Abstract
Lipopolysaccharide (LPS) causes apoptotic deletion of CD4+ CD8+ thymocytes, a phenomenon that has been linked to immune dysfunction and poor survival during sepsis. Given the abundance of thromboxane-prostanoid (TP) receptors in CD4+ CD8+ thymocytes and in vitro evidence that thromboxane A2 (TXA2) causes apoptosis of these cells, we tested whether enhanced generation of TXA2 plays a role in LPS-induced thymocyte apoptosis. Mice injected with 50 μg of LPS intraperitoneally displayed a marked increase in generation of TXA2 and prostaglandin E2 in the thymus as well as apoptotic deletion of CD4+ CD8+ thymocytes. Administration of indomethacin or rofecoxib inhibited prostanoid synthesis but did not affect thymocyte death. In contrast, thymocyte apoptosis in response to LPS was significantly attenuated in TP-deficient mice. These studies indicate that TXA2 mediates a portion of apoptotic thymocyte death caused by LPS. The absence of an effect of global inhibition of prostanoid synthesis suggests a complex role for prostanoids in this model.
Bacterial sepsis is associated with apoptotic cell death in a variety of organs and tissues, including the thymus (16, 19, 42). The LPS component of gram-negative bacteria appears to mediate most of these effects, since injection of LPS into mice is followed by a dramatic reduction in thymocyte numbers, simulating the situation during sepsis. LPS does not cause thymocyte apoptosis directly, as addition of LPS to in vitro cultures of thymocytes does not result in apoptosis of these cells (43). This result is not surprising, as thymocytes are not known to express the main LPS receptor, TLR4. Therefore, thymocyte apoptosis following LPS injection in vivo is likely mediated by soluble factors produced by TLR4-expressing cells of the innate immune system. The identification of such mediators could have implications for therapy, since recent evidence suggests that lymphocyte apoptosis during sepsis is deleterious because it might impair immune responses and contribute to mortality (20).
Prostanoids are produced by cells of the innate immune system exposed to LPS (27). TXA2 is a prostanoid mediator derived by the sequential metabolism of cell membrane phospholipids by phospholipases, COX, and thromboxane synthase. During inflammatory states, this metabolic pathway is induced, resulting in enhanced TXA2 production (39). TXA2 has long been known for its vasoconstrictive and procoagulant properties, but a recent study revealed a novel and important role for this molecule in cell-mediated immunity (38). In addition, several lines of investigation point toward TXA2 as a potential mediator of thymocyte apoptosis. First, Nusing et al. showed that the activity of TXA2 synthase in dendritic cells of the thymic stroma is as high as it is in platelets (26). Second, Namba and coworkers demonstrated that the thymus is the organ with the highest concentration of TP receptors (24). In the thymus, immature CD4+ CD8+ thymocytes have the highest concentrations of TP receptors among cells; when they are exposed to a TXA2 agonist, these cells undergo apoptosis in a concentration-dependent manner, a phenomenon that can be inhibited by a specific TXA2 antagonist (41). Finally, it has been shown that pharmacologic blockade of TP receptors in vivo abrogates donor-specific tolerance induced by thymic injection of alloantigen, suggesting that TXA2 is involved in the apoptotic deletion of alloreactive thymocytes (30).
Given that LPS enhances the production of TXA2 (and other prostanoids) (27) and the accumulating evidence that TXA2 is involved in thymocyte apoptosis in vitro, we performed experiments to determine whether TXA2 plays a role in LPS-induced thymocyte apoptosis in vivo. Our studies suggest that the generation of TXA2 and the consequent activation of the TP receptor contribute to the loss of thymocytes following exposure to LPS.
MATERIALS AND METHODS
Abbreviations.
BAD, Bcl-2 antagonist of cell death; BAK, Bcl-2 homologous antagonist/killer; BAX, Bcl-2 associated x protein; BCL-2, B-cell lymphoma/leukemia 2 protein; BCL-W, Bcl-2-like 2 protein; BCL-X, Bcl-2-like 1 protein; BFL, Bcl-2 family protein isolated from human fetal liver; Casp, caspase; COX, cyclooxygenase; DMEM, Dulbecco's modified Eagle's medium; FACS buffer, 2% fetal calf serum, 0.01% sodium azide in PBS; FADD, Fas-associated death domain; FAF, Fas-associated factor; Indo, indomethacin; FITC, fluorescein isothiocyanate; i.p., intraperitoneal; LPS, lipopolysaccharide; m-APO, mouse apoptosis; PBS, phosphate-buffered saline; PE, phycoerythrin; PGE2, prostaglandin E2; RIA, radioimmunoassay; RIP, receptor-interacting protein; Rofe, rofecoxib; RPA, RNase protection assay; SEM, standard error of the mean; TLR4, Toll-like receptor 4; TNF, tumor necrosis factor; TNF-α, tumor necrosis factor alpha; TNFR, TNF receptor; TP, thromboxane-prostanoid; TRADD, TNFR1-associated death domain; TRAIL, TNF-related apoptosis-inducing ligand; TXA2, thromboxane A2.
Animals.
Mice lacking TP receptors were generated by gene targeting, as reported previously (37). To eliminate the confounding effects of background genes in our studies, the TP mutation was backcrossed into C57BL/6 mice for more than 10 generations. The genotypes of individual mice were determined by Southern blotting, as described previously (37). All mice were bred and maintained in the Association for Assessment and Accreditation of Laboratory Animal Care-accredited animal facility of the Durham Veterans Affairs Medical Center, according to the guidelines of the National Institutes of Health.
LPS-induced thymocyte deletion.
Fifty micrograms of LPS (catalog no. L-4391; Sigma Chemical) dissolved in 100 μl of PBS (n = 9 per group) or a similar volume of PBS alone (n = 5 TP+/+ mice and n = 4 TP−/− mice) was administered to mice by i.p. injection. Twenty-four hours after i.p. injection, the animals were killed by CO2 asphyxiation and weighed; the thymuses and spleens were harvested, weighed, and placed in separate petri dishes containing 10 ml of 10% DMEM. The organs were gently ground between glass slides, and the cell suspension was transferred to 15-ml conical tubes. The tubes were placed on ice, and the cells rested for 10 min to allow fat separation. The supernatants were then poured into new 15-ml conical tubes and centrifuged at 1,200 rpm for 5 min at 4°C. The supernatants were discarded, and the pellets were resuspended with red blood cell lysis buffer (100 ml water, 0.829 g NH4Cl, 0.1 g KHCO3, 3.72 mg EDTA, pH 7.4); the thymic pellets were lysed with 3 ml for 3 min, whereas the spleens were lysed with 5 ml for 5 min. Then, 5 ml of 10% DMEM was added to each sample to stop the lysis reaction, and the cells were centrifuged at 1,200 rpm for 5 min at 4°C. The pellets were resuspended in PBS, and any remaining tissue fragments were removed by allowing them to adhere to the walls of a 5-ml pipette. The cells were centrifuged at 1,200 rpm for 5 min at 4°C, resuspended in 10 ml of 10% DMEM, and counted. Approximately 5 million cells were then pelleted and resuspended in FACS buffer for flow cytometry analysis. Splenocytes were pretreated with 5 μg of Fc Block (CD16/CD32; Pharmingen) to avoid nonspecific binding. Thymocytes were stained with anti-CD4 FITC and anti-CD8 PE; splenocytes were stained with anti-CD3 FITC and anti-B220 PE. Staining was performed in the dark at 4°C for 30 min. The cells were then washed twice in FACS buffer and fixed in 250 μl of 2% formalin in PBS. A FACSCalibur system scanner (Becton Dickinson, San Jose, CA) was used for analysis. Results were expressed as the mean percentage of gated cells ± SEM.
Separate groups of wild-type C57BL/6 animals were pretreated with indomethacin, rofecoxib, or vehicle control on the day prior to LPS or PBS injection. Both COX inhibitors were added to the drinking water at a concentration of 0.03 mg/ml, and the mice were left to drink ad libitum (28). Based on the spontaneous water consumption by the animals, the dose of COX inhibitor was approximately 7.5 mg/kg of body weight/day per 20-g mouse.
RNA isolation from thymocytes.
Eight-week-old wild-type C57BL/6 mice were injected with 50 μg of LPS as described above. At the designated time points (3, 6, and 12 h postinjection), the mice were killed by CO2 asphyxiation and the thymuses were harvested. Age-matched littermates injected with PBS served as the 0-h controls. Each thymus was placed in a petri dish containing 5 ml of red blood cell lysis buffer and was gently ground between two glass slides. After 3 min, the cells were aspirated and passed through a 70-μm-pore-size filter into a 50-ml conical tube, and 10 ml of PBS was immediately added to stop the lysis reaction. The cells were mixed, divided into three tubes (5 ml/tube), and centrifuged at 1,200 rpm and 4°C for 5 min. The supernatant was completely removed, and the RNA was extracted from the cell pellets with an RNeasy mini kit (QIAGEN), according to the manufacturer's instructions, and stored in RNase-free water at −70°C.
Detection of genes involved in apoptosis.
To detect mRNA for a variety of apoptotic genes, commercially available multiprobe template sets (Riboquant; Pharmingen) were labeled with [α-32P]UTP (Perkin-Elmer), according to the manufacturer's instructions, and then diluted to a concentration of 300,000 cpm/μl of hybridization buffer. All reagents used in probe synthesis were obtained from Pharmingen (In Vitro Transcription kit, catalog no. 45004K). RNA samples were thawed on ice and 5- to 10-μg samples were used for these studies. RNA samples were completely dried on a vacuum evaporator centrifuge without heat and solubilized in 8 μl of hybridization buffer by gently vortexing them for 3 min. The samples were mixed with 2 μl of the diluted probe, and the mixture was transferred to a hybridization oven set at 90°C. The temperature was immediately reduced to 56°C, and the samples were allowed to hybridize with the probe for 12 to 16 h. The following probe sets were used: m-APO1 (Casp-8, Casp-6, Casp-7, Casp-1, Casp-3, Casp-11, Casp-12, Casp-14), m-APO2 (BCL-W, BFL-1, BCL-X, BAK, BAX, BCL-2, BAD), and m-APO3 (Casp-8, FAS-L, FAS, FADD, FAF, TRAIL, TNFR, TRADD, RIP). RPAs were performed by using an RPA kit (catalog no. 45014K; Pharmingen) and by following the protocol suggested by the manufacturer. Briefly, RNase-protected samples were removed from the oven and subjected to sequential digestion with RNase and proteinase K. After treatment with chloroform-isoamyl alcohol, the aqueous phase was removed, the RNA was precipitated with 4 M ammonium acetate and 100% ethanol, and the samples were incubated for 30 min at −70°C. The RNA was then pelleted, washed with 90% ethanol, air dried, and resuspended in 5 μl of 1× loading buffer. The samples were heat blocked (90°C) for 3 min and then run on acrylamide gels. The gels were transferred to Whatman paper, covered with Saran wrap, and dried under vacuum at 80°C for 30 min. The dried gels were placed on film in a cassette with an intensifying screen and exposed at −70°C. The exposure time ranged from 2 h (for the housekeeping genes) to 5 days (for faint bands). The films were developed and scanned, and the bands were analyzed as a ratio of the mean density of the target RNA over that of the GAPDH control by using the Scion Image for Windows program.
Detection of prostanoids in thymic supernatants.
Wild-type C57BL/6 mice (3-month-old littermates) were allocated to receive regular tap water, 0.03 mg/ml of indomethacin, or 0.03 mg/ml of rofecoxib. Twenty-four hours later, half of the mice receiving tap water alone were injected with PBS vehicle. The remaining animals were injected with LPS, as described above. Twenty-four hours after injection, the animals were killed with an overdose of pentobarbital (100 mg/kg), followed by cervical dislocation. Detection of prostanoid levels in thymic supernatants was performed as described previously (36). Briefly, the thymuses were harvested and placed inside a glass homogenizer containing 6 ml of Krebs-Henseleit medium. The thymuses were manually homogenized and the homogenates were placed in a 37°C water bath incubator in a 5% CO2 atmosphere. After 30 min of incubation, the homogenates were transferred to 5-ml polypropylene snap-cap tubes (no. 352063, Falcon) and centrifuged at 3,000 rpm and 4°C for 10 min. The supernatant was then transferred into 2-ml polypropylene tubes (no. MCT-200; United Scientific Products, San Leandro, CA), and the pellet was resuspended in 2 ml of Krebs medium. The PGE2 and TXB2 concentrations in the supernatants were measured by RIA, and the results are expressed in pg/ml. The pellets were then sonicated and the protein concentrations were measured by using a Coomassie Plus Protein Assay Reagent kit (Pierce Biotechnology), according to the manufacturer's instructions. The results for PGE2 and TXB2 concentrations were expressed as pg/μg protein to normalize the prostanoid concentration in the supernatant to the amount of protein present in each sample.
Statistics.
The results are expressed as means ± SEMs. The Student t test was used to compare mean values between the control and the experimental groups. A P value ≤0.05 was considered statistically significant.
RESULTS
LPS causes thymocyte apoptosis.
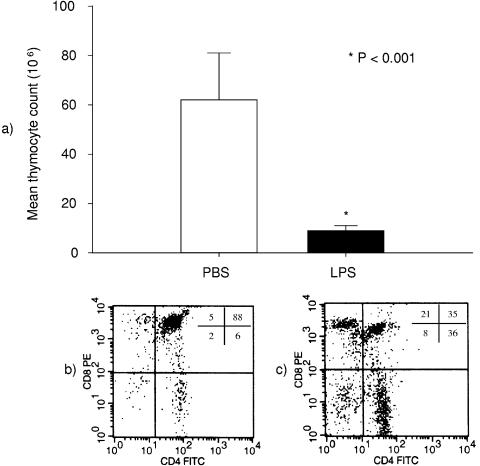
To document the effects of LPS on the thymus in vivo, wild-type C57BL/6 mice were treated with 50 μg of LPS intraperitoneally or a similar volume of PBS control. Twenty-four hours later, the animals were killed, the thymuses were harvested, the thymocytes were counted, and the cell surface phenotypes were determined by flow cytometry. At this dose of LPS, the animals developed diarrhea, piloerection, and reduced activity levels. However, this dose of LPS did not cause any mortality in this or any other experimental group. After LPS injection, there was a dramatic, ≈85% reduction in total thymocytes from (62 ± 19) × 106 to (9 ± 2) × 106 cells (Fig. 1a). As illustrated in Fig. 1b and c, the percentage of the population of CD4+ CD8+ immature T-cell precursors decreased by ≈53%, from 88% ± 1% to 41% ± 5% of total gated cells (P < 0.001).
FIG. 1.
Marked reduction in the CD4+ CD8+ thymocyte population in response to LPS. (a) The bar graph shows the total thymocyte count of wild-type C57BL/6 mice 24 h after a single i.p. injection of 50 μg of LPS (n = 9) or a similar volume of PBS control (n = 5). LPS injection caused a dramatic reduction in total thymocyte count (from 62 ± 19 million to 9 ± 2 million cells; P < 0.001). The flow cytometry plots below each bar illustrate the thymocyte phenotype of animals treated with PBS (b) or LPS (c). The numbers inside the plot represent the contribution (%) of each thymocyte population to the total of gated cells: left lower quadrant, CD4− CD8− cells; left upper quadrant, CD4− CD8+ cells; right lower quadrant, CD4+ CD8− cells; right upper quadrant, CD4+ CD8+ cells. These plots are representative of those from five experiments with control cells and nine experiments with thymocytes from LPS-injected animals. LPS-induced reduction in thymocyte count was due to a contraction of the CD4+ CD8+ population (from 88% ± 1% to 41% ± 5% of gated cells; P < 0.001).
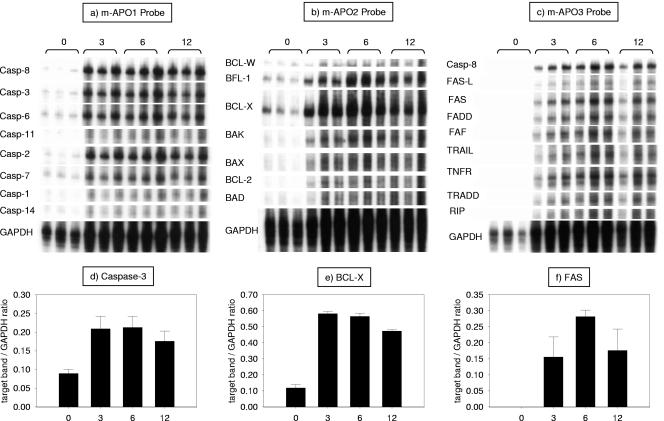
This marked reduction in thymocyte numbers was accompanied by increased expression of a panel of apoptotic genes (Fig. 2). At the baseline, the expression of most genes involved in apoptosis was very low or undetectable. Three hours after an i.p. injection of 50 μg of LPS, there was a marked increase in the expression of caspases and genes of the mitochondrial and FAS pathways (Fig. 2a to c). For example, the expression of caspase-3, a cysteine protease that has a distinct role in the final induction of apoptosis, increased from 0.09 ± 0.01 at the baseline to 0.21 ± 0.03 at 3 h (P = 0.027; mean density of target band/mean density of housekeeping gene) (Fig. 2d). The expression of proapoptotic genes (BAX, BAK, BAD) and antiapoptotic genes (BCL-W, BCL-X, BLF-1, BCL-2) of the Bcl-2 family was also markedly increased in response to LPS. As depicted in Fig. 2e, expression of the prosurvival gene BCL-X increased from 0.12 ± 0.02 to 0.58 ± 0.01 (P < 0.001) 3 h after LPS injection. Genes of the FAS pathway were similarly induced by LPS administration; the expression of the death receptor FAS was undetectable at the baseline but increased rapidly after LPS injection, achieving peak levels (0.28 ± 0.02) at 6 h (Fig. 2f).
FIG. 2.
Increased thymocyte expression of apoptotic genes in response to LPS. Wild-type mice received an i.p. injection of 50 μg of LPS, and their thymuses were harvested at 3, 6, and 12 h later. The 0-h time point presents data from animals injected with a similar volume of PBS. RNA was extracted from thymocytes, and the genes involved in apoptosis were detected by RPAs. Here we show the autoradiograph of three RPA gels done with commercially available probe sets. The bands were analyzed with an imaging program. Below each gel is a bar graph that corresponds to the analysis of a chosen gene (d to e, respectively). The genes were chosen based on their relevance (caspase-3 and FAS) or level of expression (BCL-X). Data are expressed as the ratio of the mean density of the target band over the mean density of the housekeeping gene GAPDH. As a rule, LPS caused a marked and significant increase in the level of expression of the mRNAs of all apoptotic genes tested.
LPS injection enhances prostanoid production in the thymus.
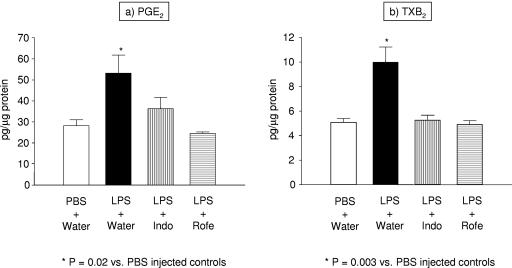
To test whether LPS injection into mice affects prostanoid production in the thymus, we measured the concentrations of PGE2 and of the stable thromboxane metabolite TXB2 in the supernatants of thymic homogenates 24 h after i.p. injection of 50 μg of LPS (n = 3) or vehicle (n = 3). As shown in Fig. 3, a single injection of LPS led to significant increases in prostanoid generation by the thymus. Twenty-four hours after LPS injection, PGE2 levels were increased almost twofold (28.25 ± 2.80 versus 53.18 ± 8.63 pg/μg protein; P = 0.02) (Fig. 3a). As shown in Fig. 3b, TXB2 levels were similarly increased (5.08 ± 0.31 versus 9.98 ± 1.24 pg/μg protein; P = 0.003).
FIG. 3.
LPS injection enhances prostanoid production in the thymus. Wild-type C57BL/6 mice received a single i.p. injection of 50 μg of LPS (n = 3) or a similar volume of PBS control (n = 3), and their thymuses were harvested 24 h later. The levels of PGE2 (a) and TXB2 (b) in thymic homogenates were determined by RIA and were adjusted for the protein concentration in the sample. LPS injection caused a doubling of the basal level of prostanoid production. To determine whether this enhanced prostanoid production could be blocked by COX inhibitors, some animals received indomethacin (n = 3) or rofecoxib (n = 3) in the drinking water starting 24 h before the injection. At the doses used, both drugs were capable of reducing prostanoid production back to baseline levels (P was not significant for the comparisons between LPS-injected mice treated with indomethacin or rofecoxib and the PBS-injected controls). Each bar represents the mean (±SEM) of six measurements (two measurements per mouse).
COX inhibition does not protect against LPS-induced thymocyte apoptosis.
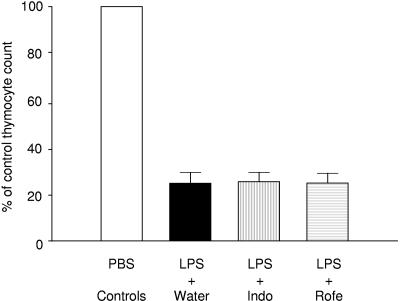
To assess whether enhanced prostanoid generation in the thymus modulates thymocyte apoptosis after LPS injection, we next tested the effect of COX inhibition. In these studies, wild-type C57BL/6 mice received the nonselective COX-1 and COX-2 inhibitor indomethacin (n = 7), the specific COX-2 inhibitor rofecoxib (n = 5), or vehicle control (n = 12) in the drinking water beginning on the day prior to LPS injection. A control group consisting of age-matched wild-type C57BL/6 animals that received indomethacin (n = 3), rofecoxib (n = 3), or vehicle control (n = 5) in the drinking water was injected with a similar volume of PBS. Twenty-four hours later, apoptotic thymocyte deletion was assessed as described above. Compared to the controls that were treated with PBS, the mice treated with LPS demonstrated an ≈75% reduction in total thymocyte count. As shown in Fig. 4, this reduction in thymocyte count induced by LPS was not affected by the administration of indomethacin or rofecoxib.
FIG. 4.
COX inhibition does not protect against LPS-induced thymocyte apoptosis. Thymocyte apoptosis was induced by injecting 50 μg of LPS in the peritoneal cavity of wild-type C57BL/6 mice. In the experimental groups, animals were treated with indomethacin (n = 7), rofecoxib (n = 5), or vehicle control (n = 12) in the drinking water beginning the day prior to LPS injection. Each experimental group had its own control, consisting of animals treated with indomethacin (n = 3), rofecoxib (n = 3), or vehicle control (n = 5) in the drinking water and injected with a similar volume of PBS. Twenty-four hours after injection, apoptotic thymocyte deletion was assessed as described in the text. The data are expressed as the percentage of the control thymocyte count (thymocyte count of experimental group divided by the mean thymocyte count of the respective PBS control and multiplied by 100). Neither indomethacin nor rofecoxib protected against the marked apoptotic thymocyte deletion induced by LPS administration.
To test whether the doses of COX inhibitors used in our experiments were sufficient to inhibit prostanoid generation, we measured the concentrations of PGE2 and TXB2 in the thymic supernatants of mice treated with a COX inhibitor 24 h after injection of LPS. The dose and mode of administration of the COX inhibitors were identical to those used in the apoptosis studies mentioned above. As shown in Fig. 3, the nonselective COX-1 and COX-2 inhibitor indomethacin caused significant reductions of both PGE2 and TXB2 to levels not different from those in the controls treated with PBS (PGE2, 36.30 ± 5.36 versus 28.25 ± 2.80 pg/μg protein; TXB2, 5.27 ± 0.40 versus 5.08 ± 0.31 pg/μg protein; P = not significant for both comparisons between LPS-injected animals treated with indomethacin and PBS-injected controls). The effect of the specific COX-2 inhibitor rofecoxib was virtually identical (PGE2, 24.59 ± 0.61 versus 28.25 ± 2.80 pg/μg protein; TXB2, 4.91 ± 0.32 versus 5.08 ± 0.31 pg/μg protein; P = not significant for both comparisons between LPS-injected animals treated with rofecoxib and PBS-injected controls). The similar levels of inhibition of these two agents suggest that the enhanced prostanoid generation by the thymus following LPS injection is primarily mediated by COX-2.
Thromboxane A2 contributes to LPS-induced thymocyte apoptosis in vivo.
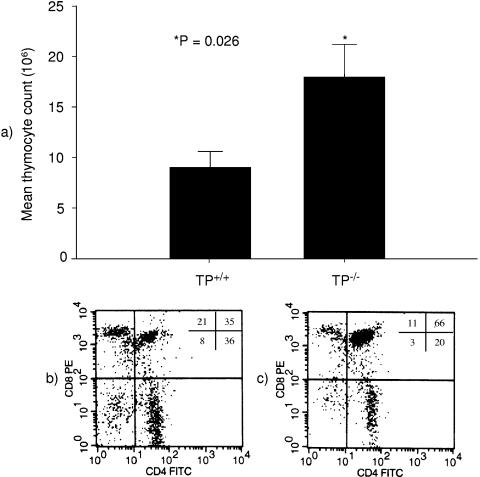
Although our studies with global COX inhibitors did not clearly reveal a role for prostanoids in thymocyte depletion after LPS treatment, the various COX products may have distinct and opposing actions on thymocyte apoptosis. Because TXA2 has been suggested to be a potential trigger for thymocyte apoptosis and TP receptors are highly expressed in the thymus, we assessed the effects of LPS injection in inbred C57BL/6 mice with targeted deletion of TP receptors. By comparison with animals treated with PBS, we found no differences in thymus weight, thymocyte count, or the percentage of CD4+ CD8+ cells between TP+/+ and TP−/− mice. These results are similar to those from our initial characterization of the TP−/− mouse line (37). After LPS injection, systemic manifestations of diarrhea, piloerection, and hypoactivity were similar in TP+/+ and TP−/− animals. However, compared to the TP+/+ animals, the effects of LPS on thymocyte apoptosis in the TP−/− mice were significantly attenuated. For example, LPS reduced the total thymocyte count by only ≈68% in TP−/− mice, from (56 ± 7) × 106 to (18 ± 3) × 106 cells. This reduction was less dramatic than the ≈85% reduction seen in wild-type mice. Twenty-four hours after LPS injection, total thymocyte counts were two times higher in TP−/− mice than in TP+/+ mice (18 ± 3 million versus 9 ± 2 million cells; P = 0.026) (Fig. 5a). Moreover, there was a significant preservation of CD4+ CD8+ cells in the TP-deficient animals. This cell compartment was reduced by only ≈30%, from 88% ± 1% to 62% ± 6% of gated cells in TP−/− mice (Fig. 2c), compared to the 53% reduction observed in the controls (Fig. 2b) (P = 0.02). Together, these data indicate an important role for TXA2, which acts through the TP receptor to promote thymocyte apoptosis following exposure to LPS.
FIG. 5.
Thromboxane A2 contributes to LPS-induced thymocyte apoptosis in vivo. (a) The bar graph shows the total thymocyte count of TP+/+ and TP−/− mice (n = 9 of each genotype) 24 h after a single i.p. injection of 50 μg of LPS. One day after LPS injection, TP−/− animals had twice as many thymocytes as TP+/+ animals (18 ± 3 million versus 9 ± 2 million cells; P = 0.026). The flow cytometry plots below each bar are representative of those from nine experiments and illustrate the thymocyte phenotype of TP+/+ (b) and TP−/− (c) animals 1 day after LPS injection. The numbers inside the plot represent the contribution (%) of each thymocyte population to the total of gated cells: left lower quadrant, CD4− CD8− cells; left upper quadrant, CD4− CD8+ cells; right lower quadrant, CD4+ CD8− cells; right upper quadrant, CD4+ CD8+ cells. Compared to the TP+/+ animals, a significantly higher percentage of CD4+ CD8+ cells remained in the thymuses of TP−/− mice 24 h after LPS injection (62% ± 6% versus 41% ± 5%; P = 0.02). PBS-treated wild-type and knockout animals exhibited similar numbers of thymocytes (62.1 ± 19.3 million versus 55.8 ± 6.6 million cells, respectively; P = not significant) and percentage of CD4+CD8+ cells (88.2 ± 0.6 vs. 87.9 ± 1.1%, respectively; P = not significant).
DISCUSSION
In our experiments, LPS administration to wild-type mice resulted in a dramatic reduction in thymocyte numbers, mostly in the CD4+ CD8+ compartment. This reduction in CD4+ CD8+ thymocytes was likely a result of apoptotic cell death, as previously demonstrated by others (25, 42, 43) and as suggested by our findings of up-regulation of proapoptotic genes of the Fas and mitochondrial pathways in thymocytes from mice exposed to LPS. The main findings of this study are that LPS exposure results in a significant increase in intrathymic levels of TXB2 and that, when thromboxane signaling is absent (as in TP−/− mice), the apoptotic response to LPS is significantly attenuated. Taken together, our data suggest that TXA2 might function as an effector molecule in LPS-induced thymocyte apoptosis.
Thymocytes harvested from mice 3 to 6 h after LPS administration demonstrated a marked up-regulation in the expression of pro- and antiapoptotic genes. Pro- and antiapoptotic family members can heterodimerize and seemingly titrate one another's function, suggesting that their relative concentration may act as a rheostat for the suicide program. Thus, the delicate balance between these competing activities determines cell fate (1). In our study, the antiapoptotic protein BCL-X was strongly upregulated by LPS; this had been previously shown by others (11, 14). However, Fig. 2 shows that proapoptotic Bcl-2 family members, FAS-related genes, and caspases were also up-regulated by LPS. Our data clearly demonstrate that LPS administration induces a marked thymocyte loss, which indicates that proapoptotic stimuli prevail in this setting.
Apoptotic cell death is a prominent feature of sepsis (4, 5, 18, 29, 40). While parenchymal cell apoptosis is thought to be detrimental during sepsis (9, 10), the role of lymphocyte apoptosis is more controversial. Some propose that lymphocyte apoptosis during sepsis might be beneficial by down-regulating the immune response and decreasing the production of pathogenic proinflammatory cytokines (12). However, experimental data by Hotchkiss et al. indicate that lymphocyte apoptosis impairs host responses during sepsis and contributes to mortality (17, 20). Several mediators, such as TNF (42), adrenal steroids (22, 25), the complement split product C5a (15, 31), and nitric oxide (44), have been implicated in sepsis- or LPS-induced thymocyte apoptosis. However, the role of prostanoid inflammatory mediators in this process had not been previously investigated.
Prostanoids are key regulators of thymocyte physiology (34, 35). The enzymes involved in prostanoid biosynthesis are abundantly present in the thymus. COX-1 and COX-2 are constitutively expressed in all cell types; COX-1 is strongly expressed in the cortical cells, whereas COX-2 is highly expressed in the medulla. COX-2 is the inducible isoform and is responsible for the surge in prostanoid production in response to inflammatory stimuli and cytokines. PGE and thromboxane synthases are also present in thymic stromal cells and are responsible for the production of PGE2 and TXA2. In vitro data suggest that these molecules have opposite effects on CD4+ CD8+ thymocytes: PGE2 appears to protect these cells from apoptosis, whereas TXA2 appears to promote apoptosis of these cells (13). In our study, the intrathymic levels of both PGE2 and TXA2 were significantly increased after LPS injection. When only thromboxane signaling was blocked (as was the case in TP−/− mice), thymocyte apoptosis was attenuated. However, global inhibition of prostanoid synthesis with indomethacin or rofecoxib did not confer similar protection. The difference in the outcomes of these experiments reflects distinct consequences of global inhibition of prostanoid synthesis (i.e., COX inhibition) compared to inhibition of a single prostanoid acting at its individual receptor. PGE2 is known to protect against thymocyte apoptosis in vitro (13), and it is therefore likely that concomitant inhibition of PGE2 synthesis (and perhaps other unmeasured protective prostanoids) by indomethacin and rofecoxib negates the beneficial effects of TXA2 inhibition in this setting. In patients with sepsis, treatment with ibuprofen reduces the levels of prostacyclin and thromboxane and decreases fever, tachycardia, oxygen consumption, and lactic acidosis; but it does not prevent the development of shock or the acute respiratory distress syndrome and does not improve survival (7). Perhaps future therapies aimed specifically at blocking TP signaling will produce better results.
Based on previous studies showing the potent actions of TP agonists in the induction of thymocyte apoptosis (41) and our findings of significant enhancement of TXA2 generation by thymocytes after LPS administration, it is very likely that TP receptors promote LPS-induced thymocyte loss through direct effects in the thymus. Nonetheless, our studies cannot completely exclude the possibility that indirect, systemic actions of TP receptors might contribute to this effect. For example, it has been shown that abrogation of TP signaling during sepsis attenuates endotoxin-mediated acute renal failure (8) and intestinal ischemia (23), reduces TNF-α levels (3), and helps preserve pulmonary and circulatory function (21). In rats receiving an endotoxin challenge, administration of the TP receptor antagonist BAY U 3405 led to an increase in the survival rate at 48 h from 0% to 45% (2).
Several strategies to block inflammatory mediators have been attempted in patients with sepsis (32, 33), but only a few have proven effective (6). In animal models, recent interventions aimed at reducing apoptosis have proven effective in improving survival. Our data suggest that by attenuating thymocyte apoptosis in response to LPS, the TP receptor signaling blockade might constitute a potential therapeutic target in sepsis.
Acknowledgments
This work was supported by the VA Research Service and grants 5 P01 DK38108-15 and AI44808 from the National Institutes of Health.
We thank Pat J. Flannery for performing the RIA for the detection of PGE2 and TXB2 in the thymic supernatants.
REFERENCES
- 1.Adams, J. M., and S. Cory. 1998. The Bcl-2 protein family: arbiters of cell survival. Science 281:1322-1326. [DOI] [PubMed] [Google Scholar]
- 2.Altavilla, D., P. Canale, F. Squadrito, A. Sardella, L. Ammendolia, G. Urna, M. Ioculano, G. Squadrito, and A. P. Caputi. 1994. Protective effects of BAY U 3405, a thromboxane A2 receptor antagonist, in endotoxin shock. Pharmacol. Res. 30:137-151. [DOI] [PubMed] [Google Scholar]
- 3.Altavilla, D., F. Squadrito, P. Canale, M. Ioculano, G. Squadrito, G. M. Campo, M. Serrano, A. Sardella, G. Urna, and G. Spignoli. 1995. G 619, a dual thromboxane synthase inhibitor and thromboxane A2 receptor antagonist, inhibits tumor necrosis factor-alpha biosynthesis. Eur. J. Pharmacol. 286:31-39. [DOI] [PubMed] [Google Scholar]
- 4.Bannerman, D. D., and S. E. Goldblum. 2003. Mechanisms of bacterial lipopolysaccharide-induced endothelial apoptosis. Am. J. Physiol. Lung Cell Mol. Physiol. 284:L899-L914. [DOI] [PubMed] [Google Scholar]
- 5.Beranek, J. T. 2002. Cardiomyocyte apoptosis contributes to the pathology of the septic shock heart. Intensive Care Med. 28:218. [DOI] [PubMed] [Google Scholar]
- 6.Bernard, G. R., J. L. Vincent, P. F. Laterre, S. P. LaRosa, J. F. Dhainaut, A. Lopez-Rodriguez, J. S. Steingrub, G. E. Garber, J. D. Helterbrand, E. W. Ely, C. J. Fisher, Jr., and Recombinant Human Protein C Worldwide Evaluation. 2001. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 344:699-709. [DOI] [PubMed] [Google Scholar]
- 7.Bernard, G. R., A. P. Wheeler, J. A. Russell, R. Schein, W. R. Summer, K. P. Steinberg, W. J. Fulkerson, P. E. Wright, B. W. Christman, W. D. Dupont, S. B. Higgins, B. B. Swindell, et al. 1997. The effects of ibuprofen on the physiology and survival of patients with sepsis. N. Engl. J. Med. 336:912-918. [DOI] [PubMed] [Google Scholar]
- 8.Boffa, J. J., A. Just, T. M. Coffman, and W. J. Arendshorst. 2004. Thromboxane receptor mediates renal vasoconstriction and contributes to acute renal failure in endotoxemic mice. J. Am. Soc. Nephrol. 15:2358-2365. [DOI] [PubMed] [Google Scholar]
- 9.Coopersmith, C. M., K. C. Chang, P. E. Swanson, K. W. Tinsley, P. E. Stromberg, T. G. Buchman, I. E. Karl, and R. S. Hotchkiss. 2002. Overexpression of Bcl-2 in the intestinal epithelium improves survival in septic mice. Crit. Care Med. 30:195-201. [DOI] [PubMed] [Google Scholar]
- 10.Coopersmith, C. M., P. E. Stromberg, W. M. Dunne, C. G. Davis, D. M. Amiot, T. G. Buchman, I. E. Karl, and R. S. Hotchkiss. 2002. Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. JAMA 287:1716-1721. [DOI] [PubMed] [Google Scholar]
- 11.Fang, W., J. J. Rivard, D. L. Mueller, and T. W. Behrens. 1994. Cloning and molecular characterization of mouse bcl-x in B and T lymphocytes. J. Immunol. 153:4388-4398. [PubMed] [Google Scholar]
- 12.Finney, S. J., and T. W. Evans. 2002. Induction of apoptosis in sepsis: cell suicide may be beneficial. Crit. Care Med. 30:261-262. [DOI] [PubMed] [Google Scholar]
- 13.Goetzl, E. J., S. An, and L. Zeng. 1995. Specific suppression by prostaglandin E2 of activation-induced apoptosis of human CD4+ CD8+ T lymphoblasts. J. Immunol. 154:1041-1047. [PubMed] [Google Scholar]
- 14.Grillot, D. A., R. Merino, J. C. Pena, W. C. Fanslow, F. D. Finkelman, C. B. Thompson, and G. Nunez. 1996. bcl-x exhibits regulated expression during B cell development and activation and modulates lymphocyte survival in transgenic mice. J. Exp. Med. 183:381-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo, R. F., M. Huber-Lang, X. Wang, V. Sarma, V. A. Padgaonkar, R. A. Craig, N. C. Riedemann, S. D. McClintock, T. Hlaing, M. M. Shi, and P. A. Ward. 2000. Protective effects of anti-C5a in sepsis-induced thymocyte apoptosis. J. Clin. Investig. 106:1271-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiramatsu, M., R. S. Hotchkiss, I. E. Karl, and T. G. Buchman. 1997. Cecal ligation and puncture (CLP) induces apoptosis in thymus, spleen, lung, and gut by an endotoxin and TNF-independent pathway. Shock 7:247-253. [DOI] [PubMed] [Google Scholar]
- 17.Hotchkiss, R. S., K. C. Chang, M. H. Grayson, K. W. Tinsley, B. S. Dunne, C. G. Davis, D. F. Osborne, and I. E. Karl. 2003. Adoptive transfer of apoptotic splenocytes worsens survival, whereas adoptive transfer of necrotic splenocytes improves survival in sepsis. Proc. Natl. Acad. Sci. USA 100:6724-6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotchkiss, R. S., and I. E. Karl. 2003. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 348:138-150. [DOI] [PubMed] [Google Scholar]
- 19.Hotchkiss, R. S., P. E. Swanson, J. P. Cobb, A. Jacobson, T. G. Buchman, and I. E. Karl. 1997. Apoptosis in lymphoid and parenchymal cells during sepsis: findings in normal and T- and B-cell-deficient mice. Crit. Care Med. 25:1298-1307. [DOI] [PubMed] [Google Scholar]
- 20.Hotchkiss, R. S., P. E. Swanson, C. M. Knudson, K. C. Chang, J. P. Cobb, D. F. Osborne, K. M. Zollner, T. G. Buchman, S. J. Korsmeyer, and I. E. Karl. 1999. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J. Immunol. 162:4148-4156. [PubMed] [Google Scholar]
- 21.Iglesias, G., S. T. Zeigler, C. W. Lentz, D. L. Traber, and D. N. Herndon. 1994. Thromboxane synthetase inhibition and thromboxane receptor blockade preserve pulmonary and circulatory function in a porcine burn sepsis model. J. Am. Coll. Surg. 179:187-192. [PubMed] [Google Scholar]
- 22.Kato, Y., A. Morikawa, T. Sugiyama, N. Koide, G. Z. Jiang, K. Takahashi, and T. Yokochi. 1995. Role of tumor necrosis factor-alpha and glucocorticoid on lipopolysaccharide (LPS)-induced apoptosis of thymocytes. FEMS Immunol. Med. Microbiol. 12:195-204. [DOI] [PubMed] [Google Scholar]
- 23.Matejovic, M., P. Radermacher, C. Zulke, A. Vlatten, J. Altherr, A. Brinkmann, U. B. Bruckner, K. W. Jauch, M. Georgieff, and K. Trager. 2000. Effects of the combined thromboxane receptor antagonist and synthase inhibitor DTTX-30 on intestinal O2-exchange and energy metabolism during hyperdynamic porcine endotoxemia. Shock 13:307-313. [DOI] [PubMed] [Google Scholar]
- 24.Namba, T., Y. Sugimoto, M. Hirata, Y. Hayashi, A. Honda, A. Watabe, M. Negishi, A. Ichikawa, and S. Narumiya. 1992. Mouse thromboxane A2 receptor: cDNA cloning, expression and Northern blot analysis. Biochem. Biophys. Res. Commun. 184:1197-1203. [DOI] [PubMed] [Google Scholar]
- 25.Norimatsu, M., T. Ono, A. Aoki, K. Ohishi, and Y. Tamura. 1995. In-vivo induction of apoptosis in murine lymphocytes by bacterial lipopolysaccharides. J. Med. Microbiol. 43:251-257. [DOI] [PubMed] [Google Scholar]
- 26.Nusing, R., R. Lesch, and V. Ullrich. 1990. Immunohistochemical localization of thromboxane synthase in human tissues. Eicosanoids 3:53-58. [PubMed] [Google Scholar]
- 27.Nusing, R., and V. Ullrich. 1992. Regulation of cyclooxygenase and thromboxane synthase in human monocytes. Eur. J. Biochem. 206:131-136. [DOI] [PubMed] [Google Scholar]
- 28.Peebles, R. S., Jr., R. Dworski, R. D. Collins, K. Jarzecka, D. B. Mitchell, B. S. Graham, and J. R. Sheller. 2000. Cyclooxygenase inhibition increases interleukin 5 and interleukin 13 production and airway hyperresponsiveness in allergic mice. Am. J. Respir. Crit. Care Med. 162:676-681. [DOI] [PubMed] [Google Scholar]
- 29.Power, C., N. Fanning, and H. P. Redmond. 2002. Cellular apoptosis and organ injury in sepsis: a review. Shock 18:197-211. [DOI] [PubMed] [Google Scholar]
- 30.Remuzzi, G., M. Noris, A. Benigni, O. Imberti, M. H. Sayegh, and N. Perico. 1994. Thromboxane A2 receptor blocking abrogates donor-specific unresponsiveness to renal allografts induced by thymic recognition of major histocompatibility allopeptides. J. Exp. Med. 180:1967-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riedemann, N. C., R. F. Guo, I. J. Laudes, K. Keller, V. J. Sarma, V. Padgaonkar, F. S. Zetoune, and P. A. Ward. 2002. C5a receptor and thymocyte apoptosis in sepsis. FASEB J. 16:887-888. [DOI] [PubMed] [Google Scholar]
- 32.Riedemann, N. C., R. F. Guo, and P. A. Ward. 2003. Novel strategies for the treatment of sepsis. Nat. Med. 9:517-524. [DOI] [PubMed] [Google Scholar]
- 33.Riedemann, N. C., R. F. Guo, and P. A. Ward. 2003. The enigma of sepsis. J. Clin. Investig. 112:460-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rocca, B., L. M. Spain, G. Ciabattoni, C. Patrono, and G. A. FitzGerald. 1999. Differential expression and regulation of cyclooxygenase isozymes in thymic stromal cells. J. Immunol. 162:4589-4597. [PubMed] [Google Scholar]
- 35.Rocca, B., L. M. Spain, E. Pure, R. Langenbach, C. Patrono, and G. A. FitzGerald. 1999. Distinct roles of prostaglandin H synthases 1 and 2 in T-cell development. J. Clin. Investig. 103:1469-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spurney, R. F., P. Y. Fan, P. Ruiz, F. Sanfilippo, D. S. Pisetsky, and T. M. Coffman. 1992. Thromboxane receptor blockade reduces renal injury in murine lupus nephritis. Kidney Int. 41:973-982. [DOI] [PubMed] [Google Scholar]
- 37.Thomas, D. W., R. B. Mannon, P. J. Mannon, A. Latour, J. A. Oliver, M. Hoffman, O. Smithies, B. H. Koller, and T. M. Coffman. 1998. Coagulation defects and altered hemodynamic responses in mice lacking receptors for thromboxane A2. J. Clin. Investig. 102:1994-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas, D. W., P. N. Rocha, C. Nataraj, L. A. Robinson, R. F. Spurney, B. H. Koller, and T. M. Coffman. 2003. Proinflammatory actions of thromboxane receptors to enhance cellular immune responses. J. Immunol. 171:6389-6395. [DOI] [PubMed] [Google Scholar]
- 39.Tilley, S. L., T. M. Coffman, and B. H. Koller. 2001. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J. Clin. Investig. 108:15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tinsley, K. W., M. H. Grayson, P. E. Swanson, A. M. Drewry, K. C. Chang, I. E. Karl, and R. S. Hotchkiss. 2003. Sepsis induces apoptosis and profound depletion of splenic interdigitating and follicular dendritic cells. J. Immunol. 171:909-914. [DOI] [PubMed] [Google Scholar]
- 41.Ushikubi, F., Y. Aiba, K. Nakamura, T. Namba, M. Hirata, O. Mazda, Y. Katsura, and S. Narumiya. 1993. Thromboxane A2 receptor is highly expressed in mouse immature thymocytes and mediates DNA fragmentation and apoptosis. J. Exp. Med. 178:1825-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, S. D., K. J. Huang, Y. S. Lin, and H. Y. Lei. 1994. Sepsis-induced apoptosis of the thymocytes in mice. J. Immunol. 152:5014-5021. [PubMed] [Google Scholar]
- 43.Zhang, Y. H., K. Takahashi, G. Z. Jiang, M. Kawai, M. Fukada, and T. Yokochi. 1993. In vivo induction of apoptosis (programmed cell death) in mouse thymus by administration of lipopolysaccharide. Infect. Immun. 61:5044-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou, X., S. A. Gordon, Y. M. Kim, R. A. Hoffman, Y. Chen, X. R. Zhang, R. L. Simmons, and H. R. Ford. 2000. Nitric oxide induces thymocyte apoptosis via a caspase-1-dependent mechanism. J. Immunol. 165:1252-1258. [DOI] [PubMed] [Google Scholar]