Abstract
In this study, we used the epidermal suction blister technique, in conjunction with multiparameter flow cytometry, to analyze the cellular and cytokine responses elicited by intradermal injection of human volunteers with synthetic analogs for spirochetal lipoproteins and compared the responses to findings previously reported from patients with erythema migrans (EM). Compared with peripheral blood (PB), lipopeptides derived from the N termini of the Borrelia burgdorferi outer surface protein C and the 17-kDa lipoprotein of Treponema pallidum (OspC-L and 17-L, respectively) elicited infiltrates enriched in monocytes/macrophages and dendritic cells (DCs) but also containing substantial percentages of neutrophils and T cells. Monocytoid (CD11c+) and plasmacytoid (CD11c−) DCs were selectively recruited to the skin in ratios similar to those in PB, but only the former expressed the activation/maturation surface markers CD80, CD83, and DC-SIGN. Monocytes/macrophages and monocytoid DCs, but not plasmacytoid DCs, displayed significant increases in surface expression of Toll-like receptor 1 (TLR1), TLR2, and TLR4. Staining for CD45RO and CD27 revealed that lipopeptides preferentially recruited antigen-experienced T-cell subsets; despite their lack of antigenicity, these agonists induced marked T-cell activation, as evidenced by surface expression of CD69, CD25, and CD71. Lipopeptides also induced significant increases in interleukin 12 (IL-12), IL-10, gamma interferon, and most notably IL-6 without corresponding increases in serum levels of these cytokines. Although lipopeptides and EM lesional infiltrates shared many similarities, differences were noted in a number of immunologic parameters. These studies have provided in situ evidence for a prominent “lipoprotein effect” during human infection while at the same time helping to pinpoint aspects of the cutaneous response that are uniquely driven by spirochetal pathogens.
Syphilis and Lyme disease (LD) are acute and chronic inflammatory disorders caused by the spirochetal pathogens Treponema pallidum and Borrelia burgdorferi, respectively (49, 74). Both are major threats to public health within the United States and globally (37, 75). Both diseases begin with a distinctive lesion at the site of inoculation (a genital sore or chancre in the case of syphilis versus erythema migrans in Lyme disease) followed by a variety of extracutaneous manifestations once the spirochetes disseminate hematogenously. Syphilis and LD also are characterized by highly similar histopathological abnormalities (24, 49), suggesting that their etiologic agents elicit inflammatory responses in skin and other tissues via common mechanisms and pathways.
T. pallidum and B. burgdorferi lack lipopolysaccharide (LPS), the proinflammatory constituent in the outer membranes of gram-negative bacteria (81), but contain an abundance of lipoproteins (11, 16, 18, 27, 28). There is now an extensive body of in vitro evidence that spirochetal lipoproteins and synthetic lipoprotein analogs (lipopeptides) are potent activators of innate immune cells and that these pathogen-associated molecular patterns (PAMPs) trigger cellular activation by binding to the pattern recognition receptors CD14 and Toll-like receptor 1 (TLR1) and TLR2 on the surfaces of monocytes/macrophages and dendritic cells (DCs) (2, 3, 12, 31, 36, 42, 47, 69, 77, 87). Most recently, Wooten and colleagues (86) showed that macrophages from TLR2-deficient mice have a dramatically diminished response to B. burgdorferi lysates, indicating that lipoproteins are the major PAMP in the spirochete. Based upon these in vitro studies, it has been surmised that treponemal and borrelial lipoproteins are major proinflammatory agonists in syphilis and LD (64, 88). While studies with knockout mice have clearly shown that these PRRs are essential for containing spirochetal infection (2, 9, 10, 48, 63, 84, 86), they constitute only indirect evidence that lipoproteins are the actual spirochetal constituents responsible for initiating protective innate responses.
Skin is a major target organ in both syphilis and LD and is easily accessible for studying tissue-based immune processes evoked by spirochetes and spirochetal products. In two prior reports (65, 70), we described the use of the epidermal suction blister technique, in conjunction with multiparameter flow cytometry, as an alternative to conventional immunohistochemistry for characterizing cellular infiltrates within spirochete-infected skin (i.e., erythema migrans [EM]) and skin injected with synthetic lipohexapeptides based on the N termini of two T. pallidum lipoproteins (TpN47 and TpN17). In this report, we used this methodology to further analyze the complex mixture of leukocyte immunophenotypes recruited into skin by spirochetal lipopeptides and we compared these results to recent findings for patients with EM, the hallmark cutaneous lesion of LD (65). These studies have provided in situ evidence for a prominent “lipoprotein effect” during human infection while at the same time helping to pinpoint aspects of the cutaneous response that are uniquely driven by spirochetal pathogens. Our work also illustrates how TLR-dependent responses set the stage for adaptive immunity at the tissue level while being subject to inherent constraints that safeguard against runaway inflammatory and autoimmune processes.
MATERIALS AND METHODS
Human subjects.
Healthy volunteers without a history of syphilis or LD were recruited by the General Clinical Research Center at the University of Connecticut Health Center (UCHC). A total of 53 participants, 26 males and 27 females, ranging in age from 18 to 65 years of age, were enrolled. After written informed consent was obtained, a complete physical examination was performed, lipopeptides were injected, and epidermal blisters were raised as described below; for some patients, peripheral blood (PB) only was drawn to establish normal values for leukocyte staining panels. LD patients were those previously described (65). The UCHC Institutional Review Board approved all of the protocols used in this study.
Synthetic lipopeptides.
Lipohexapeptides corresponding to the N termini of outer surface protein C of B. burgdorferi B31 (BBB19) and the 17-kDa lipoprotein immunogen of T. pallidum (TP0435), designated OspC-L and 17-L, respectively, were synthesized and purified by Bachem Bioscience, Inc. (King of Prussia, PA). All reagents had negligible amounts of LPS (<1 pg LPS/μg protein) as measured by QCL-1000 quantitative, chromogenic Limulus assay (BioWhittaker, Inc., Walkersville, MD) and were tested for sterility by the Laboratory Medicine core facility at UCHC. Lyophilized lipopeptides were suspended by extensive vortexing in sterile normal saline (Abbott Laboratories, North Chicago, IL).
Elicitation of epidermal suction blisters.
For the lipopeptide portion of the study, visits took place over three consecutive days. On the first day, subjects were injected intradermally with 0.1 ml of lipopeptide solution (1.0 mg/ml) in each of three sites on the volar surface of the arm. Twenty-four hours later, epidermal blisters were raised as previously described (65, 70) by applying mild suction (200 mm Hg) through acrylic cups applied to the skin surface and gentle warming with a 125-W infrared lamp for 2 h. One suction cup was applied to each of the injection sites. A thin coating of high-vacuum silicone lubricant (Dow Corning, Midland, MI) was applied to the underside of the suction cup to ensure an airtight seal with the surface of the skin. Fluid (BF) was aspirated from the blisters 24 h later, at which time 10 ml of blood also was drawn. Blisters were raised over EM lesions as described above, and blood was drawn either on the first visit or 24 h later when the blisters were aspirated (65).
Serological assays.
Subjects were tested for serologic evidence of LD or syphilis depending on whether they received T. pallidum or B. burgdorferi lipopeptides. Enzyme-linked immunosorbent assay and immunoblot assays for LD and Venereal Disease Research Laboratory tests for syphilis were performed by the UCHC clinical laboratory using standard methodologies. Immunoblots for LD were interpreted based on standard criteria established by the Centers for Disease Control and Prevention (17).
Antibody conjugates.
The majority of antibody conjugates were purchased from BD Biosciences Immunocytometry Systems (BDIS) (San Jose, CA) or PharMingen (Sacramento, CA). CD1a-phycoerythrin (PE) was obtained from Caltag Laboratories (Burlingame, CA). CD45RO-fluorescein isothiocyanate was purchased from DAKO Corporation (Carpinteria, CA). DC-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-PE was purchased from R&D Systems (Minneapolis, MN). TLR-PE conjugates were purchased from eBioscience (San Diego, CA). Isotype-matched antibody conjugates were obtained from BDIS. The antibody panels used for four-color staining of immunophenotypes in BF and PB are described in Table 1. Each panel was used on a minimum of four normal volunteers or patients.
TABLE 1.
Antibody staining panels utilized in this studya
| Panel | Target(s) of staining
|
Expression or cell populations identified | |||
|---|---|---|---|---|---|
| FITC | PE | PerCP | APC | ||
| 1 | CD14 | CD20 | CD45 | CD38 | Major leukocyte populations |
| 2 | Lineage cocktail | DC-SIGN and CCR5 | HLA DR | CD11c | Activation/maturation markers on DCs |
| 3 | Lineage cocktail | CD83, CD83, and CD86 | CD86 | CD11c | Costimulatory molecules on DCs |
| 4 | TCR gamma/delta | CD8 | CD3 | CD4 | Basic T-cell subsets |
| 5 | CD45RO | CD27 | CD3 | CD4 | Differentiation of T cells |
| 6 | CD69 | CD25 | CD3 | CD4 | Activated T cells |
| 7 | CD45RO | CD71 | CD3 | CD4 | Early T-cell proliferation marker |
| 8 | Lineage cocktail | TLR1, TLR2, or TLR4 | HLA DR | CD11c | Expression of TLRs by PBMCs |
TCR, T-cell receptor; PBMCs, peripheral blood mononuclear cells; APC, antigen-presenting cells.
Cell staining and flow cytometry.
Erythrocyte-depleted leukocytes from PB and cells from BF were prepared for fluorescence-activated cell sorting (FACS) analysis as illustrated in our earlier publications (65, 70). Cells were incubated with 10 μg of purified human immunoglobulin G, followed by incubation with fluorochrome-conjugated antibodies. Aliquots of erythrocyte-depleted leukocytes also were incubated with a single fluorochrome-conjugated antibody or isotype-matched control antibodies to compensate for fluorescence emission overlap and nonspecific fluorescence, respectively. FACS analysis was performed on a FACSCalibur dual-laser flow cytometer (BDIS) using a threshold of 52 and appropriate forward and side scatter gates to exclude dead cells, cellular debris, and residual erythrocytes. A minimum of 60,000 events were collected from BFs for each staining panel. List mode multiparameter files (consisting of forward and orthogonal scatter and three or four fluorescence parameters) were analyzed using PAINT-A-GATEPRO (version 3.0) software (BDIS). Subpopulations of interest were quantified as percentages of total events (or a gated subset thereof), and their mean fluorescence intensities (MFIs) were calculated.
Cytokine measurements.
The Cytokine Bead Array kit (BD Biosciences, San Diego, CA) was used for simultaneous measurement of gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), interleukin 10 (IL-10), IL-6 or IL-5, IL-4, and IL-2 in sera and BFs (21). Fifty-microliter portions of each specimen were added to equal volumes of the cytokine bead mixture and detection reagent, followed by 3 h of incubation at room temperature in the dark. Ten additional tubes, each containing equal volumes of beads, detection reagent, and graded amounts of the six cytokines, were prepared in parallel to generate a standard curve for each cytokine. Unstained, fluorescein isothiocyanate- or PE-labeled cytometer setup beads were prepared towards the end of the sample incubation period. At the end of the incubation period, beads were washed with the buffer provided in the kit and centrifuged at 200 × g for 5 min, and the supernatants were carefully aspirated. The pellets were resuspended in 300 μl of the wash buffer provided in the kit and assayed immediately on the FACSCalibur instrument; cytokine concentrations were determined using the software provided. IL-12 was measured using the Cytoscreen Ultrasenstitve solid-phase sandwich enzyme-linked immunosorbent assay from Biosource International (Camarillo, CA).
Statistics.
Statistical analysis was performed utilizing Analyze-it general statistical software (Analyze-it Software, Ltd., Leeds, England) for Microsoft Excel. For each analysis, we utilized either a paired or unpaired t test or the appropriate nonparametric alternative (Wilcoxon). All tests were two tailed and were carried out to the 0.05 level of significance. For all values we calculated both the standard deviations and 95% confidence intervals of the mean. Cytokine values were also compared by paired t tests for blister fluid and its serum counterparts and unpaired t tests between groups.
RESULTS
T. pallidum and B. burgdorferi lipopeptides elicit cellular infiltrates enriched in DCs and activated monocytes/macrophages.
We previously reported (70) that intradermal injection of synthetic lipopeptides based on the N termini of TpN17 and TpN47 induced dose-dependent erythema and induration observable within 24 h. BFs obtained during the peak response, approximately 48 h postinoculation, were highly cellular, whereas fluids aspirated from sites injected with the corresponding nonlipidated peptides contained on the order of 100-fold-lower cell concentrations that precluded accurate flow-cytometric analysis. Based on these results, parallel injections with nonlipidated peptides were discontinued. Because a principal objective of the present study was to assess EM lesions for the presence of potential lipoprotein-mediated responses, we began by synthesizing a surrogate for OspC, a major lipoprotein antigen expressed by B. burgdorferi within feeding ticks and during early infection (45, 78), and comparing its in vivo proinflammatory activity to that of the well-characterized TpN17 lipopeptide (17-L). One-hundred-microgram doses of either lipopeptide elicited infiltrates that were enriched in monocytes/macrophages and DCs compared to donor-matched PB (Table 2). The threefold-greater enrichment of DCs in the 17-L BFs suggested that the T. pallidum lipopeptide might be slightly more biologically active than its borrelial counterpart. With both lipopeptides, monocytes/macrophages in BFs expressed greater levels of CD14 (BF MFI, 878.2, versus PB MFI, 217.1; P = 0.001) and HLA-DR (BF MFI, 371.0, versus PB MFI, 74.0; P = 0.03) on their surfaces and were larger and more granular by forward and side scatter characteristics than their circulating counterparts, indicating that the microenvironment established by these agonists promotes monocyte-to-macrophage differentiation. Blisters were not raised over uninjected healthy skin, because in our experience the cellular infiltrate obtained from these lesions is so sparse that it is not amenable to flow-cytometric analysis (70).
TABLE 2.
Mean percentages of leukocyte populations in PB and BFa
| Cell type | % Cell population
|
||||
|---|---|---|---|---|---|
| PB-NL (n = 5) | OspC-1
|
17-L
|
|||
| PB (n = 13) | BF (n = 13) | PB (n = 9) | BF (n = 9) | ||
| PMNd | 56.6 (46.9-66.4) | 46.2 (36.7-55.7) | 36.4 (26.3-46.4) | 42.6 (26.1-59.1) | 39.3 (27.3-51.3) |
| T cells | 24.4 (15.1-33.3) | 32.4 (23.8-41.0) | 30.02 (15.9-44.1) | 34.2 (23.5-44.8) | 25.1 (20.6-29.6) |
| Monocytes | 8.1 (5.1-10.9) | 8.7 (6.7-10.6) | 18.0 (10.5-25.5)c | 11.0 (8.3-13.6) | 18.6 (11.2-25.9)c |
| Plasma cells | 0.05 (0.03-0.07) | 0.02 (0.01-0.04) | 0.02 (0-0.05) | ND | ND |
| B cells | 2.5 (0-14.5) | 2.8 (1.1-4.4) | 0.5 (0.1-0.8) | 4.4 (0.2-8.5) | 0.8 (0.05-1.2) |
| Dendritic cells | 0.5 (0.3-0.6) | 0.4 (0.3-0.5) | 3.1 (1.9-4.2)bc | 0.4 (0.2-0.5) | 9.3 (3.4-15.2)c |
Comparison between healthy control PB (PB-NL) and PB and BF from subjects injected with either OspC-1 or 17-l. Numbers shown are mean percentages with 95% confidence intervals in parentheses.
Significantly different (P = 0.02), OspC-1 BF DCs versus 17-l BF DCs.
Significantly different (P < 0.05), BF versus PB.
Polymorphonuclear leukocytes.
Spirochetal lipopeptides selectively activate monocytoid DCs in vivo and induce upregulation of DC-SIGN.
We next characterized the DC subpopulations in OspC-L BFs by staining with a panel of markers previously used to assess the activation/maturation states of DCs elicited by injection of 17-L (70). The results of these experiments are summarized in Table 3. Surface expression of CD11c distinguishes the two principal DC subsets in PB (20). OspC-L BFs contained both monocytoid (CD11c+ or mDCs) and plasmacytoid (CD11c− or pDCs) subsets in ratios very similar to those in PB. Surface expression of CD83 and the costimulatory molecules CD80 and CD86 was greatly enhanced on monocytoid but not on plasmacytoid DCs. The DC staining results for OspC-L were so similar to those previously observed for 17-L (70) that we did not consider it necessary to conduct head-to-head comparisons of the two lipopeptides on skin-infiltrating DCs. Instead, we extended our panel of DC activation/maturation markers by examining the effect of lipopeptide (17-L) on surface expression of DC-SIGN, a member of the C-type lectin family that has attracted considerable attention in recent years because of its involvement in DC-T-cell interactions during antigen priming, microbial recognition and signaling, and transmission of human immunodeficiency virus (HIV) (14, 59, 82). As shown in Table 3, injection of OspC-L and 17-L induced a highly significant upregulation of DC-SIGN on mDCs and monocytes/macrophages but not on pDCs.
TABLE 3.
Mean percentages of dendritic cell immunophenotypes in PB and BFa
| Surface antigen | % Cells with immunophenotype
|
|||
|---|---|---|---|---|
| Lipopeptide-injected subjects
|
Control blood (PB-NL) (n = 6) | |||
| Sample size | PB | BF | ||
| CD11c+ DCs | 22 | 50.2 (41.9-58.5) | 60.6 (50.7-70.7) | 61.0 (47.7-74.2) |
| CD83+ | 5 | 0.4 (−0.4-0.9) | 53.0 (5.4-100.6)b | 0.17 (−0.3-0.6) |
| CD86+ | 5 | 13.8 (−0.8-28.4) | 59.9 (32.01-87.6)b | 5.6 (−7.9-19.2) |
| CD80+ | 5 | 1.5 (−0.2-3.3) | 38.8 (14.5-63.1)b | 2.5 (−0.26-5.3) |
| DC-SIGNc | 6 | 5.0 (0.4-9.7) | 46.7 (19.5-73.8)c | 7.3 (0.4-14.2) |
| CD11c− DCs | 19 | 47.7 (39.1-56.2) | 38.1 (27.5-48.6) | 38.7 (22.7-54.6) |
| CD83+ | 5 | 0 | 1.9 (−1.8-5.7) | 0.3 (−0.5-1.1) |
| CD86+ | 5 | 12.6 (−2.7-27.9) | 9.3 (−5.9-24.6) | 2.9 (−1.9-7.6) |
| CD80+ | 5 | 15.1 (−6.0-36.2) | 1.7 (0.1-3.2) | 11.3 (−1.1-23.8) |
| DC-SIGNc | 6 | 3.2 (−1.2-7.8) | 12.2 (−9.5-33.9) | 6.8 (0.5-13.2) |
Numbers shown are mean percentages with 95% confidence intervals.
Significantly different (P < 0.05), PB versus BF.
DC-SIGN analysis includes three samples for OspC-1 and three samples for 17-l.
Lipopeptides induce enhanced expression of TLRs on monocytes/macrophages and mDCs.
In vitro studies have established that cellular activation by TLR-dependent ligands influences both TLR expression profiles and responsiveness to cognate and noncognate PAMPs (26, 67, 73). To learn more about the in vivo effects of PAMPs on TLR expression, we assessed how lipopeptides affect TLRs on skin-infiltrating leukocytes. In addition to TLR1 and TLR2, we analyzed expression of TLR4, the LPS receptor (60), as a means of detecting cross talk between TLR signaling pathways. The results of these experiments are presented in Table 4. Marked increases in the percentages of mDCs and monocytes/macrophages expressing all three TLRs were observed in BFs. Small increases in surface staining for TLR2 also were observed on BF T cells, with the increases for OspC-L being statistically significant. This finding is provocative in light of recent work suggesting that TLR2 serves as a costimulatory receptor for antigen-specific T-cell development and participates in the maintenance of T-cell memory (41).
TABLE 4.
Mean percentage expression of Toll-like receptors (TLR-1, -2, and -4) on dendritic cells, macrophages, and T cellsa
| Cell group and TLR | % Expression of TLR
|
|||||
|---|---|---|---|---|---|---|
| PB-NL (n = 6) | OspC-1
|
17L
|
EM in BF (n = 5) | |||
| PB (n = 4) | BF (n = 4) | PB (n = 5) | BF (n = 5) | |||
| CD11c+ DCs | ||||||
| TLR1 | 1.0 (0.3-1.7) | 1.2 (−0.2-2.5) | 37.4 (−19.3-94.1)b | 0.34 (0-0.7) | 50.5 (23.4-77.7)b | 74.3 (54.8-93.6) |
| TLR2 | 2.9 (0-5.7) | 3.0 (−1.7-7.9) | 56.6 (20.2-92.9)b | 1.6 (−0.1-.3) | 60.7 (41.3-80.1)b | 80.9 (65.4-96.4) |
| TLR4 | 1.7 (0-3.9) | 2.1 (−0.9-5.1) | 45.8 (25.6-65.9)b | 5.6 (−5.8-17.0) | 55.8 (36.8-74.7)b | 80.6 (68.49-92.6) |
| CD11c− DCs | ||||||
| TLR1 | 2.7 (0-5.0) | 1.9 (−1.3-5.6) | 4.1 (0.2-8.0) | 2.9 (−3.0-8.8) | 3.9 (−1.6-9.3) | 33.2 (9.6-56.7) |
| TLR2 | 2.7 (0-5.7) | 0.8 (−0.4-1.9) | 6.6 (−10.0-23.2) | 0.5 (−0.2-1.3) | 6.5 (−5.2-18.3) | 29.9 (6.7-53.1) |
| TLR4 | 5.2 (0-3.9) | 1.2 (0.2-2.2) | 1.7 (−0.4-3.7) | 1.0 (−0.8-2.9) | 10.7 (1.3-20.1) | 30.2 (1.3-58.9) |
| Macrophages | ||||||
| TLR1 | 1.3 (0.4-2.17) | 5.7 (−1.3-12.7) | 49.4 (−36.1-134.9)b | 3.6 (0.9-6.4) | 44.9 (0.3-89.5)b | 57.2 (30.7-83.6) |
| TLR2 | 1.9 (0.2-3.5) | 6.2 (−4.7-17.1) | 58.9 (−1.7-119.4)b | 3.3 (−0.3-6.7) | 70.8 (30.5-111.0)b | 60.1 (40.2-87.8) |
| TLR4 | 12.4 (10.8-13.9) | 7.7 (−4.4-19.7) | 52.4 (11.0-93.7)b | 1.8 (0.6-3.0) | 55.6 (31.1-80.0)b | 53.3 (24.4-82.1) |
| T cells | ||||||
| TLR1 | 0.8 (0.2-1.3) | 0.7 (0.4-0.8) | 1.3 (0.1-2.7) | 1.9 (−2.9-6.8) | 1.4 (−0.7-3.5) | 1.6 (0.69-2.4) |
| TLR2 | 0.4 (0.09-0.69) | 0.6 (0.4-0.8) | 2.8 (0.5-5.1)b | 0.9 (−0.1-1.8) | 3.3 (0.4-6.2) | 3.2 (0-7.1) |
| TLR4 | 0.6 (0-1.2) | 0.6 (0.07-1.2) | 1.3 (0.4-2.1) | 0.9 (0-1.7) | 5.3 (−4.1-14.6) | 2.2 (0.51-4.9) |
Results are shown for peripheral blood from normal volunteers (PB-NL), OspC-1, and 17L elicited BF and corresponding PB and EM lesions. Mean percentages with 95% confidence intervals in parentheses.
Significantly different (P < 0.05), OspC-1 PB versus OspC-1 BF.
Significantly different (P < 0.05), PB versus BF.
Selective recruitment and activation of antigen (Ag)-experienced T-cell immunophenotypes by lipopeptides.
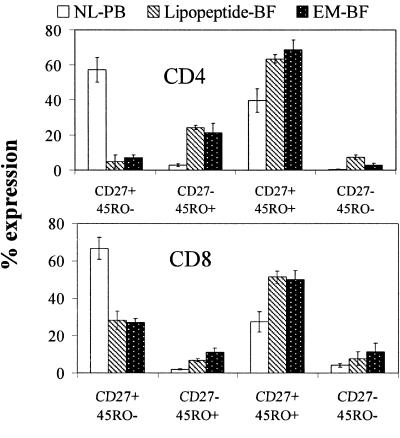
We previously noted that a large majority (ca. 80%) of the T cells in lipopeptide BFs expressed the skin homing receptor cutaneous lymphocyte antigen (CLA) (70). While CLA expression is known to be restricted to T cells that were Ag sensitized in skin-draining lymph nodes (43), this result provided little insight into the types of memory and effector-T-cell subsets that are recruited to skin in our experimental human model. To obtain this information, we stained T cells in PB and BFs for CD27 and CD45RO, surface markers commonly used to distinguish naive from Ag-experienced T lymphocytes; it should be noted that the functions of T-cell subpopulations expressing combinations of these markers have been extensively studied during human infection (4, 34, 35). As shown in Fig. 1, with both CD4+ and CD8+ T cells, there was unambiguous skewing towards Ag-sensitized immunophenotypes within the cutaneous milieu. This trend was particularly notable for CD4+ T cells, which, compared to their PB counterparts, were significantly enriched for cells belonging to the memory (CD27+/CD45RO+), memory-effector (CD27−/CD45RO+), and effector (CD27−/CD45RO−) subsets.
FIG. 1.
Characterization (percentage of cells and standard error of the mean) by flow cytometry of CD4+ and CD8+ T lymphocytes based upon surface expression of CD45RO and CD27. Cells in PB of a normal volunteer (NL-PB) or in blister fluid from lipopeptide-injected sites (lipopeptide-BF) or erythema migrans lesions from a Lyme disease patient (EM-BF) were stained with antibody conjugates specific for CD45R0, CD27, CD4, and CD3. CD8+ T lymphocytes were identified as CD3+/CD4− cells.
We also previously observed that approximately 10% of the T cells in lipopeptide BFs were HLA-DR+ as opposed to only 1 to 2% of circulating T cells (70). This result, suggesting that T lymphocytes become activated in the inflammatory milieu established by lipopeptides, was intriguing given that these agonists, unlike their full-length, native counterparts, are not bona fide antigens. We therefore stained BF and PB T cells for surface expression of CD69 and CD25 in order to more fully assess the activation states of the skin-infiltrating T cells. As shown in Table 5, approximately 40% of CD4+ and CD8+ T cells expressed one or both activation markers, percentages far greater than those in PB. The greater percentages of both CD4+ and CD8+ cells expressing CD25 were consistent with the more transient expression kinetics of CD69 when T cells are stimulated with mitogen or antigen in vitro (15). Small but statistically significant percentages of both CD4+ and CD8+ T cells also expressed the early proliferation marker CD71 (33); expression of this antigen was largely confined to CD45RO+ (i.e., memory) T-cell subsets (Table 5).
TABLE 5.
Characterization of T-cell immunophenotypes in PB and BFa
| Surface antigens or phenotype | % Cells with immunophenotype
|
||
|---|---|---|---|
| PB-NL | OspC-1 and 17-lc
|
||
| PB | BF | ||
| CD4/CD8d | |||
| CD3+ CD4+ | 57.0 (48.1-65.9) | 62.7 (56.6-68.9) | 67.7 (61.9-73.5) |
| CD3+ CD8+ | 34.1 (22.7-45.3) | 37.2 (30.9-43.4) | 32.3 (26.5-38.1) |
| CD25/CD69e | |||
| CD4+ CD25+ CD69− | 6.25 (0.8-11.6) | 7.9 (4.5-11.3) | 21.3 (13.9-28.6)b |
| CD4+ CD25− CD69+ | 0.3 (0.04-0.6) | 1.2 (−1.2-3.6) | 13.9 (7.1-20.7)b |
| CD4+ CD25+ CD69+ | 0.02 (0-0.06) | 0.1 (0.00-0.2) | 10.8 (0.5-21.1)b |
| CD4+ CD25− CD69− | 93.4 (88.2-98.6) | 90.5 (88.8-92.2) | 54.0 (37.8-70.2)b |
| CD8+ CD25+ CD69− | 0.5 (0.02-0.99) | 0.8 (−0.1-1.6) | 27.4 (4.5-50.5)b |
| CD8+ CD25− CD69+ | 1.5 (−0.3-3.4) | 0.5 (0.1-0.9) | 3.8 (0.2-7.4) |
| CD8+ CD25+ CD69+ | 0 (0-0) | 0.1 (0.001-0.1) | 6.3 (0.2-7.4) |
| CD8+ CD25− CD69− | 97.9 (96.1-99.8) | 98.7 (97.9-99.5) | 62.4 (35.7-89.0)b |
| CD45RO/CD71f | |||
| CD4+ CD71+ CD45RO− | 0.1 (0-0.2) | 0.2 (−0.2-0.3) | 0 (0-0) |
| CD4+ CD71− CD45RO+ | 51.5 (40.1-52.9) | 30.7 (21.3-40.2) | 77.2 (67.2-87.1)b |
| CD4+ CD71+ CD45RO+ | 0.62 (0.4-0.9) | 0.6 (0.4-0.9) | 10.7 (5.5-15.8)b |
| CD4+ CD71− CD45RO− | 49.46 (36.1-59.2) | 68.3 (59.1-77.6) | 11.5 (4.5-18.5)b |
| CD8+ CD71+ CD45RO− | 0.12 (0-0.3) | 0.1 (0.02-0.2) | 1.1 (0.2-1.9) |
| CD8+ CD71− CD45RO+ | 32.4 (23.7-41.1) | 20.7 (13.9-27.4) | 45.8 (31.5-60.1)b |
| CD8+ CD71+ CD45RO+ | 0.2 (0.1-0.3) | 0.2 (0.1-0.2) | 4.6 (2.4-6.8)b |
| CD8+ CD71− CD45RO− | 67.3 (58.6-76.1) | 79.1 (72.3-85.7) | 48.5 (32.8-64.2)b |
Numbers shown are mean percentages with 95% confidence intervals in parentheses. PB-NL, normal (control) blood.
Significantly different (P < 0.05), PB versus BF.
Each T-cell panel is derived from a combination of subjects injected with either 17-l or OspC-1.
For PB-NL, n = 11 samples; for PB, n = 12 samples; for BF, n = 12 samples.
For PB-NL, n = 5 samples; for PB, n = 7 samples; for BF, n = 7 samples.
For PB-NL, n = 5 samples; for PB, n = 7 samples; for BF, n = 6 samples.
In vivo cytokine responses elicited by lipopeptides. Measurement of cytokine levels was performed to further elucidate lipopeptide-induced responses in vivo (Table 6). Not unexpectedly, serum cytokine levels in volunteers receiving lipopeptides were extremely low and did not differ significantly from levels in uninjected controls. Compared to PB, BFs from lipopeptide-injected sites contained modest but significantly increased levels of IL-10 and strikingly increased concentrations of IL-6. It should be noted that these IL-6 values are 5- to 10-fold greater than those measured in BFs from normal skin (65). Interestingly, although microbial lipoproteins/lipopeptides are strong in vitro inducers of TNF-α and IL-12 secretion by DCs and monocytes/macrophages (42, 62, 79), TNF-α was not detected in lipopeptide BFs, while IL-12 showed only a minimal increase. Moreover, despite the flow-cytometric evidence for T-cell activation, we were unable to detect increases in the T-cell-derived cytokine IL-4. Using an in vitro system in which DCs are preactivated with PAMPs, Agrawal et al. (1) found that the generic microbial lipopeptide Pam3Cys-Ser-Lys4 skewed T-cell differentiation towards a limited Th2 response based on detection of IL-5 but not IL-4 in culture supernatants. To be sure we were not overlooking an analogous, limited Th2 response in our human model, we also assayed IL-5 levels in BFs from six OspC-L-injected recipients. Levels of this cytokine in BFs were no greater than those in the corresponding donor sera (Table 6).
TABLE 6.
Cytokine levels in blister fluid from lipopeptide-injected subjects (OspC-1 and 17-L) and erythema migrans lesions (EM)a
| Cytokine | Cytokine level in BF
|
|||
|---|---|---|---|---|
| Control blood (n = 6) | OspC-1 (n = 14) | 17-L (n = 7) | EM (n = 20) | |
| IFN-γ | 12.6 (7.8-17.3) | 73.9 (21.4-126.5)b | 55.2 (8.6-101.7)b | 3590.4 (390.8-6790.9)c |
| TNF-α | 1.9 (0.9-2.9) | 11.7 (2.0-21.5) | 3.7 (1.8-5.5) | 31.0 (9.4-52.6) |
| IL-2 | 4.5 (2.9-6.0) | 11.5 (0.1-23.0) | 6.5 (2.7-9.1) | 12.6 (5.4-19.8) |
| IL-4 | 5.7 (2.3-9.1) | 11.4 (2.2-20.7) | 7.1 (3.3-10.9) | 15.1 (10.2-20.1) |
| IL-5 | 6.9 (−2.3-16.2) | 12.2 (−2.8-27.2) | ND | 24.9 (19.8-30.1) |
| IL-6 | 11.7 (−11.8-35.3) | 1448 (34.6-2863.2)b | 2309.1 (412.7-4205.5)b | 3535.9 (1207.8-5864.1)b |
| IL-10 | 2.5 (1.9-3.2) | 47.1 (22.4-71.4)b | 303.4 (−64.4-671.2)b | 84.1 (7.4-169.8)b |
| IL-12 | 21.4 (−8.0-50.9) | 52.3 (0.8-103.9) | 88.5 (49.1-127.9)b | 1495.7 (−7.9-2999.7)d |
Numbers shown are mean pictogram values with 95% confidence intervals in parentheses.
Significantly different (P < 0.05), lipopeptide-induced BF versus control PB.
Significantly different (P < 0.05), lipopeptide-induced BF versus EM.
P = 0.058, OspC1 versus EM from Lyme disease patients; and P = 0.06 (17-l versus LD).
Lipopeptides and LD spirochetes elicit overlapping but distinct cutaneous cellular and cytokine responses.
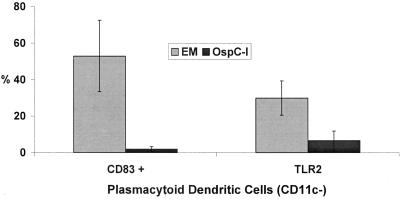
Finally, we compared the leukocyte subsets and cytokines elicited by lipopeptides with those observed during actual infection (i.e., EM) as a means of identifying components of the local response to B. burgdorferi that are likely caused by borrelial lipoproteins. Both stimuli elicited cellular infiltrates with similar composition, the principal difference being the statistically significant enrichment for T cells observed in EM BFs (65). The percentages of memory, memory/effector, and effector T cells within the two set of BFs, on the other hand, were remarkably similar (Fig. 1). Monocytoid DCs in both sets of BFs showed similarly high levels of expression of CD83 and CD80. CD80 was not detected on plasmacytoid DCs in either group of fluids. A striking dichotomy was observed for the maturation marker CD83, which was significantly upregulated only on EM-derived plasmacytoid DCs (Fig. 2). A similar, though less pronounced, dichotomy also was observed for expression of TLRs on plasmacytoid DCs from the two sets of BFs (Fig. 2 and Table 4). Differences also were evident in the cytokine profiles of the two sets of fluids. IL-6 levels, already quite elevated in lipopeptide BFs, were even higher in EM BFs; even more notable were the markedly elevated levels of IFN-γ and IL-12 in EM BFs (Table 6).
FIG. 2.
Characterization of CD83 and TLR-2 expression by plasmacytoid dendritic (CD11c-) cells from blister fluid derived from erythema migrans lesions (EM) and blister fluid from lipopeptide (OspC-l)-injected skin lesions. DCs were identified as lineage-negative, HLA-DR+ cells and then further gated with respect to the expression of CD11c to identify plasmacytoid (CD11c−) subsets. Bar graphs shown represent the means and corresponding standard errors.
DISCUSSION
Despite numerous reports documenting the proinflammatory activities of microbial lipoproteins and corresponding synthetic analogs, strategies for assessing the in situ biological activities of these proinflammatory agonists and evaluating their contribution to the response elicited by the virulent pathogen have been hard to devise, particularly with humans. Whereas in vitro studies of innate or adaptive responses tend to focus on one or a limited number of immune cell types, the suction blister methodology affords a comprehensive picture of cellular responses elicited by inflammatory stimuli within a complex tissue environment highly relevant to spirochetal infection, human skin. Moreover, as noted below, in a number of instances, findings from our human model differed, sometimes strikingly, from those obtained in analogous in vitro or ex vivo investigations. Ideally we would have liked to obtain specimens from secondary syphilis patients for comparison with EM lesions and sites injected with lipopeptides. However, even with the current upsurge among men who have sex with men, incidence rates for new cases of syphilis have dropped substantially in the United States during the past decade (25). Nevertheless, we believe that our results can be extrapolated to syphilis given the abundance of lipoproteins in both T. pallidum and B. burgdorferi, the stereotypical nature of responses to these TLR2/1-dependent agonists in vitro and in vivo (42, 50, 51, 53, 54, 61), corroborated further herein using OspC-L and 17-L, and the similar histopathological abnormalities induced by these two pathogens (24, 49).
Intradermal injection of lipopeptides and cutaneous infection with B. burgdorferi elicit a complex mixture of leukocytes derived from both the innate and adaptive arms of the cellular immune response. These results contrast with the overwhelming predominance of T lymphocytes in BFs from tuberculin skin reactions (57) and cells eluted from biopsies of contact hypersensitivity reactions (8). The substantial overlap in the composition of lipopeptide-induced and EM lesional infiltrates supports our principal hypothesis that during infection, spirochetal lipoproteins create a proinflammatory milieu that recruits from PB diverse leukocyte immunophenotypes capable of trafficking into inflamed skin (64). The chemotactic and homing signals that attract this mixed infiltrate presumably are generated initially by lipoprotein-responsive elements resident in uninflamed skin (43), such as vascular endothelium (29, 68), keratinocytes (58), and Langerhans cells (76), and then subsequently amplified as extravasating leukocytes join the local inflammatory cascade.
Monocytes/macrophages and mDCs are highly responsive to microbial lipoproteins and lipopeptides in vitro. The replication of these findings in our human model is in line with the premise that spirochetal lipoproteins are directly involved in the activation as well as the recruitment of these two principal innate immune cell types during infection. A novel observation along these lines was the upregulation of DC-SIGN on skin-infiltrating mDCs and monocytes/macrophages. Cross talk between DC-SIGN and TLR signaling pathways during microbial sensing and recognition already has been documented (82); to our knowledge, however, ours is the first report directly linking the stimulation of TLR2/1 pathways with upregulation of this C-type lectin. T. pallidum and treponemal lipoproteins/lipopeptides also induce expression of CCR5, the principal coreceptor for macrophage-tropic (i.e., sexually transmitted) HIV strains, on macrophages and DCs, and CCR5+ CD4+ T cells are recruited selectively into lipopeptide-injected skin (70, 71). The findings described herein for DC-SIGN, along with these earlier observations, demonstrate that spirochetes and/or spirochetal products have the capacity to bring together all of the cellular elements and surface molecules required for highly efficient transmission of the AIDS virus during sexual activity.
While activation of monocytes/macrophages and mDCs by lipopeptides in vivo was not unexpected, the cytokine profile of the lipopeptide BFs, most notably the absence of TNF-α and the modestly elevated levels of IL-12, was surprising in light of in vitro and ex vivo studies with these same or related agonists (23, 42, 62, 79). In contrast, elevated levels of TNF-α and markedly increased concentrations of IL-12 were observed in EM BFs. Consistent with our own findings, a number of investigators have reported that spirochetes and spirochetal lipoproteins stimulate macrophages to produce the anti-inflammatory mediator IL-10 (13, 22, 30, 32). IFN-γ, a hallmark cytokine of adaptive immunity, markedly augments production of IL-12 and TNF-α by monocytes and mDCs stimulated with diverse PAMPs and infectious agents (42, 46, 80). These two sets of observations provide a plausible explanation for the disparate cytokine profiles in lipopeptide and EM BFs. The anti-inflammatory effect of IL-10, acting unopposed during lipopeptide reactions, could be counterbalanced by the high levels of IFN-γ produced by Ag-stimulated T cells within infected skin.
Because monocytes isolated from PB will differentiate into DCs during in vitro incubation with monocyte-conditioned medium or combinations of cytokines, typically IL-4 and granulocyte-macrophage colony-stimulating factor, one might surmise that the mDCs in lipopeptides and EM BFs arose from extravasated monocytes. Multiple lines of evidence collectively argue, instead, that these two cell types, already diverged from a common progenitor in bone marrow (6), continue along distinct maturation pathways within inflamed skin in order to fulfill specialized functions in pathogen clearance (i.e., macrophages) and pathogen sensing (i.e., mDCs). Among these are (i) the higher levels of CD14 expressed on monocytes/macrophages in BF than in PB; (ii) the nearly identical ratios of mDC to pDC in BF and PB; (iii) the lack of appreciable IL-4 in both sets of BFs; and most importantly, (iv) the extremely high levels of IL-6, a cytokine known to promote differentiation of monocytes to macrophages rather than to DCs (19). The upregulation of surface TLRs observed on both mDCs and monocytes/macrophages indicates that these two leukocyte subpopulations continue to share a lipopeptide-mediated positive feedback loop that potentially enhances their responsiveness to the inciting agonist, as well as to other PAMPs, during a perceived time of danger.
Plasmacytoid DCs are a newly recognized DC subset with poorly defined roles in Ag presentation, immunoregulation, and pathogen sensing (20). Current notions about pDC function have been strongly influenced by their distinctive cytokine/chemokine secretory profiles, most notably their ability to produce copious amounts of IFN-α/β in response to viruses and viral nucleic acids, their limited ability to prime naive T cells, and immunocytochemical studies localizing them to secondary lymphoid tissues (20). As a result, it is widely believed that pDCs migrate from PB to inflamed lymph nodes, where they influence T-cell clonal expansion and polarization via the production of IFN-α and other inflammatory mediators (e.g., IL-12); mDCs, by contrast, are thought to have a unique capacity to traffic into infected peripheral sites, where they acquire Ag and then migrate to draining lymph nodes for T-cell priming (7, 20, 52, 56). Our findings that pDCs are enriched in lipopeptide-elicited and EM lesional infiltrates and that the ratios of pDCs and mDCs in the two sets of BFs mirror those in PB indicate that the two DC subsets manifest comparable skin-trafficking capabilities at least in response to some proinflammatory stimuli. Indeed, recent reports identifying pDCs in infiltrates associated with chronic inflammatory skin conditions (8, 85), synovial fluids from patients with rheumatoid and psoriatic arthritis (44), and cerebrospinal fluids from patients with inflammatory neurological disorders (55) are further evidence that these cells routinely recognize homing signals generated by nonlymphoid compartments. While lipopeptides and spirochetes were similarly capable of attracting circulating pDCs into skin, only the latter provided the requisite maturation signals for this DC subset. The inability of lipopeptides to activate pDCs is consistent with ex vivo studies showing that immature pDCs isolated from PB do not express TLR2 or -1 and are unresponsive to TLR2/1-dependent agonists (39, 40). Immature pDCs do, however, express TLR9, the receptor for unmethylated CpG motifs in bacterial DNA (39, 40), and B. burgdorferi DNA could, therefore, be responsible for the selective activation of pDCs in EM lesions. A striking and unexpected observation was that sizable percentages of pDCs in EM BFs stained positively for TLR1, -2 and -4, molecules which are not considered to be part of their TLR repertoire (38-40). Although the infection-specific signals inducing this infection-specific maturation program are unidentified, it is clear that pDCs possess a much greater plasticity in the TLR expression profile, and presumably corresponding PAMP responsiveness, than has been previously discerned. Still enigmatic is the function of these cells at the site of infection given their lack of expression of costimulatory molecules and the low levels of IFN-α in EM BFs.
In summary, we found that lipopeptides recruit to the skin all of the cellular elements required for a flexible and highly coordinated immune response. In addition to containing large numbers of innate effector cells (e.g., neutrophils and macrophages), the host's early line of defense, lipopeptide infiltrates also were enriched in activated DCs and memory/effector T cells. Consistent with the Th1 character of syphilitic and EM lesions (65, 83), the recruited T cells express CCR5 and CLA, Th1-associated surface markers (70), and, as shown here, secrete IFN-γ even in the absence of exogenous Ag. At the outset of infection, a situation analogous to the purely innate microenvironment induced by lipopeptide, a primary cellular response would ensue because the infiltrating memory/effector T cells do not see their cognate Ag. As infection progresses, however, there would be progressive recruitment of recently sensitized spirochete-specific T cells capable of participating in a local secondary reaction. The increased percentage of T cells in EM BFs, the markedly increased concentrations of IFN-γ in EM lesions, and the observation that EM patients have in their circulation CD27hi CD4+ and CD8+ T cells (65), immunophenotyes consistent with neosensitized T cells (5), argue in favor of this scenario. Upregulation of DC-SIGN, and perhaps other C-type lectin adhesions, would expedite the secondary reaction by facilitating transient binding of recruited T cells and antigen-presenting cells. Based on the widespread activation of T cells observed in response to lipopeptide stimulation, we can further postulate that the proinflammatory microenvironment not only drives the recruitment of memory T cells with diverse Ag specificities but also enhances their state of readiness for a possible encounter with Ag. The lowered threshold for Ag stimulation also could augment the ability of macrophages, which are less efficient at presenting Ag than DCs (66), to participate in the local secondary response.
One of the dangers inherent in an inflammatory response is that it will be uncontrolled, causing unnecessary tissue damage, or even worse, give rise to a self-perpetuating autoimmune state. The relatively rapid dissipation of the lipopeptide reaction is clear-cut evidence at the gross level for the existence of built-in safeguards to prevent innate induced, runaway events. Modlin and colleagues (72) observed that peripheral blood mononuclear cells incubated with bacterial lipopeptides produced large amounts of IFN-γ in a major histocompatibility complex-dependent manner, and they conjectured that the cytokine was induced by the presentation of endogenous peptides by macrophages. While the production of IFN-γ by T cells infiltrating lipopeptide injection sites observed herein is consistent with their ex vivo observations, the relatively modest levels of this cytokine induced by lipopeptides in situ imply either that the representation of self-reactive T cells at the lipopeptide injection is too low to sustain a response and/or that their responsiveness is dampened by other mediators and cell types at the reaction site. In either case, juxtaposition of the lipopeptide and EM BFs makes clear that the combination of PAMP and exogenous Ag, in the form of a replicating bacterial pathogen, is responsible for the positive feedback loops involving IFN-γ and IL-12, a watershed event that marks the transition from a purely innate response to a sustaining cellular reaction that will cause disease manifestations until the proinflammatory and Ag stimuli represented by the pathogen are eradicated.
Acknowledgments
We are indebted to Gene Pizzo for excellent technical assistance with flow cytometry.
This work was partially supported by Public Health Service grants AI-38894 (J.D.R.), General Clinical Research Center grant M01RR06192 from the National Institutes of Health, and grant DF 00-014 (J.C.S.) from The Donaghue Medical Research Foundation.
REFERENCES
- 1.Agrawal, S., A. Agrawal, B. Doughty, A. Gerwitz, J. Blenis, T. Van Dyke, and B. Pulendran. 2003. Cutting edge: different toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J. Immunol. 171:4984-4989. [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulou, L., V. Thomas, M. Schnare, Y. Lobet, J. Anguita, R. T. Schoen, R. Medzhitov, E. Fikrig, and R. A. Flavell. 2002. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat. Med. 8:878-884. [DOI] [PubMed] [Google Scholar]
- 3.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 4.Appay, V., P. R. Dunbar, M. Callan, P. Klenerman, G. M. Gillespie, L. Papagno, G. S. Ogg, A. King, F. Lechner, C. A. Spina, S. Little, D. V. Havlir, D. D. Richman, N. Gruener, G. Pape, A. Waters, P. Easterbrook, M. Salio, V. Cerundolo, A. J. McMichael, and S. L. Rowland-Jones. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379-385. [DOI] [PubMed] [Google Scholar]
- 5.Appay, V., and S. L. Rowland-Jones. 2004. Lessons from the study of T-cell differentiation in persistent human virus infection. Semin. Immunol. 16:205-212. [DOI] [PubMed] [Google Scholar]
- 6.Ardavin, C., S. Amigorena, and C. Reis e Sousa. 2004. Dendritic cells: immunobiology and cancer immunotherapy. Immunity 20:17-23. [DOI] [PubMed] [Google Scholar]
- 7.Banchereau, J., S. Paczesny, P. Blanco, L. Bennett, V. Pascual, J. Fay, and A. K. Palucka. 2003. Dendritic cells: controllers of the immune system and a new promise for immunotherapy. Ann. N. Y. Acad. Sci. 987:180-187. [DOI] [PubMed] [Google Scholar]
- 8.Bangert, C., J. Friedl, G. Stary, G. Stingl, and T. Kopp. 2003. Immunopathologic features of allergic contact dermatitis in humans: participation of plasmacytoid dendritic cells in the pathogenesis of the disease? J. Investig. Dermatol. 121:1409-1418. [DOI] [PubMed] [Google Scholar]
- 9.Benhnia, M. R., D. Wroblewski, M. N. Akhtar, R. A. Patel, W. Lavezzi, S. C. Gangloff, S. M. Goyert, M. J. Caimano, J. D. Radolf, and T. J. Sellati. 2005. Signaling through CD14 attenuates the inflammatory response to Borrelia burgdorferi, the agent of lyme disease. J. Immunol. 174:1539-1548. [DOI] [PubMed] [Google Scholar]
- 10.Bolz, D. D., R. S. Sundsbak, Y. Ma, S. Akira, C. J. Kirschning, J. F. Zachary, J. H. Weis, and J. J. Weis. 2004. MyD88 plays a unique role in host defense but not arthritis development in Lyme disease. J. Immunol. 173:2003-2010. [DOI] [PubMed] [Google Scholar]
- 11.Brandt, M. E., B. S. Riley, J. D. Radolf, and M. V. Norgard. 1990. Immunogenic integral membrane proteins of Borrelia burgdorferi are lipoproteins. Infect. Immun. 58:983-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 13.Brown, J. P., J. F. Zachary, C. Teuscher, J. J. Weis, and R. M. Wooten. 1999. Dual role of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect. Immun. 67:5142-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cambi, A., and C. G. Figdor. 2003. Dual function of C-type lectin-like receptors in the immune system. Curr. Opin. Cell Biol. 15:539-546. [DOI] [PubMed] [Google Scholar]
- 15.Caruso, A., S. Licenziati, M. Corulli, A. D. Canaris, M. A. De Francesco, S. Fiorentini, L. Peroni, F. Fallacara, F. Dima, A. Balsari, and A. Turano. 1997. Flow cytometric analysis of activation markers on stimulated T cells and their correlation with cell proliferation. Cytometry 27:71-76. [DOI] [PubMed] [Google Scholar]
- 16.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. M. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. 1995. Recommendations for test performance and interpretation from the second national conference on serologic diagnosis of Lyme disease. Morb. Mortal. Wkly. Rep. 44:590-591. [PubMed] [Google Scholar]
- 18.Chamberlain, N. R., M. E. Brandt, A. L. Erwin, J. D. Radolf, and M. V. Norgard. 1989. Major integral membrane protein immunogens of Treponema pallidum are proteolipids. Infect. Immun. 57:2872-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chomarat, P., J. Banchereau, J. Davoust, and A. K. Palucka. 2000. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat. Immunol. 1:510-514. [DOI] [PubMed] [Google Scholar]
- 20.Colonna, M., G. Trinchieri, and Y. J. Liu. 2004. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5:1219-1226. [DOI] [PubMed] [Google Scholar]
- 21.Cook, E. B., J. L. Stahl, L. Lowe, R. Chen, E. Morgan, J. Wilson, R. Varro, A. Chan, F. M. Graziano, and N. P. Barney. 2001. Simultaneous measurement of six cytokines in a single sample of human tears using microparticle-based flow cytometry: allergics vs. non-allergics. J. Immunol. Methods 254:109-118. [DOI] [PubMed] [Google Scholar]
- 22.Diterich, I., L. Harter, D. Hassler, A. Wendel, and T. Hartung. 2001. Modulation of cytokine release in ex vivo-stimulated blood from borreliosis patients. Infect. Immun. 69:687-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diterich, I., C. Rauter, C. J. Kirschning, and T. Hartung. 2003. Borrelia burgdorferi-induced tolerance as a model of persistence via immunosuppression. Infect. Immun. 71:3979-3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duray, P. H. 1989. Histopathology of clinical phases of human Lyme disease. Rheum. Dis. Clin. N. Am. 15:691-710. [PubMed] [Google Scholar]
- 25.Erbelding, E. 2003. 2001 syphilis rates show increase: does this portend a new wave of HIV infection? Hopkins HIV Rep. 15:15. [PubMed] [Google Scholar]
- 26.Flo, T. H., O. Halaas, S. Torp, L. Ryan, E. Lien, B. Dybdahl, A. Sundan, and T. Espevik. 2001. Differential expression of Toll-like receptor 2 in human cells. J. Leukoc. Biol. 69:474-481. [PubMed] [Google Scholar]
- 27.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidman, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fujii, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 28.Fraser, C. M., S. J. Norris, G. M. Weinstock, O. White, G. G. Sutton, R. Dodson, M. Gwinn, E. K. Hickey, R. Clayton, K. A. Ketchum, E. Sodergren, J. M. Hardham, M. P. McLeod, S. Salzberg, J. Peterson, H. Khalak, D. Richardson, J. K. Howell, M. Chidambaram, T. Utterback, L. McDonald, P. Artiach, C. Bowman, M. D. Cotton, and J. C. Venter. 1998. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 281:375-388. [DOI] [PubMed] [Google Scholar]
- 29.Gergel, E. I., and M. B. Furie. 2004. Populations of human T lymphocytes that traverse the vascular endothelium stimulated by Borrelia burgdorferi are enriched with cells that secrete gamma interferon. Infect. Immun. 72:1530-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giambartolomei, G. H., V. A. Dennis, B. L. Lasater, P. K. Murthy, and M. T. Philipp. 2002. Autocrine and exocrine regulation of interleukin-10 production in THP-1 cells stimulated with Borrelia burgdorferi lipoproteins. Infect. Immun. 70:1881-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giambartolomei, G. H., V. A. Dennis, B. L. Lasater, and M. T. Philipp. 1999. Induction of pro- and anti-inflammatory cytokines by Borrelia burgdorferi lipoproteins in monocytes is mediated by CD14. Infect. Immun. 67:140-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giambartolomei, G. H., V. A. Dennis, and M. T. Philipp. 1998. Borrelia burgdorferi stimulates the production of interleukin-10 in peripheral blood mononuclear cells from uninfected humans and rhesus monkeys. Infect. Immun. 66:2691-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glasova, M., E. Konikova, J. Stasakova, and O. Babusikova. 1998. The relationship of HLA-DR, CD38 and CD71 markers to activation, proliferation and differentiation of some human leukemia and lymphoma cells. Neoplasma 45:88-95. [PubMed] [Google Scholar]
- 34.Hamann, D., P. A. Baars, M. H. Rep, B. Hooibrink, S. R. Kerkhof-Garde, M. R. Klein, and R. A. Van Lier. 1997. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 186:1407-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hintzen, R. Q., R. De Jong, S. M. Lens, M. Brouwer, P. Baars, and R. A. Van Lier. 1993. Regulation of CD27 expression on subsets of mature T-lymphocytes. J. Immunol. 151:2426-2435. [PubMed] [Google Scholar]
- 36.Hirschfeld, M., C. J. Kirschning, R. Schwandner, H. Wesche, J. H. Weis, R. M. Wooten, and J. J. Weis. 1999. Inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J. Immunol. 163:2382-2386. [PubMed] [Google Scholar]
- 37.Hook, E. W., III, and R. W. Peeling. 2004. Syphilis control—a continuing challenge. N. Engl. J. Med. 351:121-124. [DOI] [PubMed] [Google Scholar]
- 38.Hornung, V., S. Rothenfusser, S. Britsch, A. Krug, B. Jahrsdorfer, T. Giese, S. Endres, and G. Hartmann. 2002. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168:4531-4537. [DOI] [PubMed] [Google Scholar]
- 39.Jarrossay, D., G. Napolitani, M. Colonna, F. Sallusto, and A. Lanzavecchia. 2001. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur. J. Immunol. 31:3388-3393. [DOI] [PubMed] [Google Scholar]
- 40.Kadowaki, N., S. Ho, S. Antonenko, M. R. de Waal, R. A. Kastelein, F. Bazan, and Y. J. Liu. 2001. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Komai-Koma, M., L. Jones, G. S. Ogg, D. Xu, and F. Y. Liew. 2004. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc. Natl. Acad. Sci. USA 101:3029-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krutzik, S. R., M. T. Ochoa, P. A. Sieling, S. Uematsu, Y. W. Ng, A. Legaspi, P. T. Liu, S. T. Cole, P. J. Godowski, Y. Maeda, E. N. Sarno, M. V. Norgard, P. J. Brennan, S. Akira, T. H. Rea, and R. L. Modlin. 2003. Activation and regulation of Toll-like receptors 2 and 1 in human leprosy. Nat. Med. 9:525-532. [DOI] [PubMed] [Google Scholar]
- 43.Kupper, T. S., and R. C. Fuhlbrigge. 2004. Immune surveillance in the skin: mechanisms and clinical consequences. Nat. Rev. Immunol. 4:211-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lande, R., E. Giacomini, B. Serafini, B. Rosicarelli, G. D. Sebastiani, G. Minisola, U. Tarantino, V. Riccieri, G. Valesini, and E. M. Coccia. 2004. Characterization and recruitment of plasmacytoid dendritic cells in synovial fluid and tissue of patients with chronic inflammatory arthritis. J. Immunol. 173:2815-2824. [DOI] [PubMed] [Google Scholar]
- 45.Liang, F. T., M. B. Jacobs, L. C. Bowers, and M. T. Philipp. 2002. An immune evasion mechanism for spirochetal persistence in Lyme borreliosis. J. Exp. Med. 195:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Libraty, D. H., L. E. Airan, K. Uyemura, D. Jullien, B. Spellberg, T. H. Rea, and R. L. Modlin. 1997. Interferon-gamma differentially regulates interleukin-12 and interleukin-10 production in leprosy. J. Clin. Investig. 99:336-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 48.Liu, N., R. R. Montgomery, S. W. Barthold, and L. K. Bockenstedt. 2004. Myeloid differentiation antigen 88 deficiency impairs pathogen clearance but does not alter inflammation in Borrelia burgdorferi-infected mice. Infect. Immun. 72:3195-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lukehart, S. A. 2004. Syphilis, p. 977-985. In E. Brauwald, A. S. Faucci, S. L. Hauser, D. L. Longo, and J. L. Jameson (ed.), Harrison's principles of internal medicine. McGraw Hill, New York, N.Y.
- 50.Ma, Y., K. P. Seiler, K. F. Tai, L. Yang, M. Woods, and J. J. Weis. 1994. Outer surface lipoproteins of Borrelia burgdorferi stimulate nitric oxide production by the cytokine-inducible pathway. Infect. Immun. 62:3663-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma, Y., and J. J. Weis. 1993. Borrelia burgdorferi outer surface lipoproteins OspA and OspB possess B-cell, mitogenic, and cytokine-stimulatory properties. Infect. Immun. 61:3843-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mazzoni, A., and D. M. Segal. 2004. Controlling the Toll road to dendritic cell polarization. J. Leukoc. Biol. 75:721-730. [DOI] [PubMed] [Google Scholar]
- 53.Norgard, M. V., L. L. Arndt, D. R. Akins, L. L. Curetty, D. A. Harrich, and J. D. Radolf. 1996. Activation of human monocytic cells by Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides proceeds via a pathway distinct from that of lipopolysaccharide but involves the transcriptional activator NF-κB. Infect. Immun. 64:3845-3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Norgard, M. V., B. S. Riley, J. A. Richardson, and J. D. Radolf. 1995. Dermal inflammation elicited by synthetic analogs of Treponema pallidum and Borrelia burgdorferi lipoproteins. Infect. Immun. 63:1507-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pashenkov, M., Y. M. Huang, V. Kostulas, M. Haglund, M. Soderstrom, and H. Link. 2001. Two subsets of dendritic cells are present in human cerebrospinal fluid. Brain 124:480-492. [DOI] [PubMed] [Google Scholar]
- 56.Penna, G., S. Sozzani, and L. Adorini. 2001. Cutting edge: selective usage of chemokine receptors by plasmacytoid dendritic cells. J. Immunol. 167:1862-1866. [DOI] [PubMed] [Google Scholar]
- 57.Picker, L. J., R. J. Martin, A. Trumble, L. S. Newman, P. A. Collins, P. R. Bergstresser, and D. Y. Leung. 1994. Differential expression of lymphocyte homing receptors by human memory/effector T cells in pulmonary versus cutaneous immune effector sites. Eur. J. Immunol. 24:1269-1277. [DOI] [PubMed] [Google Scholar]
- 58.Pivarcsi, A., L. Bodai, B. Rethi, A. Kenderessy-Szabo, A. Koreck, M. Szell, Z. Beer, Z. Bata-Csorgoo, M. Magocsi, E. Rajnavolgyi, A. Dobozy, and L. Kemeny. 2003. Expression and function of Toll-like receptors 2 and 4 in human keratinocytes. Int. Immunol. 15:721-730. [DOI] [PubMed] [Google Scholar]
- 59.Pohlmann, S., F. Baribaud, and R. W. Doms. 2001. DC-SIGN and DC-SIGNR: helping hands for HIV. Trends Immunol. 22:643-646. [DOI] [PubMed] [Google Scholar]
- 60.Poltorak, A., X. He, I. Smirnova, M.-Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57Bl/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 61.Radolf, J. D., L. L. Arndt, D. R. Akins, L. L. Curetty, M. E. Levi, Y. Shen, L. S. Davis, and M. V. Norgard. 1995. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytes/macrophages. J. Immunol. 154:2866-2877. [PubMed] [Google Scholar]
- 62.Radolf, J. D., M. V. Norgard, M. E. Brandt, R. D. Isaacs, P. A. Thompson, and B. Beutler. 1991. Lipoproteins of Borrelia burgdorferi and Treponema pallidum activate cachectin/tumor necrosis factor synthesis: analysis using a CAT reporter construct. J. Immunol. 147:1968-1974. [PubMed] [Google Scholar]
- 63.Rafii-El-Idrissi Benhia, M., D. Wroblewski, M. N. Akhtar, R. A. Patel, W. Lavezzi, S. Gangloff, S. M. Goyert, M. J. Caimano, J. D. Radolf, and T. J. Sellati. 2005. Signaling through CD14 attenuates the inflammatory response to Borrelia burgdorferi, the agent of Lyme disease. J. Immunol. 174:1539-1548. [DOI] [PubMed] [Google Scholar]
- 64.Salazar, J., K. R. O. Hazlett, and J. Radolf. 2002. The immune response to infection with Treponema pallidum, the stealth pathogen. Microbes. Infect. 4:1133. [DOI] [PubMed] [Google Scholar]
- 65.Salazar, J. C., C. D. Pope, T. J. Sellati, H. M. Feder, Jr., T. G. Kiely, K. R. Dardick, R. L. Buckman, M. W. Moore, M. J. Caimano, J. G. Pope, P. J. Krause, and J. D. Radolf. 2003. Coevolution of markers of innate and adaptive immunity in skin and peripheral blood of patients with erythema migrans. J. Immunol. 171:2660-2670. [DOI] [PubMed] [Google Scholar]
- 66.Santin, A. D., P. L. Hermonat, A. Ravaggi, M. Chiriva-Internati, M. J. Cannon, J. C. Hiserodt, S. Pecorelli, and G. P. Parham. 1999. Expression of surface antigens during the differentiation of human dendritic cells vs macrophages from blood monocytes in vitro. Immunobiology 200:187-204. [DOI] [PubMed] [Google Scholar]
- 67.Sato, S., F. Nomura, T. Kawai, O. Takeuchi, P. F. Muhlradt, K. Takeda, and S. Akira. 2000. Synergy and cross-tolerance between toll-like receptor (TLR) 2- and TLR4-mediated signaling pathways. J. Immunol. 165:7096-7101. [DOI] [PubMed] [Google Scholar]
- 68.Sellati, T. J., L. D. Abrescia, J. D. Radolf, and M. B. Furie. 1996. Outer surface lipoproteins of Borrelia burgdorferi activate vascular endothelium in vitro. Infect. Immun. 64:3180-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sellati, T. J., D. A. Bouis, R. L. Kitchens, R. P. Darveau, J. Pugin, R. J. Ulevitch, S. C. Gangloff, S. M. Goyert, M. V. Norgard, and J. D. Radolf. 1998. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytic cells via a CD14-dependent pathway distinct from that used by lipopolysaccharide. J. Immunol. 160:5455-5464. [PubMed] [Google Scholar]
- 70.Sellati, T. J., S. L. Waldrop, J. C. Salazar, P. R. Bergstresser, L. J. Picker, and J. D. Radolf. 2001. The cutaneous response in humans to Treponema pallidum lipoprotein analogues involves cellular elements of both innate and adaptive immunity. J. Immunol. 166:4131-4140. [DOI] [PubMed] [Google Scholar]
- 71.Sellati, T. J., D. A. Wilkinson, J. S. Sheffield, R. A. Koup, J. D. Radolf, and M. V. Norgard. 2000. Virulent Treponema pallidum, lipoprotein, and synthetic lipopeptides induce CCR5 on human monocytes and enhance their susceptibility to infection by human immunodeficiency virus type 1. J. Infect. Dis. 181:283-293. [DOI] [PubMed] [Google Scholar]
- 72.Sieling, P. A., W. Chung, B. T. Duong, P. J. Godowski, and R. L. Modlin. 2003. Toll-like receptor 2 ligands as adjuvants for human Th1 responses. J. Immunol. 170:194-200. [DOI] [PubMed] [Google Scholar]
- 73.Reference deleted.
- 74.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345:115-125. [DOI] [PubMed] [Google Scholar]
- 75.Steere, A. C., J. Coburn, and L. Glickstein. 2004. The emergence of Lyme disease. J. Clin. Investig. 113:1093-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takeuchi, J., E. Watari, E. Shinya, Y. Norose, M. Matsumoto, T. Seya, M. Sugita, S. Kawana, and H. Takahashi. 2003. Down-regulation of Toll-like receptor expression in monocyte-derived Langerhans cell-like cells: implications of low-responsiveness to bacterial components in the epidermal Langerhans cells. Biochem. Biophys. Res. Commun. 306:674-679. [DOI] [PubMed] [Google Scholar]
- 77.Takeuchi, O., S. Sato, T. Horiuchi, K. Hoshino, K. Takeda, Z. Dong, R. L. Modlin, and S. Akira. 2002. Cutting edge: role of toll-like receptor 1 in mediating immune response to microbial lipoproteins. J. Immunol. 169:10-14. [DOI] [PubMed] [Google Scholar]
- 78.Templeton, T. J. 2004. Borrelia outer membrane surface proteins and transmission through the tick. J. Exp. Med. 199:603-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thoma-Uszynski, S., S. M. Kiertscher, M. T. Ochoa, D. A. Bouis, M. V. Norgard, K. Miyake, P. J. Godowski, M. D. Roth, and R. L. Modlin. 2000. Activation of toll-like receptor 2 on human dendritic cells triggers induction of IL-12, but not IL-10. J. Immunol. 165:3804-3810. [DOI] [PubMed] [Google Scholar]
- 80.Trinchieri, G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3:133-146. [DOI] [PubMed] [Google Scholar]
- 81.Ulevitch, R. J., and P. S. Tobias. 1999. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Curr. Opin. Immunol. 11:19-22. [DOI] [PubMed] [Google Scholar]
- 82.van Kooyk, Y., and T. B. Geijtenbeek. 2003. DC-SIGN: escape mechanism for pathogens. Nat. Rev. Immunol. 3:697-709. [DOI] [PubMed] [Google Scholar]
- 83.Van Voorhis, W. C., L. K. Barrett, D. M. Koelle, J. M. Nasio, F. A. Plummer, and S. A. Lukehart. 1996. Primary and secondary syphilis lesions contain mRNA for Th1 cytokines. J. Infect. Dis. 173:491-495. [DOI] [PubMed] [Google Scholar]
- 84.Wang, G., Y. Ma, A. Buyuk, S. McClain, J. J. Weis, and I. Schwartz. 2004. Impaired host defense to infection and Toll-like receptor 2-independent killing of Borrelia burgdorferi clinical isolates in TLR2-deficient C3H/HeJ mice. FEMS Microbiol. Lett. 231:219-225. [DOI] [PubMed] [Google Scholar]
- 85.Wollenberg, A., M. Wagner, S. Gunther, A. Towarowski, E. Tuma, M. Moderer, S. Rothenfusser, S. Wetzel, S. Endres, and G. Hartmann. 2002. Plasmacytoid dendritic cells: a new cutaneous dendritic cell subset with distinct role in inflammatory skin diseases. J. Investig. Dermatol. 119:1096-1102. [DOI] [PubMed] [Google Scholar]
- 86.Wooten, R. M., Y. Ma, R. A. Yoder, J. P. Brown, J. H. Weis, J. F. Zachary, C. J. Kirschning, and J. J. Weis. 2002. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 168:348-355. [DOI] [PubMed] [Google Scholar]
- 87.Wooten, R. M., T. B. Morrison, J. H. Weis, S. D. Wright, R. Thieringer, and J. J. Weis. 1998. The role of CD14 in signaling mediated by outer membrane lipoproteins of Borrelia burgdorferi. J. Immunol. 160:5485-5492. [PubMed] [Google Scholar]
- 88.Wooten, R. M., and J. J. Weis. 2001. Host-pathogen interactions promoting inflammatory Lyme arthritis: use of mouse models for dissection of disease processes. Curr. Opin. Microbiol. 4:274-279. [DOI] [PubMed] [Google Scholar]