Abstract
The effect of immunization with five lipopeptides, three containing T-helper (Th) epitopes and two with both Th and cytotoxic T-lymphocyte (CTL) epitopes, on equine infectious anemia virus (EIAV) challenge was evaluated. Peripheral blood mononuclear cells from EIAV lipopeptide-immunized horses had significant proliferative responses to Th peptides compared with those preimmunization, and the responses were attributed to significant responses to peptides Gag from positions 221 to 245 (Gag 221-245), Gag 250-269, and Pol 326-347; however, there were no consistent CTL responses. The significant proliferative responses in the EIAV lipopeptide-immunized horses allowed testing of the hypothesis that Th responses to immunization would enhance Th and CTL responses following EIAV challenge and lessen the viral load and the severity of clinical disease. The EIAV lipopeptide-immunized group did have a significant increase in proliferative responses to Th peptides 1 week after virus challenge, whereas the control group did not. Two weeks after challenge, a significant CTL response to virus-infected cell targets occurred in the EIAV lipopeptide-immunized group compared to that in the control group. These Th and CTL responses did not significantly alter either the number of viral RNA copies/ml or disease severity. Thus, lipopeptide-induced proliferative responses and enhanced Th and CTL responses early after virus challenge were unable to control challenge virus load and clinical disease.
Equine infectious anemia virus (EIAV) is a lentivirus that causes characteristic persistent infections similar to those caused by other lentiviruses, including human immunodeficiency virus (HIV) type 1 (HIV-1). However, unlike HIV-1, EIAV has been demonstrated to infect only monocytes, macrophages, and endothelial cells and not T lymphocytes. The hallmark signs of clinical EIAV disease include fever, thrombocytopenia, and anemia. During the acute phase of disease, peak plasma viremia levels vary between 103.6 and >106 50% tissue culture infective doses (TCID50s) per milliliter (18, 27). The initial viremia decreases to low or undetectable levels 3 to 4 weeks after infection, concurrent with detectable virus-specific cytotoxic T-lymphocyte (CTL) responses (17, 32). In contrast, virus-specific neutralizing antibodies do not usually appear until 2 or 3 months or more after infection (17). Recurrent episodes of acute disease and high titers of plasma viremia, associated with emerging novel antigenic variants, often occur within the first year (26, 33, 35). However, most horses eventually enter a life-long inapparent carrier state, with no clinical signs of disease and undetectable or low plasma virus loads. Thus, EIAV-infected inapparent carrier horses resemble HIV-1-infected long-term nonprogressors. Nonetheless, EIAV infection is never cleared, since viremia and acute clinical disease recur in immunocompromised animals (25, 56). Additionally, transfer of whole blood from inapparent carrier horses into naïve horses causes acute disease in the recipient animals (21).
Cellular immune responses are critical in the control of lentiviral infections. For example, CD8+ cells were absolutely required for the control of viremia in both acute and chronic simian immunodeficiency virus (SIV) infection (47). HIV-1-specific CTL activity had a significant inverse correlation with plasma RNA viral load (9, 37); however, this correlation was not present in every study (5). In EIAV infection, CTL responses occur coincident with initial viremia (32) and before the appearance of neutralizing antibody. Although virus-specific CD8+ CTL responses may occur independently, particularly when high frequencies of CTL precursors are present (34), successful defense against viral challenge may often depend on concurrent CD4+ T-helper (Th) lymphocyte responses. Specifically, Th lymphocyte responses that result in increased interleukin-2 and gamma interferon (IFN-γ) secretion (Th1 responses) may be particularly beneficial. In fact, recent studies have shown that CD4+ Th lymphocytes are required during priming to generate functional CD8+ CTL memory (23, 48, 54). Moreover, in HIV-1 and SIV studies, Th lymphocyte responses to Gag proteins correlated with the control of viremia in untreated individuals (19, 24, 43, 46). Finally, in several model systems, vaccines that have combined Th lymphocyte and CTL epitopes have resulted in increased CTL responses, along with subsequent vaccine effectiveness (29, 39, 40). Thus, the induction of strong Th lymphocyte responses is a part of current vaccine designs against viral infections where strong CTL responses are desired.
Peptide-based vaccine strategies are of particular interest for lentiviruses because of the increased safety concerns about the reversion to virulence of the virus in modified live lentivirus vaccines. Additional advantages to the use of peptide-based vaccines include the ability to readily distinguish between infected and immunized animals and the targeting of relevant rather than broad immune responses that may, in some cases, exacerbate disease (22, 30, 44). Nonetheless, a major impediment to the use of peptide-based vaccine strategies is defining a group of immunologically relevant epitopes that are recognized by the majority of the affected population and that are highly conserved both within and across virus strains (58).
To date, only a few EIAV-specific CTL epitopes and the presenting major histocompatibility complex (MHC) class I molecules are known (31, 33, 59). Even less is known about the horse MHC class II molecules that present EIAV peptides; however, this problem may be partially overcome by finding broadly recognized MHC class II-restricted CD4+ Th epitopes (12, 13). Broadly recognized MHC class II-restricted CD4+ Th epitopes may be more common than broadly recognized MHC class I-restricted CD8+ CTL epitopes. For example, several common human leukocyte antigen DR class II molecules exhibit overlapping peptide binding repertoires (51), and several promiscuous class II-restricted peptides were previously identified in HIV-1 Gag and Pol (58). Therefore, initial efforts focused on defining Th epitopes that were broadly recognized by a group of MHC class II disparate EIAV-infected horses. To that end, four EIAV peptides that contained Th epitopes recognized by peripheral blood mononuclear cells (PBMCs) from 60 to 93% of 15 MHC class II disparate EIAV-infected horses were identified (12, 13). These peptides were highly conserved among known EIAV strains, and three of these stimulated increased IFN-γ mRNA levels (13). Additionally, two peptides containing CTL epitopes restricted by the equine leukocyte antigen (ELA) class I molecule ELA-A1 were previously characterized (33, 45). A slightly longer version of one also contained a Th epitope recognized by PBMCs from 33% of the EIAV-infected horses mentioned above (12). Based on the availability of defined EIAV Th and CTL peptides, horses were immunized with lipopeptide versions of these peptides and the proliferative and CTL responses were evaluated. The lipopeptide immunization induced significant proliferative responses to Th peptides but did not consistently induce CTL responses. Therefore, we tested the hypothesis that Th responses to immunization would enhance both Th and CTL responses following EIAV challenge and lessen the viral load and the clinical severity of disease.
MATERIALS AND METHODS
MHC typing of horses.
All nine experimental horses, as well as their sires and dams, were typed for ELA-A locus alleles by a standard complement-mediated lymphocyte microcytotoxicity assay with serologically defined antisera for alleles ELA-A1 to ELA-A10 and W11 (4, 55). The ELA-A locus is the only defined polymorphic classical class I MHC locus in horses. PCR-single-strand conformation polymorphism analysis of exon 2 was used to type the minimally polymorphic MHC class II DRA locus and the more highly polymorphic DQA locus according to previously published protocols (1, 11). The MHC typing results for the experimental horses are shown in Table 1. When possible, the heterozygosity or the homozygosity of horses that typed for only a single allele at the ELA-A or DQA locus was inferred by pedigree information.
TABLE 1.
MHC class I ELA-A haplotypes and class II DRA and DQA alleles of experimental horses
| Horse | Age (mo)a | Group | ELA-A typeb | DRA type | DQA type | Dam |
|---|---|---|---|---|---|---|
| H637 | 17 | EIAV | A1/W11 | 1/1 | 5/12 | H504 |
| H640 | 6 | EIAV | A1′/A6′ | 1/1 | 4/11 | H607 |
| H641 | 6 | EIAV | A1′/NDc | 1/1 | 4/ND | H604 |
| H646 | 5 | EIAV | A1′/A5 | 1/1 | 4/ND | H615 |
| H651 | 4.5 | EIAV | A1′/A5 | 1/xd | 4 | H563 |
| H643 | 5.5 | Control | A1′/A5 | 1/1 | 4/ND | H606 |
| H648 | 5 | Control | A1′/A5 | 1/1 | 4/ND | H623 |
| H649 | 5 | Control | A1′/A5 | 1/x | 4 | H545 |
| H652 | 3.5 | Control | A1/W11 | 1/1 | 5/12 | H504 |
The age listed is the age at the first immunization.
Horses H637 and H652 shared the maternally derived ELA-A1/DRA*0101/DQA*1201 haplotype, whereas the remaining seven horses shared the paternally derived ELA-A1′/DRA*0101/DQA*0401 haplotype. Different designations were used because of apparent functional differences between ELA-A1 and ELA-A1′ described in the results.
ND, an allele was not detected by the available serological reagents (ELA-A) or available primer set (DQA), although pedigree information indicated that the individual was heterozygous.
The type x in DRA indicates a nonsequenced allele with a unique single-strand conformation polymorphism pattern.
Horses H640, H641, H643, H646, H648, H649, and H651 shared an identical by descent (IBD) haplotype designated ELA-A1′/DRA*0101/DQA*0401 (Table 1) and originated from a common sire, horse H600. Although the dams of these seven horses shared various MHC class I and class II alleles, only the dams of horses H649 and H651 were known to be related. Horses H637 and H652 shared both an IBD haplotype designated ELA-A1/DRA*0101/DQA*1201 from a common dam and an IBD haplotype designated ELA-W11/DRA*0101/DQA*0501 from a common grandsire (Table 1). At the onset of the experiment, potential differences in the presentation and/or immunodominance of the selected CTL epitopes between the class I molecules representing ELA-A1 and ELA-A1′ were unknown.
Synthetic peptides and lipopeptides.
Peptides and lipopeptides were synthesized at the Washington State University Laboratory of Biotechnology and Bioanalysis by the solid-phase method, based on standard 9-fluorenylmethoxy carbonyl chemistry (for a review, see reference 10). The purities of the peptides used in this study (Table 2) were determined by high-pressure liquid chromatography to be >80%, and those of most peptides were >90%. Peptide stocks (2 mg/ml) were dissolved in RPMI 1640 or Dulbecco's modified Eagle medium (DMEM) with 10% dimethyl sulfoxide (DMSO) and stored at −20°C. Lipopeptides were constructed as described previously by coupling a palmitic acid molecule to each of the free NH2 groups on the lysine-serine-serine elongated peptides (57) and contained <5% free peptide. Stock solutions of lipopeptides (20 mg/ml) were dissolved in 100% DMSO and stored at −20°C.
TABLE 2.
Amino acid sequences of EIAV peptides and the A. marginale peptide (Ana-P16a) used in this study
| Peptidea | Response (reference) | Amino acid sequenceb |
|---|---|---|
| Gag 221-245 | Th1 (13) | QGPIPMTARFIRGLGVPRERQMEPA |
| Gag 250-269 | Th1 (13) | RQTYRQWIIEAMSEGIKVMI |
| Pol 326-347 | Th1 (13) | LPQGFVLSPYIYQKTLQEILQP |
| Pol 704-719c | Th (12) | EIVYFAWVPGHKGIYG |
| Gag 13-32 | Th (12) and CTL (33) | KLEKVTVQGSQKLTTGNCNW |
| Env 195-206PVd | CTL (33) | RVEDVMNTTEYW |
| Pol 704-Env 195PV | Th (12) and CTL (33) | EIVYFAWVPGHKGIYGPGPGRVEDVMNTTEYW |
| Ana-P16a | Unknown | LYLDTGIASFNFAYFGGELGVRFA |
Amino acid numbers for EIAV peptides are published elsewhere (53).
Minimal ELA-A1-restricted CTL epitope sequences are underlined. The artificial linker amino acid residues in the hybrid peptide Pol 704-Env 195PV are in boldface and italic.
The corresponding peptide in EIAVWSUS has a C at position 718; however, this peptide is recognized by horses infected with various strains including EIAVPV, EIAVWSUS, EIAVWY (12).
A hybrid lipopeptide, Pol position 704 (Pol 704)-Env 195 from the pony-virulent strain (Env 195PV), that contained a Pol Th lymphocyte epitope and an Env CTL epitope separated by four linker amino acid residues (Table 2) was made to decrease the likelihood of junctional epitope formation (28). The two epitopes were selected for artificial linkage based on the observations that PBMCs from 11 of 15 EIAV-infected horses proliferated in response to the Pol 704-719, while none proliferated in response to peptide Env 195-206PV (12), which contained an ELA-A1-restricted CTL epitope (33). The physical linkage of Th and CTL epitopes was shown in a previous study to be required for in vivo priming of HIV-specific CD8+ CTLs (49). Before use in immunization studies, hybrid peptide Pol 704-Env 195PV was evaluated in vitro to determine if it would stimulate lymphocyte proliferation and CTLs in PBMCs from EIAVWSU5-infected horse 2140 (13, 33). Stimulated A2140 PBMCs proliferated equally well in response to the hybrid peptide and Pol 704-719 but not in response to Env 195-206PV (data not shown). In a CTL assay, A2140 PBMCs stimulated with either the hybrid peptide Pol 704-Env 195PV or Env 195-206PV significantly lysed equine kidney (EK) cell targets in an ELA-A1-restricted manner when they were pulsed with Pol 704-Env 195PV or Env 195-206PV but not Pol 704-719 (data not shown).
Experimental horse groups for immunization and infection.
The nine horses used were related mixed-breed ponies ranging in age from 3.5 to 17 months at the time of the first immunization (see the ages in Table 1). Horses H640, H641, H643, H646, H648, H649, and H651 shared the IBD ELA-A1′ allele. They were subgrouped by additional MHC similarities and were then assigned randomly to either the EIAV or the control peptide immunization groups. For horses H637 and H652, which shared the IBD ELA-A1 allele, H637 was assigned to the EIAV immunization group because H637 EK cell targets were known a priori to present both of the ELA-A1-restricted CTL epitopes used in the study.
The horses were immunized five times at 2-week intervals prior to viral challenge either with EIAV lipopeptides (horses H637, H640, H641, H646, and H651) or with control lipopeptide Ana-P16a (horses H643, H648, H649, and H652), a characterized Th peptide for cattle derived from Anaplasma marginale (6). The EIAV lipopeptides included Th peptides Gag 221-245, Gag 250-269, Pol 326-347 plus two peptides containing both Th and CTL epitopes, Gag 13-32, and the artificially constructed hybrid peptide Pol 704-Env 195PV (Table 2). A quantity equivalent to 1 mg free peptide was used for each lipopeptide, resulting in a dose of approximately 10 μg of each peptide per kilogram of body weight. By accounting for the palmitic acid and linker amino acid residues, the total amount of lipopeptide in the EIAV immunization group was 8.9 mg. The initial immunization mixture consisted of lipopeptides in a 1-ml total volume containing 40% complete Freund's adjuvant (CFA; Sigma, St. Louis, MO), 40% phosphate-buffered saline (PBS), and 20% DMSO. Subsequent immunizations were identical, except that incomplete Freund's adjuvant (Sigma) was used in place of CFA in order to reduce the possibility of abscess formation. All parameters for the control group immunization were identical to those for the EIAV immunization group, except that the total amount of Ana-P16a was 1.3 mg. All immunization mixtures were emulsified in bulk on the day on which they were needed by mixing between two glass syringes. To minimize abscess formation, the 1-ml total for each horse was injected intramuscularly into three sites on either side of the neck and two sites on the chest (approximately 0.12 ml/site).
For EIAV challenge, both immunized horse groups were injected intravenously 2 weeks after the final immunization with a dose of 300 TCID50s of the pathogenic pony-virulent strain of EIAV (EIAVPV) (16, 44) diluted in 1 ml DMEM without serum or antibiotics. The Washington State University Animal Care and Use Committee approved all experimental procedures involving animals.
Measurement of EIAV disease parameters.
Morning rectal temperatures were taken daily beginning 3 days prior to EIAVPV challenge and continuing throughout the 8-week trial. Temperatures above 101.5°F were considered a fever, and a febrile episode was defined as two or more consecutive days of fever. Whole blood was collected by venipuncture at 1 week and 3 days before viral challenge and placed in tubes with heparin and EDTA anticoagulant. After challenge, blood was collected three times per week for the first 4 weeks and then twice per week. Platelet count and packed cell volume were determined each time that blood was collected. The remaining plasma was stored at −80°C. Platelet counts below 100,000/μl whole blood were considered indicative of thrombocytopenia. Real-time reverse transcription-PCR (RT-PCR) was used to quantify the plasma viral RNA load after EIAVPV challenge as described previously (33, 45), except that the following oligonucleotides were used: forward primer, 5′-AGCCAGGACATTTATCTAGTCAATGTAGAGACAC-3′; reverse primer, 5′-GTGCTGACTCTTCTGTTGTATCGGGAAAGTTTG-3′; and TaqMan (Applied Biosystems, Foster, CA) probe, 5′-ACGGGAAGCAAGGGGCTCAAGGGAGGCC-3′. The minimum amount of standard RNA detected ranged from 10 to 100 copies; therefore, the minimum amount detectable in plasma ranged from 430 to 4,300 copies per ml, based on a correction factor of 43 (33).
Lymphocyte proliferation assays.
PBMCs were isolated from horse blood prior to lipopeptide immunization, at various times during immunization, and weekly after EIAVPV challenge (13). Two cryovials containing 5 × 107 to 1 × 108 PBMCs each in 90% fetal bovine serum (FBS; Atlanta Biologicals; Norcross, GA) plus 10% DMSO were frozen at −80°C and were then transferred to liquid nitrogen storage for later use. The remaining PBMCs were used for proliferation and CTL assays. PBMC proliferative responses to the free EIAV-specific peptides listed in Table 2 and to Ana-P16a were measured as described previously (13), except that fresh complete RPMI 1640 medium that contained 300 μg/ml l-glutamine, 25 mM HEPES, 50 μg/ml gentamicin, and 10% heat-inactivated autologous serum was made for each horse prior to each assay. Serum was collected in bulk from each horse before immunization and infection, filtered through a 0.2-μm-pore-size filter, heat inactivated for 30 min at 56°C, and frozen in smaller aliquots at −20°C. The PBMC preparations were assayed in quadruplicate against 10, 1, and 0.1 μg/ml of each peptide. Sixteen wells of PBMCs in complete RPMI 1640 were used as controls. For positive controls, 2.5 μg/ml pokeweed mitogen (PWM; Sigma), a known T-lymphocyte mitogen in horses (2), was added to four control wells 48 h prior to the addition of [3H]thymidine. The remaining 12 wells served as unstimulated negative controls. PBMCs were incubated for a total of 6 days at 37°C with 5% CO2 and then labeled with 0.25 μCi [3H]thymidine per well (Dupont, NEN, Boston, MA) and incubated for an additional 16 to 20 h. The cells were harvested onto a glass fiber filter and counted by liquid scintillation. The mean and standard deviation (SD) of negative control wells were calculated, and if the SD was ≥75% of the mean, the most disparate value was dropped and the mean was recalculated. No values were discarded for the peptide- or PWM-stimulated wells.
CTL assays.
PBMCs were obtained prior to immunization, during immunization, prior to viral challenge, and weekly after viral challenge for CTL assays with EIAVPV or Gag 13-32 and Env 195-206PV peptides. Briefly, 0.5 × 108 to 1 × 108 PBMCs were incubated at 37°C with 5% CO2 with repeated gentle mixing either for 1 h with 40 μM of either peptide or for 2 h with EIAVWSU5 at a multiplicity of infection for monocytes equal to 2. EIAVWSU5 was derived from a tissue culture-adapted strain of the Wyoming wild-type virus strain (EIAVWY) (38) and has an overall nucleotide sequence homology of 99.4% with EIAVPV. The peptide Gag 13-32 is invariant between the two strains. The peptide Env 195-206 differs by two amino acid residues between virus strains (Table 2); however, the two peptide versions are cross-recognized by CTLs from ELA-A1-typed horses infected with either EIAVWSU5 or EIAVPV (45). After incubation, the PBMCs were washed with Hank's balanced salt solution and were then resuspended to 1 × 106 to 4 × 106 PBMCs/ml in RPMI medium with 25 mM HEPES, 10% FBS, 10 μg/ml gentamicin, and 10 μM 2-mercaptoethanol (2-ME). The PBMCs were transferred to 24-well tissue culture plates or 75-cm2 tissue culture flasks and were incubated undisturbed for 7 days.
For CTL targets, EK cells were obtained from kidney biopsy specimens prior to infection, as described previously (32), and were maintained as frozen cell lines. Autologous, ELA-A half-matched or ELA-A-mismatched EK target cells were prepared by labeling 3 × 104 uninfected or EIAVPV-infected EK cells per well in collagen-coated 96-well plates for 3 to 5 h with 2.5 μCi 51Cr in 50 μl DMEM plus 5% calf serum and 10 μg/ml gentamicin. Target cell infection was confirmed by direct immunofluorescent staining with polyclonal antibodies from an EIAV-infected horse serum sample which recognize EIAV Env and Gag proteins (8). Additionally, portions of uninfected EK cells were pulsed with 200 μg/ml of each peptide during labeling. Labeled target cells were washed three times with DMEM to remove any remaining 51Cr in the supernatant. PBMC effectors were removed from the culture, centrifuged, and resuspended to a final concentration of 3 × 106 PBMCs/ml in fresh RPMI medium with 10% FBS, gentamicin, and 2-ME, as described above. Effectors were added to targets at a 20:1 effector cell-to-target cell ratio, in a 200-μl total volume, and then incubated at 37°C with 5% CO2 for 17 h.
To determine 51Cr release, 100 μl of supernatant was removed from each well and counted by liquid scintillation. Percent specific lysis was calculated as [(E − S)/(M − S)] × 100, where E is the mean release from six experimental wells, S is the mean spontaneous release from six wells of target cells without effector cells, and M is the mean maximal release from six wells of target cells containing 2 to 3% Triton X-100. Standard error (SE) estimates accounted for the variability in E, S, and M (50). Only assays with a spontaneous lysis of less than 30% were used.
CTL assays with previously frozen PBMCs as effectors were similarly performed with the following additions. Fresh autologous PBMCs from each horse were irradiated with 3 kilorads from a 60Co source. The fresh irradiated PBMCs, used as a source of antigen-presenting cells, were stimulated with virus for 2 h, as described above. Virus-stimulated, freshly irradiated PBMCs were mixed at a 1:1 ratio with frozen PBMCs in complete RPMI 1640, as described above, plus 10 units/ml of recombinant human interleukin-2 and were then incubated undisturbed for 7 days.
Statistical analyses.
The lymphocyte proliferative responses of the EIAV lipopeptide-immunized group measured pre- and postimmunization were compared by using a nonparametric repeated-measures analysis of variance (Friedman's test) with a posttest by using Dunn's multiple-comparisons test. This analysis used the uncorrected counts per minute as described previously (20) and was also used to compare the responses postimmunization and 1 week after virus challenge. Similar analyses were done for the control lipopeptide-immunized group responses. Stimulation indices (SIs) for peptide- or PWM-stimulated PBMCs were calculated by dividing the mean counts per minute for the stimulated PBMC replicate wells by the mean for the negative control wells. Proliferation assays were considered valid if the overall SI for PWM-stimulated PBMCs was ≥20. Furthermore, the proliferative responses to a particular peptide were considered significantly positive when the mean counts per minute minus the background counts per minute (medium control alone) was >1,000 and the SI was ≥3. For individual CTL assays to be significant they had to have ≥10% specific lysis after subtraction of the percent specific lysis of the appropriate noninfected or non-peptide-pulsed control EK cell targets, and the results for the CTL assays that met this definition were also ≥3 SEs above those for the noninfected or non-peptide-pulsed EK cell targets. A one-sided Mann-Whitney rank sum test was used to compare clinical parameters as well as the number of viral RNA copies/ml at peak levels and the time to reach the peak number of viral RNA copies/ml between the EIAV- and control lipopeptide-immunized groups.
RESULTS
Mild to moderate side effects were noted with lipopeptide immunization in adjuvant.
Firm nodules approximately 1 cm in diameter were noted at a few injection sites on three of five control immunized horses. No redness, heat, or soreness was detected; and all of the nodules disappeared by 2 weeks after EIAV challenge. In addition, horse H643 had a moderate fever (temperature, 103°F) for 24 h after the third injection. In contrast, horses in the EIAV peptide immunization group exhibited increased side effects. All five horses in this group developed small, hard nodules approximately 1 to 2 cm in diameter at all of the injection sites. Again, no redness or heat was associated with the nodules, but most horses exhibited increased sensitivity to touch at the injection sites following immunizations three through five. One to two nodules per horse abscessed and drained, although no secondary infections were noted. Only one horse in the EIAV peptide immunization group, H640, had a moderate fever (temperature, 103.5°F) for 24 h after the third injection. The sizes of the nodules in this group of horses decreased, but they did not disappear completely by 8 weeks after EIAV challenge.
Immunization with EIAV lipopeptides induced strong lymphocyte proliferative responses.
PBMC responses to 10, 1, and 0.1 μg/ml free EIAV and Anaplasma peptides were evaluated before and after lipopeptide immunization. The responses to 10 μg/ml had higher means, and Tables 3 and 4 contain the results obtained with this concentration to stimulate PBMCs obtained preimmunization and 1 week after the fifth and final immunization. Despite the use of freshly prepared medium containing 10% autologous serum from each horse, the mean counts per minute of the unstimulated preimmunization PBMC control varied among horses, ranging from 70 cpm for horse H641 (Table 3) to 2,836 cpm for horse H652 (Table 4). However, there were only three significantly positive responses of preimmunization PBMCs among 63 assays (nine horses with seven peptides) (Table 3 and 4).
TABLE 3.
Lymphocyte proliferation to free peptides by PBMCs obtained preimmunization, 1 week after the last EIAV lipopeptide immunization, and 1 and 3 weeks after virus challenge in the EIAV lipopeptide immunized group
| Time | Horse | Mean response (cpm)a
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag 221-245 | Gag 250-269 | Gag 13-32 | Pol 326-347 | Pol 704-719 | Pol 704-Env 195 | EnvPV 195-206 | Ana-P16a | PWM | Medium alone | ||
| Pre-immunization | H637 | 1,463 | 2,276 | 489 | 7,154 | 3740 | ND | 2,764 | 2,602 | 60,650 | 1,626 |
| H640 | 429 | 663 | 156 | 312 | 2,184 | ND | 1,599 | 507 | 58,695 | 390 | |
| H641 | 224 | 784 | 364 | 252 | 245 | ND | 98 | 175 | 63,182 | 70 | |
| H646 | 1,479 | 789 | 592 | 296 | 1,578 | ND | 1,676 | 592 | 65,667 | 986 | |
| H651 | 285 | 110 | 613 | 131 | 1,182 | ND | 263 | 175 | 54,458 | 219 | |
| Post-immunization | H637 | 3,658 | 11,083 | 1,310 | 7,426 | 7,207 | 6,770 | 983 | 710 | 24,133 | 546 |
| H640 | 25,465 | 1,978 | 3,175 | 3,263 | 2,104 | 4,712 | 340 | 340 | 26,397 | 126 | |
| H641 | 8,400 | 7,207 | 2,047 | 2,153 | 1,998 | 1,736 | 97 | 87 | 20,205 | 97 | |
| H646 | 2,391 | 3,959 | 941 | 1,999 | 902 | 1,764 | 510 | 745 | 24,696 | 392 | |
| H651 | 2,988 | 2,699 | 2,699 | 1,735 | 868 | 2,313 | 1,157 | 771 | 29,306 | 964 | |
| 1 wk after viral | H637 | 11,316 | 21,771 | 5,675 | 15,493 | 4,885 | 14,809 | 1,381 | 531 | 34,373 | 118 |
| challenge | H640 | 34,707 | 7,216 | 5,151 | 11,022 | 4,395 | 9,100 | 73 | 118 | 27,882 | 91 |
| H641 | 13,339 | 25,704 | 2,033 | 5,994 | 5,566 | 6,136 | 202 | 55 | 25,355 | 92 | |
| H646 | 10,852 | 8,214 | 2,738 | 5,254 | 3,848 | 6,142 | 1,184 | 3,034 | 48,470 | 740 | |
| H651 | 11,998 | 8,275 | 4,816 | 4,959 | 3,905 | 5,472 | 86 | 247 | 27,465 | 95 | |
| 3 wk after viral | H637 | 15,609 | 19,584 | 339 | 13,794 | 8,083 | 8,881 | 605 | 1,236 | 29,500 | 242 |
| challenge | H640 | 27,293 | 13,409 | 983 | 11,697 | 6,055 | 13,060 | 634 | 380 | 37,089 | 317 |
| H641 | 12,245 | 9,248 | 1,581 | 11,461 | 2,917 | 1,269 | 797 | 540 | 41,917 | 135 | |
| H646 | 3004 | 12,017 | 2,170 | 7,010 | 3,839 | 9,847 | 1,836 | 3,004 | 34,715 | 1,669 | |
| H651 | 4,894 | 9,853 | 1,288 | 7,342 | 3,091 | 6,054 | 1,352 | 1,610 | 38,318 | 644 | |
The values are mean counts per minute of four replicates of PBMC stimulated with either 10 μg/ml of peptide or 2.5 μg/ml PWM. The last column contains the mean counts per minute for medium alone. Peptide responses in boldface were significant positive responses, defined as a mean counts per minute minus mean counts per minute with medium alone of ≥1,000 and also an SI ≥3 (neither calculation is shown in the table). ND, not done.
TABLE 4.
Lymphocyte proliferation in response to free peptides by PBMCs obtained preimmunization, 1 week after the last control lipopeptide (Ana-P16a) immunization, and 1 and 3 weeks after virus challenge in the control lipopeptide immunized group
| Time | Horse | Mean response (cpm)a
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag 221-245 | Gag 250-269 | Gag 13-32 | Pol 326-347 | Pol 704-719 | Pol 704-Env 195 | EnvPV 195-206 | Ana-P16a | PWM | Medium alone | ||
| Pre-immunization | H643 | 666 | 333 | 1,331 | 1,999 | 1,836 | ND | 333 | 2,499 | 56,144 | 1,666 |
| H648 | 443 | 258 | 3,136 | 123 | 369 | ND | 160 | 898 | 60,036 | 123 | |
| H649 | 1,807 | 761 | 1,712 | 1,331 | 761 | ND | 761 | 1,046 | 70,279 | 951 | |
| H652 | 2,836 | 1,418 | 1,985 | 1,134 | 3,120 | ND | 255 | 3,403 | 56,436 | 2,836 | |
| Post-immunization | H643 | 2,218 | 2,016 | 907 | 6,048 | 1,512 | 3,125 | 605 | 2,822 | 27,144 | 1,008 |
| H648 | 3,994 | 5,769 | 1,997 | 4,216 | 6,213 | 7,988 | 5,769 | 3,550 | 47,487 | 2,219 | |
| H649 | 1,436 | 766 | 479 | 2,807 | 2,169 | 2,935 | 1,180 | 3,892 | 26,190 | 319 | |
| H652 | 271 | 738 | 172 | 148 | 369 | 197 | 541 | 517 | 15,793 | 246 | |
| 1 wk after viral | H643 | 1,995 | 1,155 | 855 | 6,330 | 2,100 | 2,295 | 30 | 11,120 | 27,670 | 150 |
| challenge | H648 | 5,524 | 1,841 | 5,933 | 4,296 | 5,115 | 11,048 | 1,637 | 10,230 | 67,723 | 2,046 |
| H649 | 4,579 | 2,451 | 2,129 | 3,676 | 3,096 | 6,063 | 3,225 | 7,417 | 43,731 | 645 | |
| H652 | 240 | 959 | 242 | 719 | 1,227 | 578 | 127 | 2,862 | 27,607 | 141 | |
| 3 wk after viral | H643 | 935 | 610 | 274 | 772 | 1,700 | 1,402 | 248 | 11,724 | 23,112 | 146 |
| challenge | H648 | 2,622 | 2,401 | 1,056 | 244 | 4,698 | 6,403 | 151 | 7,111 | 38,558 | 116 |
| H649 | 2,201 | 2,341 | 1,010 | 3,351 | 4,039 | 3,213 | 230 | 8,858 | 31,809 | 459 | |
| H652 | 77 | 111 | 75 | 76 | 247 | 85 | 78 | 3,459 | 23,851 | 85 | |
The values are mean counts per minute of four replicates of PBMCs stimulated with either 10 μg/ml of peptide or 2.5 μg/ml PWM. The last column contains the mean counts per minute for medium alone. Peptide responses in boldface were significant positive responses, defined as a mean counts per minute minus mean counts per minute with medium alone of ≥1,000 and also an SI ≥3 (neither calculation is shown in the table). ND, not done.
The postimmunization proliferative responses of PBMCs from the EIAV lipopeptide-immunized group were compared with the preimmunization responses to the five Th peptides, Gag 221-245, Gag 250-269, Pol 326-347, Pol 704-719, and Gag 13-32 (Table 3). By using a nonparametric repeated-measures analysis of variance (Friedman's test), there was a significant increase in postimmunization counts per minute (P = 0.0006). The Dunn's multiple-comparisons posttest results were significant (P < 0.05) for three Th peptides: Gag 221-245, Gag 250-269, and Pol 326-347.
In contrast, there was no significant difference in the proliferative responses of PBMCs from the control group pre- and postimmunization to the five Th peptides (Freidman's test P > 0.05) (Table 4). Only PBMCs from H649 had a significant positive response to Ana-P16a, used to immunize the control group; and this horse also responded to three of the five EIAV Th peptides (Table 4). There is no homology between Ana-P16a and the EIAV peptides that could explain these results. Furthermore, H649 tested negative for EIAV antibodies, indicating that it was not infected (41).
Lack of a consistent CTL response to immunization with EIAV lipopeptides.
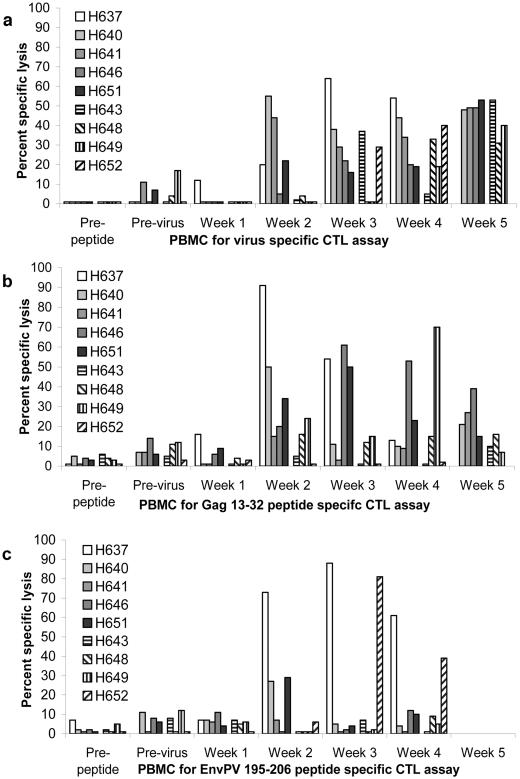
CTL responses in PBMCs from the EIAV and control lipopeptide-immunized groups were evaluated before and after immunization. There was no significant CTL activity in preimmunization PBMCs from any of the nine horses when they were stimulated with EIAV and assayed on EIAV-infected EK cell targets (Fig. 1a), when they were stimulated with Gag 13-32 and assayed on Gag 13-32-pulsed EK cell targets (Fig. 1b), or when they were stimulated with Env 195-206PV and assayed on Env 195-206PV-pulsed targets (Fig. 1c). The Gag 13-32 and Env 195-206PV peptides were used because they contained optimal CTL epitopes known to be ELA-A1 restricted (31, 33). Gag 13-32 also contained a Th epitope, and Env 195-206PV was linked to a Th epitope for immunization. PBMCs from ELA-A1 horses H637 and H652 were tested in all CTL assays by using H637 EK cell targets because H637 and H652 had identical MHC class I haplotypes (Table 1). PBMCs from the remaining seven horses were tested for CTLs by using half-matched EK cells from their sire (H600), since these seven horses inherited this sire's ELA-A1′ allele.
FIG. 1.
CTL activities of PBMCs from horses before immunization (prepeptide), after five lipopeptide immunizations (previrus), and following EIAVPV challenge (weeks 1 to 5). The first five horses listed in each legend (H637, H640, H641, H646, and H651) were immunized with EIAV lipopeptides, and the next four horses (H643, H648, H649, and H652) were immunized with an Anaplasma control lipopeptide. (a) EK cell targets were EIAVPV infected, and prepeptide and previrus PBMC effectors were stimulated with EIAVPV, whereas PBMCs obtained after challenge (weeks 1 to 5) were stimulated with EIAVWSU5. (b) The Gag 13-32 peptide was used to stimulate PBMCs and pulse EK cell targets. (c) The EnvPV 195-206 peptide was used to stimulate PBMCs and pulse EK cell targets. In panel a, the percent specific lysis of noninfected targets was subtracted from the percent specific lysis of infected targets; in panels b and c, the percent specific lysis of non-peptide-pulsed targets was subtracted from that of peptide-pulsed targets. The corrected specific lysis in this figure of ≥10% was considered a significant positive assay result, as these assay results were also ≥3 SEs above those for the noninfected or nonpulsed targets. Peptide targets for H637 previrus had levels of spontaneous lysis that were too high and were not used. Neither H637 PBMCs nor H652 PBMCs were available for testing at week 5 (a and b), and no assay was performed in week 5 with EnvPV 195-206 (c). The effector cell-to-target cell ratio was 20:1.
CTL assays were done with the Ana-P16a control lipopeptide-immunized horses 1 week after the fifth and final immunization. Stimulated PBMCs from some horses in both immunization groups caused significant but low-level lysis of either EIAV-infected or peptide-pulsed EK cell targets (Fig. 1a to c, previrus); however, there was no significant difference between the groups (one-sided Mann-Whitney rank sum test, P > 0.05). The assay with H637 PBMCs and peptide-pulsed targets after the fifth immunization was not used (Fig. 1b and c, previrus) because the rate of spontaneous lysis of the target cells exceeded 30%; however, these PBMCs did not significantly lyse virus-infected targets, which had an acceptable level of spontaneous lysis (Fig. 1a, previrus). PBMCs from control horse H649 had weak but significant CTL responses to both virus-infected and peptide-pulsed target cells. As indicated in the preceding section on lymphocyte proliferation, the reactivity of H649 PBMCs to EIAV was not explained either by shared amino acid sequences between EIAV and the Anaplasma peptide or by inadvertent infection of H649 with EIAV.
Proliferation of PBMCs from EIAV lipopeptide-immunized horses was enhanced after virus challenge.
Proliferation assays were performed weekly after EIAVPV challenge, and the results from 1 and 3 weeks after challenge are presented in Tables 3 and 4. Comparison of the postimmunization proliferative responses to the five Th peptides with the responses at 1 week after virus challenge demonstrated a significant enhancement of responses (Freidman's test, P = 0.0001). The Dunn's multiple-comparisons posttest was used to determine if the individual peptides causing the significant group response could be identified, but none of the five individual Th peptides were significant in the posttest (P > 0.05). However, all 25 individual assays at 1 week after challenge were positive, and each one had higher counts per minute than those postimmunization (Table 3). Three weeks after challenge, 19 of the 25 assays were significantly positive (Table 3).
There was no significant enhancement of the proliferative response to Th peptides in the control group 1 week after virus challenge (Freidman's test, P > 0.05). The results of only 10 of the 20 assays were significantly positive at this time, and the results of 8 assays were positive 3 weeks after challenge. All four horses responded to the control Ana-P16a peptide at 1 and 3 weeks after challenge.
Appearance of CTL responses after virus challenge.
PBMCs from all horses in both groups developed CTL responses in which the CTLs significantly lysed the virus-infected targets by 5 weeks after EIAVPV challenge (Fig. 1a, week 5). However, these CTL responses appeared sooner (Fig. 1a, week 2) in horses immunized with EIAV lipopeptides than in control horses (one-sided Mann-Whitney rank sum test, P < 0.05). The transient loss of CTL activity in horse H643 at week 4 (Fig. 1a) was during a febrile episode, and there was a concurrent transient loss of the proliferative response (data not shown). The transient loss of immune responses has been well described in EIAV and other viral infections (14, 36).
The CTL lysis of virus-infected cell targets by PBMCs from horses H637 and H652 could have been restricted by the MHC class I molecules expressed from either haplotype (ELA-A1 or W11) since homologous EK cell targets were used. For H640, H641, H643, H646, H648, H649, and H651, the CTL activity measured was restricted to MHC class I molecules from their IBD ELA-A1′-containing haplotype because EK targets from the common sire (H600 ELA A1′/W11) were used (Table 1). Therefore, PBMCs from these seven horses frozen at week 2 after virus challenge were tested for CTL activity against EIAVPV-infected EK cell targets from each dam. None had significant CTL activity against infected targets from the dam (data not shown). All the frozen PBMCs were capable of responding, because lysis of EIAVPV-infected H600 EK cell targets mirrored those responses obtained with fresh PBMCs against these cell targets (data not shown). When the total ELA-A haplotype was considered, none of four control horses had CTLs that would lyse virus-infected targets 2 weeks after viral challenge, whereas four of five EIAV lipopeptide-immunized horses had these responses.
There were some differences in CTL responses after viral challenge to Gag 13-32 and Env 195-206PV, depending on the source of the ELA-A1 allele. Horses H637 and H652 shared the maternally derived ELA-A1/DRA*0101/DQA*1201 haplotype and were assigned to different immunization groups. PBMCs from H637 in the EIAV lipopeptide-immunized group responded 2 weeks after viral challenge to both Env 195-206PV and Gag 13-32 (Fig. 1b and c). PBMCs from H652 in the control lipopeptide-immunized group responded to Env 195-206PV by week 3 (Fig. 1c) but did not respond to Gag 13-32 at any time (Fig. 1b). Despite being in two different immunization groups, the other seven horses (H640, H641, H646, H651, H643, H648, and H649), which shared the paternally derived ELA-A1′/DRA*0101/DQA*0401 haplotype, all had CTL responses to Gag 13-32 at some time point after viral challenge (Fig. 1b), whereas only three of these horses (H640, H646, and H651 in the EIAV lipopeptide group) had occasional significant CTL responses to Env 195-206PV.
Clinical disease and viral load following virus challenge of immunized horses.
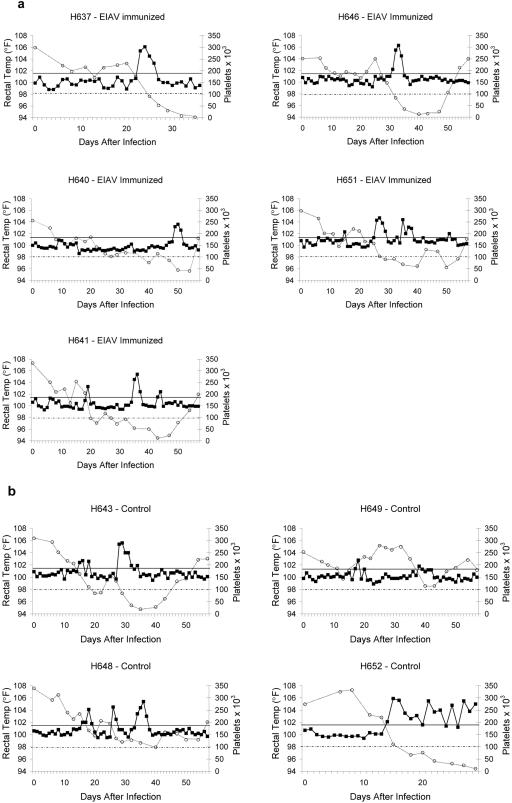
Both horse groups were challenged intravenously with 300 TCID50s EIAVPV 2 weeks after the final immunization. All the horses experienced fevers and decreased platelet counts (Fig. 2a and b), and there was no significant difference between the two groups in these parameters (one-sided Mann-Whitney rank sum test, P > 0.05). Horse H652 (control group) experienced the most severe clinical disease (Fig. 2b) and died on day 30. Horse H637 (EIAV peptide immunization group) developed persistently low platelet counts (Fig. 2a) and was euthanized on day 37. Horse H649 in the control group had a subclinical EIAV infection with only two nonconsecutive days of mild fever and no thrombocytopenia (Fig. 2b). The five EIAV lipopeptide-immunized horses had their first febrile episode at 23, 49, 35, 31, and 26 days after viral challenge, respectively (Fig. 2a), whereas the four control peptide-immunized horses had their first febrile episode at 15, 16, >57, and 14 days after viral challenge, respectively (Fig. 2b). The apparent delay in the EIAV lipopeptide-immunized group was not statistically significant (one-sided Mann-Whitney rank sum test, P > 0.05). Viral loads were measured by real-time RT-PCR with plasma samples obtained during the first 47 days after infection (Table 5), and there was no significant difference between the two groups in either the number of viral RNA copies/ml at peak levels or the time to reach the peak number of viral RNA copies/ml (one-sided Mann-Whitney rank sum test, P > 0.05). Further, no association was found between the CTL responses and any of the disease parameters in either group. Thus, there was no evidence of better control of virus load or clinical disease in the EIAV lipopeptide-immunized group.
FIG. 2.
Disease parameters for EIAV lipopeptide-immunized (a) and control lipopeptide-immunized (b) horses following EIAV challenge. Morning rectal temperatures (in °F) are on the left scale (closed squares) and periodic platelet counts × 1,000 per μl blood are on the right scale (open circles). Rectal temperatures above the dark solid horizontal line were considered fevers, and platelet counts below the lighter dotted horizontal line were considered thrombocytopenic.
TABLE 5.
Plasma viral load determined by real-time RT-PCR
| Days PVa | Plasma viral load (no. of viral RNA copies/ml)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| EIAV lipopeptide group
|
Control lipopeptide group
|
||||||||
| H637 | H640 | H641 | H646 | H651 | H643 | H648 | H649 | H652 | |
| 8 | NDb | ND | 2,540 | ND | ND | 2,770 | ND | ND | ND |
| 11 | 8,000 | 8,100 | 12,500 | ND | 810 | 49,000 | 6,670 | 2,090 | 8,890 |
| 13 | 12,000 | 13,000 | 57,000 | ND | ND | 47,700 | 14,900 | 2,880 | 42,200 |
| 15 | 6,000 | 15,900 | 135,000 | 2,690 | ND | 2,40,000 | 12,700 | 1,650 | 192,000 |
| 18 | 35,000 | 29,500 | 1,370,000 | 1,230 | ND | 1,390,000 | 349,000 | 920 | 416,000 |
| 25 | 7,270,000c | 16,100 | 538,000 | 2,790,000 | 259,000 | 568,000 | 5,590 | 3,200 | 3,760,000 |
| 32 | 1,190,000 | 900 | 320,000 | 13,900,000 | 311,000 | 299,000 | 1,250,000 | 31,800 | 10,300,000e |
| 47 | NAd | 226,000 | 361,000 | ND | 392,000 | ND | 1,320,000 | 92,900 | NA |
Days PV, days after viral challenge.
ND, not detected.
The peak viral load for each horse is in boldface.
NA, not available at that time point due to death or euthanasia.
This determination was done with the day 29 sample instead of the day 32 sample for horse H652.
DISCUSSION
The horse group immunized with EIAV lipopeptides had significant postimmunization proliferative responses to Th peptides compared to those preimmunization, with the responses to peptides Gag 221-245, Gag 250-260, and Pol 326-347 being significant. The CTL response to EIAV lipopeptide immunization was not significantly different from that to the control immunization. These results permitted evaluation of immunization-induced Th responses on the Th and CTL responses after virus challenge and on the viral load and subsequent clinical disease. The lack of postimmunization CTL responses prevented a similar evaluation of CTL effects.
One week after virus challenge, the EIAV lipopeptide-immunized group had a significantly increased proliferative response to the Th peptides compared to the response postimmunization. PBMCs from all five horses had significantly positive responses to all five Th peptides, demonstrating that these horses had MHC class II molecules that could present the five Th peptides. Stimulated PBMCs from four of these horses obtained 2 weeks after virus challenge had CTL activity when they were tested on EIAV-infected EK cell targets; whereas none of the control group had CTL responses. Despite the Th responses to the Gag and Pol peptides before EIAV challenge, enhanced proliferation 1 week after challenge, and CTL responses 2 weeks after challenge, there was no significant protection, as measured by the peak number of RNA copies/ml plasma, the time to the peak number of RNA copies/ml, platelet counts, or fever. Macaques with a CD4 T-cell response to the SIV envelop protein without a CTL response had enhanced SIV replication and accelerated progression of AIDS following SIV challenge (52). This was attributed to CD4 T-cell tropic SIV infection of activated CD4 T cells (52). No enhancement of viremia was noted in horses with Th responses in the current experiment, likely because EIAV does not infect lymphocytes.
The ability of horse MHC class II molecules to present peptide Ana-P16a, from the bovine pathogen Anaplasma marginale (6), was unknown prior to this study. PBMCs from all four control horses immunized with peptide Ana-P16a had significant proliferative responses; however, the response occurred after virus challenge in three horses. Peptide Ana-P16a does not share sequences with EIAV, so it is unlikely that EIAV challenge stimulated responses to this peptide. This interpretation is supported by the observation that none of the EIAV lipopeptide-immunized horses responded to Ana-P16a after virus challenge.
The dose of lipopeptides and the use of Freund's adjuvant were based on a previous preliminary experiment with horses. That experiment used a dose that induced strong cellular immune responses to lipopeptides without adjuvant in both chimpanzees and humans (3, 57) and the induction of strong B, Th, and CTL responses to HIV-1 lipopeptides in HIV-1-seronegative volunteers (15, 42). Three horses (adult mixed-breed ponies) were immunized subcutaneously with 5 μg per kilogram of each lipidated peptide, including Gag 221-245, Gag 242-261, and Pol 323-344, in 1 ml PBS with 10% DMSO (unpublished results). No side effects occurred; however, the proliferative response to immunization was either inconsistent or nonexistent, even though these horses responded to all three peptides after EIAV challenge. Thus, in the current experiment, the lipopeptide quantity was increased and Freund's adjuvant was included to induce maximal stimulation with the lipopeptides. While this adjuvant increased the Th responses to the lipopeptides, it also increased the side effects, especially in the EIAV peptide-immunized group. The increased side effects in this group may have been due to increased lipopeptide or the immune response to lipopeptides, or both.
In contrast to the strong lymphocyte proliferative responses after immunization with EIAV lipopeptides, consistent CTL responses were not detected. Since only PBMCs were evaluated, it is possible that CTLs were present in lymphoid or other tissues. In a previous study, CTLs were present transiently in PBMCs from three ELA-A1 horses immunized with a Env peptide coupled to a complex lipopeptide from Escherichia coli (45). In the current study, CTL responses measured with EIAV-infected cell targets developed sooner in EIAV lipopeptide-immunized horses than in controls. Assays were done weekly, so the earlier development of CTL activity may have been under- or overestimated. A group of closely related horses was chosen to minimize some of the variables in outbred horses; however, the genetic variability among this group far exceeded that in inbred strains of mice. Still, the earlier onset of CTL activity in the EIAV lipopeptide-immunized horses was unable to significantly affect the outcome of EIAV challenge.
There were differences in CTL responses associated with the source of the ELA-A1 haplotype in immunized horses. Even though all nine horses had an ELA-A1 serotype, there were apparently two different subtypes. Gag 13-32 stimulated an immunodominant CTL response in horses sharing the ELA-A1′/DRA*0101/DQA*0401 haplotype that originated from their common sire, horse H600, compared to the responses to Env 195-206PV. The two horses sharing the ELA-A1/DRA*0101/DQA*1201 haplotype from their common dam, horse H504, had the highest responses to Env 195-206PV, whereas one responded to Gag 13-32 and one did not. Given that other unrelated ELA-A1 serotypes have differences in their abilities to present these two peptides (31) and that a common ELA-A1 sequence has not been found (7), sequence-based typing is needed for future studies with ELA-A1 horses. Horses H637 and H652 had the highest Env 195-206PV CTL responses and had the most severe disease following EIAV challenge (Fig. 2). More severe disease could be caused by CTL epitope variation, since variants of Env 195-206 occur in EIAV-infected horses that escape CTL recognition (33).
In conclusion, this study evaluated horses immunized with lipidated versions of five broadly reactive EIAV Th peptides (12, 13). Three of these peptides induce both CD4+ T-lymphocyte proliferation and increased IFN-γ mRNA production in PBMCs from EIAV-infected horses (13). Two lipopeptides containing CTL epitopes presented by an MHC class I molecule likely representing ELA-A1 (31, 33) were included in the immunization of ELA-A1 horses. One CTL epitope was linked to a Th peptide; the other was made longer than optimum to include a Th epitope. The group immunized with EIAV lipopeptides had significant PBMC proliferation compared with that preimmunization, and there was a further significant increase in the PBMC proliferative response 1 week after virus challenge. Consistent CTL responses were not induced by lipopeptide immunization; however, CTL responses to virus-infected targets appeared earlier after viral challenge in the EIAV lipopeptide-immunized group than in controls. Despite these differences, the overall clinical outcome and the viral load were not significantly different in the two groups. Accordingly, the hypothesis that Th responses to immunization would enhance both the Th and the CTL responses following EIAV challenge was supported, whereas there was no support for the hypothesis that these enhanced responses would affect disease outcome. The lack of protective immunity in this trial with outbred horses is disappointing but reflects the difficulty of inducing consistent CTL responses by immunization in outbred species, even when some of the major variables, including MHC class I and class II haplotypes and peptides, are optimized. Nevertheless, the results clearly demonstrate that significant proliferative responses to Th peptides before EIAV challenge were insufficient to alter the viral load or the severity of clinical disease.
Acknowledgments
This work was supported by U.S. Public Health Service Individual National Research Service Award AI10528 (to D. G. Fraser) and National Institutes of Health research grants AI24291 and AI47660 (to T. C. McGuire).
We thank E. Karel and A. Edwards for horse care, M. Littke for technical assistance, K. Kegerreis for help choosing the Anaplasma peptide, and G. Munske for peptide synthesis. We also thank E. Bailey, University of Kentucky, for providing ELA-A typing antisera.
REFERENCES
- 1.Albright-Fraser, D. G., R Reid, V. Gerber, and E. Bailey. 1996. Polymorphism of DRA among equids. Immunogenetics 43:315-317. (Erratum, 44:487.) [PubMed] [Google Scholar]
- 2.Allen, G., M. Yeargan, L. R. Costa, and R. Cross. 1995. Major histocompatibility complex class I-restricted cytotoxic T lymphocyte responses in horses infected with equine herpesvirus 1. J. Virol. 69:606-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.BenMohamed, L., H. Gras-Masse, A. Tartar, P. Daubersies, K. Brahimi, M. Bossus, A. Thomas, and P. Druilhe. 1997. Lipopeptide immunization without adjuvant induces potent and long-lasting B, T helper, and cytotoxic T lymphocyte responses against a malaria liver stage antigen in mice and chimpanzees. Eur. J. Immunol. 27:1242-1253. [DOI] [PubMed] [Google Scholar]
- 4.Bernoco, D., D. F. Antczak, E. Bailey, K. Bell, R. W. Bull, G. Byrns, G. Guerin, S. Lazary, J. McClure, J. Templeton, and H. Varewyck. 1987. Joint report of the fourth international workshop on lymphocyte alloantigens of the horse, Lexington, Kentucky, 12-22 October 1985. Anim. Genet. 18:81-94. [PubMed] [Google Scholar]
- 5.Betts, M. R., D. R. Ambrozak, D. C. Douek, S. Bonhoeffer, J. M. Brenchley, J. P., Casazza, R. A. Koup, and L. J. Picker. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 75:11983-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, W. C., T. C. McGuire, D. Zhu, H. A. Lewin, J. Sosnow, and G. H. Palmer. 2001. Highly conserved regions of the immunodominant major surface protein 2 (MSP2) of the ehrlichial pathogen Anaplasma marginale are rich in naturally derived CD4+ T lymphocyte epitopes that elicit strong recall responses. J. Immunol. 166:114-124. [DOI] [PubMed] [Google Scholar]
- 7.Chung, C., S. R. Leib, D. G. Fraser, S. A. Ellis, and T. C. McGuire. 2003. Novel classical MHC class I alleles identified in horses by sequencing clones of reverse transcription-PCR products. Eur. J. Immunogenet. 30:387-396. [DOI] [PubMed] [Google Scholar]
- 8.Crawford, T. B., T. C. McGuire, and J. B. Henson. 1971. Detection of equine infectious anemia by immunofluorescence. Arch. Gesamte Virusforsch. 34:332-339. [DOI] [PubMed] [Google Scholar]
- 9.Edwards, B. H., A. Bansal, S. Sabbaj, J. Bakari, M. J. Mulligan, and P. A. Goepfert. 2002. Magnitude of functional CD8+ T-cell responses to the Gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J. Virol. 76:2298-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fields, G. B., and R. L. Noble. 1990. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int. J. Pept. Protein Res. 35:161-164. [DOI] [PubMed] [Google Scholar]
- 11.Fraser, D. G., and E. Bailey. 1998. Polymorphism and multiple loci for the horse DQA gene. Immunogenetics 47:487-490. [DOI] [PubMed] [Google Scholar]
- 12.Fraser, D. G., R. H. Mealey, and T. C. McGuire. 2003. Selecting peptides to optimize Th1 responses to an equine lentivirus using HLA-DR binding motifs and defined HIV-1 Th peptides. Immunogenetics 55:508-514. [DOI] [PubMed] [Google Scholar]
- 13.Fraser, D. G., J. L. Oaks, W. C. Brown, and T. C. McGuire. 2002. Identification of broadly recognized, T helper 1 lymphocyte epitopes in an equine lentivirus. Immunology 105:295-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuller, M. J., and A. J. Zajac. 2003. Ablation of CD8 and CD4 T cell responses by high viral loads. J. Immunol. 170:477-486. [DOI] [PubMed] [Google Scholar]
- 15.Gahery-Segard, H., G. Pialoux, B. Charmeteau, S. Sermet, H. Poncelet, M. Raux, A. Tartar, J.-P. Levy, H. Gras-Masse, and J.-G. Guillet. 2000. Multiepitopic B- and T-cell responses induced in humans by a human immunodeficiency virus type 1 lipopeptide vaccine. J. Virol. 74:1694-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammond, S. A., S. J. Cook, L. D. Falo, C. J. Issel, and R. C. Montelaro. 1999. A particulate viral protein vaccine reduces viral load and delays progression to disease in immunized ponies challenged with equine infectious anemia virus. Virology 254:37-49. [DOI] [PubMed] [Google Scholar]
- 17.Hammond, S. A., S. J. Cook, D. L. Lichtenstein, C. J. Issel, and R. C. Montelaro. 1997. Maturation of the cellular and humoral immune responses to persistent infection in horses by equine infectious anemia virus is a complex and lengthy process. J. Virol. 71:3840-3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrold, S. M., S. J. Cook, R. F. Cook, K. E. Rushlow, C. J. Issel, and R. C. Montelaro. 2000. Tissue sites of persistent infection and active replication of equine infectious anemia virus during acute disease and asymptomatic infection in experimentally infected equids. J. Virol. 74:3112-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hel, Z., J. Nacsa, E. Tryniszewska, W.-P. Tsai, R. W. Parks, D. C. Montefiori, B. K. Felber, J. Tartaglia, G. N. Pavlakis, and G. Franchini. 2002. Containment of simian immunodeficiency virus infection in vaccinated macaques: correlation with the magnitude of virus-specific pre- and postchallenge CD4+ and CD8+ T cell responses. J. Immunol. 169:4778-4787. [DOI] [PubMed] [Google Scholar]
- 20.Herbert, T. B., M. Coriell, and S. Cohen. 1994. Analysis of lymphocyte proliferation data: do different approaches yield the same results? Brain Behavior Immun. 8:153-162. [DOI] [PubMed] [Google Scholar]
- 21.Issel, C. J., W. V. Adams, L. Meek, and R. Ochoa. 1982. Transmission of equine infectious anemia virus from horses without clinical signs of disease. J. Am. Vet. Med. Assoc. 180:272-275. [PubMed] [Google Scholar]
- 22.Issel, C. J., D. W. Horohov. D. F. Lea, W. V. Adams, S. D. Hagius, J. M. McManus, A. C. Allison, and R. C. Montelaro. 1992. Efficacy of inactivated whole-virus and subunit vaccines in preventing infection and disease caused by equine infectious anemia virus. J. Virol. 66:3398-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janssen, E. M., E. E. Lemmens, T. Wolfe, U. Christen, M. G. von Herrath, and S. P. Schoenberger. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421:852-856. [DOI] [PubMed] [Google Scholar]
- 24.Kalams, S. A., S. P. Buchbinder, E. S. Rosenberg, J. M. Billingsley, D. S. Colbert, N. G. Jones, A. K. Shea, A. K. Trochea, and B. D. Walker. 1999. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J. Virol. 73:6715-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kono, Y., K. Hirasawa, Y. Fukunaga, and T. Taniguchi. 1976. Recrudescence of equine infectious anemia by treatment with immunosuppressive drugs. Natl. Inst. Anim. Health Q. (Tokyo) 16:8-15. [PubMed] [Google Scholar]
- 26.Kono, Y., K. Kobayashi, and Y. Fukunaga. 1973. Antigenic drift of equine infectious anemia virus in chronically infected horses. Arch. Gesamte Virusforsch. 41:1-10. [DOI] [PubMed] [Google Scholar]
- 27.Kono, Y. 1969. Viremia and immunological responses in horses infected with equine infectious anemia virus. Natl. Inst. Anim. Health Q. (Tokyo) 9:1-9. [PubMed] [Google Scholar]
- 28.Livingston, B. D., C. Crimi, M. Newman, Y. Higashimoto, E. Appella, J. Sidney, and A. Sette. 2002. A rational strategy to design multiepitope immunogens based on multiple Th lymphocyte epitopes. J. Immunol. 168:5499-5506. [DOI] [PubMed] [Google Scholar]
- 29.Maeker, H. T., D. T. Umetsu, R. H. DeKruyff, and S. Levy. 1998. Cytotoxic T cell responses to DNA vaccination: dependence on antigen presentation via class II MHC. J. Immunol. 161:6532-6536. [PubMed] [Google Scholar]
- 30.McGuire, T. C., D. S. Adams, G. C. Johnson, P. Klevjer-Anderson, D. D. Barbee, and J. R. Gorham. 1986. Acute arthritis in caprine arthritis-encephalitis virus challenge exposure of vaccinated or persistently infected goats. Am. J. Vet. Res. 47:537-540. [PubMed] [Google Scholar]
- 31.McGuire, T. C., S. R. Leib, R. H. Mealey, D. G. Fraser, and D. J. Prieur. 2003. Presentation and binding affinity of equine infectious anemia virus CTL envelope and matrix protein epitopes by an expressed equine classical MHC class I molecule. J. Immunol. 171:1984-1993. [DOI] [PubMed] [Google Scholar]
- 32.McGuire, T. C., D. B. Tumas, K. M. Byrne, M. T. Hines, S. R. Leib, A. L. Brassfield, K. I. O'Rourke, and L. E. Perryman. 1994. Major histocompatibility complex-restricted CD8+ cytotoxic T lymphocytes from horses with equine infectious anemia virus recognize Env and Gag/PR proteins. J. Virol. 68:1459-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mealey, R., H., B. Zhang, S. R. Leib, M. H. Littke, and T. C. McGuire. 2003. Epitope specificity is critical for high and moderate avidity cytotoxic T lymphocytes associated with control of viral load and clinical disease in horses with equine infectious anemia virus. Virology 313:537-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mintern, J. D., G. M. Davey, G. T. Belz, F. R. Carbone, and W. R. Heath. 2002. Cutting edge: frequency affects the helper dependence of cytotoxic T cells. J. Immunol. 168:977-980. [DOI] [PubMed] [Google Scholar]
- 35.Montelaro, R. C., B. Parekh, A. Orrego, and C. J. Issel. 1984. Antigenic variation during persistent infection by equine infectious anemia virus, a retrovirus. J. Biol. Chem. 259:10539-10544. [PubMed] [Google Scholar]
- 36.Newman, M. J., C. J. Issel, R. E. Truax, M. D. Powell, D. W. Horohov, and R. C. Montelaro. 1991. Transient suppression of equine immune responses by equine infectious anemia virus (EIAV). Virology 184:55-66. [DOI] [PubMed] [Google Scholar]
- 37.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 38.O'Rourke, K., L. E. Perryman, and T. C. McGuire. 1988. Antiviral, anti-glycoprotein and neutralizing antibodies in foals with equine infectious anaemia virus. J. Gen. Virol. 69:667-674. [DOI] [PubMed] [Google Scholar]
- 39.Ossendorp, F., E. Mengede, M. Camps, R. Filius, and C. J. Melief. 1998. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J. Exp. Med. 187:693-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Partidos, C. D., P. Vohra, and M. W. Steward. 1996. Induction of measles virus-specific cytotoxic T-cell responses after intranasal immunization with synthetic peptides. Immunology 87:179-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pearson, J. E., and L. Coggins. 1979. Protocol for the immunodiffusion (Coggins) test for equine infectious anemia. Proc. Am. Assoc. Vet. Lab. Diagn. 22:449-462. [Google Scholar]
- 42.Pialoux, G., H. Gahery-Segard, S. Sermet, H. Poncelet, S. Fournier, L. Gerard, A. Tartar, H. Gras-Masse, J.-P. Levy, and J.-G. Guillet for the ANRS VAC 04 Study Team. 2001. Lipopeptides induce cell-mediated anti-HIV immune responses in seronegative volunteers. AIDS 15:1239-1249. [DOI] [PubMed] [Google Scholar]
- 43.Pontesilli, O., P. Carotenuto, S. R. Kerkhof-Garde, M. T. Roos, I. P. Keet, R. A. Coutinho, J. Goudsmit, and F. Miedema. 1999. Lymphoproliferative response to HIV type 1 p24 in long-term survivors of HIV type 1 infection is predictive of persistent AIDS-free infection. AIDS Res. Hum. Retrovir. 15:973-981. [DOI] [PubMed] [Google Scholar]
- 44.Raabe, M. L., Issel, C. J. Cook, S. J. Cook, R. F. Cook, B. Woodson, and R. C. Montelaro. 1998. Immunization with a recombinant envelope protein (rgp90) of EIAV produces a spectrum of vaccine efficacy ranging from lack of clinical disease to severe enhancement. Virology 245:151-162. [DOI] [PubMed] [Google Scholar]
- 45.Ridgely, S. L., B. S. Zhang, and T. C. McGuire. 2003. Response of ELA-A1 horses immunized with lipopeptide containing an equine infectious anemia virus ELA-A1-restricted CTL epitope to virus challenge. Vaccine 21:491-506. [DOI] [PubMed] [Google Scholar]
- 46.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with the control of viremia. Science 278:1447-1450. [DOI] [PubMed] [Google Scholar]
- 47.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 238:857-860. [DOI] [PubMed] [Google Scholar]
- 48.Shedlock, D. J., and H. Shen. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300:337-339. [DOI] [PubMed] [Google Scholar]
- 49.Shirai, M., C. D. Pendleton, J. Ahlers, T. Takeshita, M. Newman, and J. A. Berzofsky. 1994. Helper-cytotoxic T lymphocyte (CTL) determinant linkage required for priming of anti-HIV CD8+ CTL in vivo with peptide vaccine constructs. J. Immunol. 152:549-556. [PubMed] [Google Scholar]
- 50.Siliciano, R. F., A. D. Keegan, R. Z. Dintzis, H. M. Dintzis, and H. S. Shin. 1985. The interaction of nominal antigen with T cell antigen receptors. Part I. Specific binding of multivalent nominal antigen to cytolytic T cell clones. J. Immunol. 135:906-914. [PubMed] [Google Scholar]
- 51.Southwood, S., J. Sidney, A. Kondo, M.-F. del Guercio, E. Appella, S. Hoffman, R. T. Kubo, R. W. Chesnut, H. M. Grey, and A. Sette. 1998. Several common HLA-DR types share largely overlapping peptide binding repertoires. J. Immunol. 160:3363-3373. [PubMed] [Google Scholar]
- 52.Staprans, S. I., A. P. Barry, G. Silvestri, J. T. Safrit, N. Kozyr, B. Sumpter, H. Nguyen, H. McClure, D. Montefiori, J. I. Cohen, and M. B. Feinberg. 2004. Enhanced SIV replication and accelerated progression to AIDS in macaques primed to mount a CD4 T cell response to the SIV envelope protein. Proc. Natl. Acad. Sci. USA 101:13026-13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stephens, R. M., J. W. Casey, and N. R. Rice. 1986. Equine infectious anemia virus gag and pol genes: relatedness to visna and AIDS virus. Science 231:589-594. [DOI] [PubMed] [Google Scholar]
- 54.Sun, J. C., and M. J. Bevan. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300:339-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Terasaki, P. I., D. Bernoco, M. S. Park, G. Ozturk, and Y. Iwaki. 1978. Microdroplet testing for HLA-A, -B, -C and -D antigens. The Philip Levine Award Lecture. Am. J. Clin. Pathol. 69:103-120. [DOI] [PubMed] [Google Scholar]
- 56.Tumas, D. B., M. T. Hines, L. E. Perryman, W. C. Davis, and T. C. McGuire. 1994. Corticosteroid immunosuppression and monoclonal antibody-mediated CD5+ T lymphocyte depletion in normal and equine infectious anaemia virus-carrier horses. J. Gen. Virol. 75:959-968. [DOI] [PubMed] [Google Scholar]
- 57.Vitiello, A., G. Ishioka, H. M. Grey, R. Rose, P. Farness, R. LaFond, L. Yuan, F. V. Chisari, J. Furze, R. Bartholomeuz, and R. W. Chesnut. 1995. Development of a lipopeptide-based therapeutic vaccine to treat chronic HBV infection. J. Clin. Investig. 95:341-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson, C. C., B. Palmer, S. Southwood, J. Sidney, Y. Higashimoto, E. Appella, R. Chesnut, A. Sette, and B. D. Livingston. 2001. Identification and antigenicity of broadly cross-reactive and conserved human immunodeficiency virus type 1-derived helper T-lymphocyte epitopes. J. Virol. 75:4195-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang, W., S. M. Lonning, and T. C. McGuire. 1998. Gag protein epitopes recognized by ELA-A restricted cytotoxic T lymphocytes from horses with long-term equine infectious anemia virus infection. J. Virol. 72:9612-9620. [DOI] [PMC free article] [PubMed] [Google Scholar]