well as with IgA, the IgG response showed characteristic patterns that could be exploited to distinguish acute from chronic infections. Even in the very early phase of infection, the recombinant antigens reacted with IgG antibodies, whereas the reference test system was still negative. This finding suggests a higher sensitivity of the recombinant line assay with these sera. At the beginning of the IgG response, IgG antibodies to GRA7 and/or GRA8 were exclusively present, whereas IgG antibodies to MAG1 and/or SAG1 were still lacking. In all time courses of infection, IgG antibodies to MAG1 and, in particular, to SAG1 were observed significantly later. In addition, the IgG antibodies against MAG1 and SAG1 matured at significantly later time points than those directed against GRA7 and GRA8. These findings can easily be observed in both time courses of infection shown as examples (Fig. 1 and Fig. 2) and were observed in all cases of this study. In previous studies, it has been also shown that the maturation characteristics of the IgG antibodies against individual antigens are substantially different (3, 19, 34), but so far no attempt has been made to take advantage of these findings in order to improve the serodiagnosis of toxoplasmosis.
ROP1 and to a lesser extent GRA8 failed to trigger the maturation of IgG antibodies to high avidity in every single case, whereas IgG antibodies directed against GRA7, MAG1, and SAG1 matured in all observed cases to high avidity. Thus, the analysis of the IgG avidity for individual recombinant antigens might enable a more accurate diagnosis of acute toxoplasmosis than that of an IgG avidity determination applying tachyzoite lysates, as has already been suggested by Beghetto et al. (3). Whereas the work of Beghetto et al. (3) focuses on one single recombinant antigen, MIC3, for avidity determination, our study includes five recombinant antigens that show different characteristics with regard to avidity maturation. This should be suitable to give a more differentiated picture of the avidity maturation process.
In cases of infections acquired long ago, IgG antibodies against SAG1 and GRA7 mainly dominated over IgG antibodies against the other recombinant antigens investigated, particularly over ROP1 and GRA8, in this study. The latter observation is consistent with previous findings (13). Nevertheless, GRA8 cannot be utilized as a marker of acute infections, because IgG antibodies to GRA8 persist far beyond the acute phase of infection. Whereas in most studies SAG1 was to found to induce IgG antibodies as soon as the acute phase of infection (4, 24), our study shows no reactivity of IgG antibodies against SAG1 in the early stages of infection. Our study findings are supported by another study (1). These eminent differences in the levels of recognition of recombinant SAG1 by human sera might be the result of variations in the preparations of this very complex molecule that were applied in the different studies. Thus, different epitopes might be presented dependent on the preparation technique.
The benefit of analyzing the human immune response against different recombinant antigens individually for characterization of infections caused by Toxoplasma gondii could be demonstrated. The combination of IgG reactivity to and IgG avidity for five individual antigens in the recombinant line assay can be regarded as a valuable tool to distinguish acute from chronic toxoplasmosis in pregnancy using a single serum sample. Nevertheless, further and more extensive studies are required and will be performed to strengthen and specify the findings presented in this study.
Acknowledgments
We acknowledge Silvia Dorn and Veronika Rilling for helpful discussions, Alexandra Pfrepper for critical reading of the manuscript, and Manfred Motz for support.
REFERENCES
- 1.Aubert, D., G. T. Maine, I. Villena, J. C. Hunt, L. Howard, M. Sheu, S. Brojanac, L. E. Chovan, S. F. Nowlan, and J. M. Pinon. 2000. Recombinant antigens to detect Toxoplasma gondii-specific immunoglobulin G and immunoglobulin M in human sera by enzyme immunoassay. J. Clin. Microbiol. 38:1144-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beghetto, E., A. Spadoni, W. Buffolano, M. Del Pezzo, O. Minenkova, E. Pavoni, A. Pucci, R. Cortese, F. Felici, and N. Gargano. 2003. Molecular dissection of the human B-cell response against Toxoplasma gondii infection by lambda display of cDNA libraries. Int. J. Parasitol. 33:163-173. [DOI] [PubMed] [Google Scholar]
- 3.Beghetto, E., W. Buffolano, A. Spadoni, M. Del Pezzo, M. Di Cristina, O. Minenkova, E. Petersen, F. Felici, and N. Gargano. 2003. Use of an immunoglobulin G avidity assay based on recombinant antigens for diagnosis of primary Toxoplasma gondii infection during pregnancy. J. Clin. Microbiol. 41:5414-5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burg, J. L., D. Perelman, L. H. Kasper, P. L. Ware, and J. C. Boothroyd. 1988. Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J. Immunol. 141:3584-3591. [PubMed] [Google Scholar]
- 5.Cesbron-Delauw, M. F., B. Guy, G. Torpier, R. J. Pierce, G. Lenzen, J. Y. Cesbron, H. Charif, P. Lepage, F. Darcy, J. P. Lecocq, and A. Capron. 1989. Molecular characterization of a 23 kilodalton major antigen secreted by Toxoplasma gondii. Proc. Natl. Acad. Sci. USA 86:7537-7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 7.Harning, D., J. Spenter, A. Metsis, J. Vuust, and E. Petersen. 1996. Recombinant Toxoplasma gondii surface antigen 1 (P30) expressed in Escherichia coli is recognized by human Toxoplasma-specific immunoglobulin M (IgM) and IgG antibodies. Clin. Diagn. Lab. Immunol. 3:355-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hedman, K., M. Lappalainen, I. Seppala, and O. Makela. 1989. Recent primary Toxoplasma infection indicated by a low avidity of specific IgG. J. Infect. Dis. 159:736-739. [DOI] [PubMed] [Google Scholar]
- 9.Huskinson, J., P. Thulliez, and J. S. Remington. 1990. Toxoplasma antigens recognized by human immunoglobulin A antibodies. J. Clin. Microbiol. 28:2632-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs, D., J. F. Dubremetz, A. Loyens, F. Bosman, and E. Saman. 1998. Identification and heterologous expression of a new dense granule protein (GRA7) from Toxoplasma gondii. Mol. Biochem. Parasitol. 91:237-249. [DOI] [PubMed] [Google Scholar]
- 11.Jenum, P. A., and B. Stray-Pedersen. 1998. Development of specific immunoglobulins G, M, and A following primary Toxoplasma gondii infection in pregnant women. J. Clin. Microbiol. 36:2907-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knapp, S., R. Ziegelmaier, and H. Küpper. June 1991. Toxoplasma gondii antigens, their production and application. European patents EP0748815, EP0748816, and EP0751147.
- 13.Li, S., G. Maine, Y. Suzuki, F. G. Araujo, G. Galvan, J. S. Remington, and S. Parmley. 2000. Serodiagnosis of recently acquired Toxoplasma gondii infection with a recombinant antigen. J. Clin. Microbiol. 38:179-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li, S., G. Galvan, F. G. Araujo, Y. Suzuki, J. S. Remington, and S. Parmley. 2000. Serodiagnosis of recently acquired Toxoplasma gondii infection using an enzyme-linked immunosorbent assay with a combination of recombinant antigens. Clin. Diagn. Lab. Immunol. 7:781-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liesenfeld, O., C. Press, R. Flanders, R. Ramirez, and J. S. Remington. 1996. Study of Abbott IMx system for detection of immunoglobulin G and immunoglobulin M toxoplasma antibodies: value of confirmatory testing for diagnosis of acute toxoplasmosis. J. Clin. Microbiol. 34:2526-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liesenfeld, O., C. Press, J. G. Montoya, R. Gill, J. L. Isaac-Renton, K. Hedman, and J. S. Remington. 1997. False-positive results in immunoglobulin M (IgM) toxoplasma antibody tests and importance of confirmatory testing: the Platelia Toxo IgM test. J. Clin. Microbiol. 35:174-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liesenfeld, O., J. G. Montoya, N. J. Tathineni, M. Davis, B. W. Brown, Jr., K. L. Cobb, J. Parsonnet, and J. S. Remington. 2001. Confirmatory serologic testing for acute toxoplasmosis and rate of induced abortions among women reported to have positive Toxoplasma immunoglobulin M antibody titers. Am. J. Obstet. Gynecol. 184:140-145. [DOI] [PubMed] [Google Scholar]
- 18.Liesenfeld, O., J. G. Montoya, S. Kinney, C. Press, and J. S. Remington. 2001. Effect of testing for IgG avidity in the diagnosis of Toxoplasma gondii infection in pregnant women: experience in a US reference laboratory. J. Infect. Dis. 183:1248-1253. [DOI] [PubMed] [Google Scholar]
- 19.Marcolino, P. T., D. A. Silva, P. G. Leser, M. E. Camargo, and J. R. Mineo. 2000. Molecular markers in acute and chronic phases of human toxoplasmosis: determination of immunoglobulin G avidity by Western blotting. Clin. Diagn. Lab. Immunol. 7:384-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mevelec, M. N., T. Chardes, O. Mercereau-Puijalon, I. Bourguin, A. Achbarou, J. F. Dubremetz, and D. Bout. 1992. Molecular cloning of GRA4, a Toxoplasma gondii dense granule protein recognized by mucosal IgA antibodies. Mol. Biochem. Parasitol. 56:227-238. [DOI] [PubMed] [Google Scholar]
- 21.Reference deleted.
- 22.Parmley, S. F., G. D. Sgarlato, J. Mark, J. B. Prince, and J. S. Remington. 1992. Expression, characterization, and serologic reactivity of recombinant surface antigen P22 of Toxoplasma gondii. J. Clin. Microbiol. 30:1127-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parmley, S. F., S. Yang, G. Harth, L. D. Sibley, A. Sucharczuk, and J. S. Remington. 1994. Molecular characterization of a 65-kilodalton Toxoplasma gondii antigen expressed abundantly in the matrix of tissue cysts. Mol. Biochem. Parasitol. 66:283-296. [DOI] [PubMed] [Google Scholar]
- 24.Pietkiewicz, H., E. Hiszczynska-Sawicka, J. Kur, E. Petersen, H. V. Nielsen, M. Stankiewicz, I. Andrzejewska, and P. Myjak. 2004. Usefulness of Toxoplasma gondii-specific recombinant antigens in serodiagnosis of human toxoplasmosis. J. Clin. Microbiol. 42:1779-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prince, J. B., F. G. Araujo, J. S. Remington, J. L. Burg, J. C. Boothroyd, and S. D. Sharma. 1989. Cloning of cDNAs encoding a 28 kDa antigen of Toxoplasma gondii. Mol. Biochem. Parasitol. 34:3-14. [DOI] [PubMed] [Google Scholar]
- 26.Redlich, A., and W. A. Müller. 1998. Serodiagnosis of acute toxoplasmosis using a recombinant form of the dense granule antigen GRA6 in an enzyme-linked immunosorbent assay. Parasitol. Res. 84:700-706. [DOI] [PubMed] [Google Scholar]
- 27.Remington, J. S., R. McLeod, P. Thulliez, and G. Desmonts. 2001. Toxoplasmosis, p. 205-346. In J. S. Remington and J. Klein (ed.), Infectious diseases of the fetus and newborn infant, 5th ed. W. B. Saunders, Philadelphia, Pa.
- 28.Reference deleted.
- 29.Smith, J. 1995. A ubiquitous intracellular parasite: the cellular biology of Toxoplasma gondii. Int. J. Parasitol. 25:1301-1309. [DOI] [PubMed] [Google Scholar]
- 30.Soutschek, E., B. Hoflacher, and M. Motz. 1990. Purification of a recombinantly produced transmembrane protein (gp41) of HIV I. J. Chromatogr. 521:267-277. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki, Y., R. Ramirez, C. Press, S. Li, S. Parmley, P. Thulliez, and J. S. Remington. 2000. Detection of immunoglobulin M antibodies to P35 antigen of Toxoplasma gondii for serodiagnosis of recently acquired infection in pregnant women. J. Clin. Microbiol. 38:3967-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tenter, A. M., A. R. Heckeroth, and L. M. Weiss. 2000. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 30:1217-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Gelder, V., F. Bosman, F. De Meuter, H. Van Heuverswyn, and P. Hérion. 1993. Serodiagnosis of toxoplasmosis by using a recombinant form of the 54-kilodalton rhoptry antigen expressed in Escherichia coli. J. Clin. Microbiol. 31:9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villavedra, M., J. Battistoni, and A. Nieto. 1999. IgG recognizing 21-24 kDa and 30-33 kDa tachyzoite antigens show maximum avidity maturation during natural and accidental human toxoplasmosis. Rev. Inst. Med. Trop. Sao Paulo 41:297-303. [DOI] [PubMed] [Google Scholar]