Abstract
There is currently no standardized serum bactericidal antibody (SBA) assay for evaluating immune responses to meningococcal outer membrane vesicle or protein vaccines. Four laboratories, Manchester Health Protection Agency (MC HPA), New Zealand Institute of Environmental Science and Research Limited (NZ ESR), Norwegian Institute of Public Health (NIPH), and Chiron Vaccines (Chiron), measured SBA titers in the same panel of human sera (n = 76) from laboratory staff (n = 21) vaccinated with MenBvac. Blood samples were collected prevaccination, prior to each of the three doses of MenBvac given at 6-week intervals, and 6 weeks following the third dose. Initial results showed a number of discrepancies in results between the four participating laboratories. The greatest effect on titers appeared to be due to differences among laboratories in the maintenance of the meningococcal serogroup B test strain, 44/76-SL. A repeat study was conducted using the same frozen isolate (meningococcal serogroup B test strain 44/76-SL), freshly distributed to all four laboratories. Using SBA titers from the tilt method for all samples, and using MC HPA as the comparator, the results were as follows for NZ ESR, NIPH, and Chiron, respectively, using log10 titers: correlation coefficients (r) were 0.966, 0.967, and 0.936; intercepts were 0.08, 0.15, and 0.17; and slopes were 0.930, 0.851, and 0.891. In both prevaccination and postvaccination samples from 15 subjects assayed by all four laboratories, similar increases in SBA (fourfold or greater) were observed (for 11, 11, 9, and 9 subjects for MC HPA, NZ ESR, NIPH, and Chiron, respectively), and similar percentages of subjects with SBA titers of ≥4 prevaccination and 6 weeks following each dose were found. The SBA assay has been harmonized between the four different laboratories with good agreement on seroconversion rates, n-fold changes in titers, and percentages of subjects with SBA titers of ≥4.
There is currently no accepted correlate of protection for human meningococcal serogroup B (MenB) disease. The importance of serum bactericidal antibody (SBA) in protection against meningococcal disease has been illustrated by the inverse relationship between SBA level and incidence of disease (6, 17). The accepted correlate of short-term protection for serogroup C disease is SBA (1, 6). SBA is currently the primary measure for the determination of immunogenicity for vaccines containing serogroups A or C or W135 or Y polysaccharides in tandem with the measurement of specific anticapsular antibodies. The poor immunogenicity of the MenB capsule has resulted in the development of outer membrane protein- or outer membrane vesicle (OMV)-based vaccines that have been tested extensively in clinical trials (2, 4, 11, 15, 16). The determination of SBA level using exogenous human complement has been used primarily to assess the responses to these vaccines, and SBA titers have been shown to correlate with the efficacies of OMV vaccines in Norwegian and Cuban teenagers (8, 15). Similar strong correlations between the efficacy of a B:15:P1.3 outer membrane protein vaccine in Chile and the percentages of subjects with fourfold or greater increases in SBA titers from prevaccination to post-second dose have been reported (3).
The lack of a standardized MenB SBA assay restricts the comparison of data produced by different laboratories and prevents the direct assessment of the immunogenicity of MenB vaccines. A multilaboratory comparison was successfully performed for serogroup A and C SBA assays (10), and a similar approach is required for MenB. A collaboration involving four laboratories, Health Protection Agency (Manchester, United Kingdom) (MC HPA), Institute for Environmental Science and Research Ltd. (Porirua, New Zealand) (NZ ESR), Norwegian Institute of Public Health (Oslo, Norway) (NIPH), and Chiron Vaccines (Emeryville, Calif.) (Chiron), was set up to compare the SBA titers generated when the same target strain and same complement source were used to test the same serum samples (but using “local” protocols). The sera were obtained from subjects immunized with a three-dose course of MenBvac produced by NIPH. The aim of the study was to standardize the SBA assay prior to trials of a “tailor-made” OMV vaccine for use in New Zealand, where a MenB epidemic caused by meningococci defined as B:4:P1.7-2,4 has been occurring since 1991 (9).
MATERIALS AND METHODS
Initial SBA assays.
NIPH supplied strain 44/76-SL (B:15:P1.7,16) (14) by swabs sent on modified Stuart transport medium to all study sites. At MC HPA, 44/76-SL was streaked for isolation from a mother culture which had originally been processed by single-colony selection and incubated overnight at 37°C with 5% CO2 on a Columbia blood agar plate with 5% horse blood (CBA) (Oxoid, Basingstoke, United Kingdom). The next morning, approximately 10 colonies were subcultured onto another CBA plate and incubated for 4 h at 37°C with 5% CO2. After 4 h, bacteria were suspended in bactericidal buffer (Hanks balanced salt solution; Gibco, Paisley, United Kingdom) containing 0.5% bovine serum albumin (BSA) (Sigma, Poole, United Kingdom) and 0.5 U/ml heparin (CP Pharmaceuticals, Wrexham, United Kingdom) and adjusted to 8 × 104 organisms/ml. Equal volumes (10 μl) of the bacterial suspension and human complement were added to 20 μl heat-inactivated test serum serially diluted twofold in bactericidal buffer in 96-well U-bottom microtiter plates (Greiner, Frickenhausen, Germany). The complement source was plasma provided by NIPH from one suitable donor with a ≤15% reduction in CFU which was used at a 25% final concentration after 60 min of incubation. The reaction mixture was mixed by gentle tapping, and the number of CFU at time zero was determined by allowing 10 μl of the reaction mixture (in the control column) to flow 8 to 10 cm, in lanes, down a CBA plate (the tilt method). Following incubation of the reaction mixture at 37°C for 60 min, 10 μl was removed from each well and plated on CBA using the tilt method to determine the number of CFU per well after 60 min of incubation. Colonies were counted after overnight incubation at 37°C with 5% CO2. SBA titers were expressed as the reciprocal of the final serum dilution step giving ≥50% killing at 60 min compared to the number of CFU at time zero. The assay was controlled by the use of an in-house serum control, and the target strain was controlled by the use of monoclonal antibodies raised against serogroup B polysaccharide (NIBSC code 95/750; National Institute for Biological Standards and Control, Potters Bar, South Mimms, Hertfordshire, United Kingdom) and serosubtypes P1.7 (NIBSC code 01/514) and P1.16 (NIBSC code 01/538).
All four laboratories used the same complement source provided by NIPH. The protocols of each laboratory differed from the protocol of MC HPA. NZ ESR cultured bacteria on CBA with 5% sheep blood, bactericidal buffer with 1% BSA, and occasional light tapping of the reaction mixture to ensure thorough mixing. NIPH used bactericidal buffer with 0.1% BSA in Hanks balanced salt solution, mixed the heparin with the complement rather than the bactericidal buffer, and mixed the reaction mixture by an occasional light tapping. For the initial study, NIPH added 120 μl of agar, equilibrated to 48°C (and liquefied by heating to 100°C), to each well of the microtiter plates (agar overlay method) to determine the number of CFU after 60 min of incubation. The original strain shipment received by Chiron contained a contaminant, and a replacement strain was sent as a frozen culture. (This frozen culture was subsequently resupplied by NIPH to the remaining labs for use in the second study.) To create its mother culture, Chiron used a sweep of an overnight growth on chocolate agar (36°C, 5% CO2; Microbiological Media, Concord, CA), which was then frozen in 50% BSA at −80°C. For use in the assay, a tube was thawed and 10 μl was added to chocolate agar, spread across the plate, and cultured overnight. After 18 h, 10 to 15 colonies were combined and subcultured for 1.5 h in Mueller-Hinton broth (Becton-Dickinson, Sparks, MD) plus 0.25% d-glucose (Sigma, St. Louis, MO) with 5% CO2 and gentle mixing. For the determination of surviving CFU, the reaction mixture was cultured in Gey's buffer with balanced salts and 1% BSA with gentle mixing in 5% CO2; surviving bacteria were then plated and grown overnight on Mueller-Hinton agar with 5% CO2.
For the initial study, MC HPA, Chiron, and NZ ESR ran each sample three times and NIPH ran each sample twice.
Repeat interlaboratory study.
Modifications to laboratory testing procedures were included in the protocols for the repeat study. Strain 44/76-SL, as a frozen culture, was redistributed to all laboratories with instructions for the production of the mother culture and working stocks. This included instructions not to pick a single colony on receipt at the receiving laboratory but to take a sweep of colonies for preparation of the mother culture. Also, the number of colonies picked for subculture for the working suspension was increased from 10 to 50 colonies.
Other changes implemented included the following. MC HPA implemented gentle rocking (for mixing) at 65 rpm in a Unitron model AJ2150 shaking incubator (Infors, Surrey, United Kingdom) during the reaction, and a control serum (NIBSC code 02/234) was used in the assay. NIPH introduced the tilt method described above for the MC HPA protocol. The two runs that NIPH performed in the repeat study differed slightly in the assay procedure; the first run used sera that were not heat inactivated, while the second run was performed with heat-inactivated sera. The results of the two runs were combined as r = 0.89, slope = 0.833, and intercept = −0.05 when the log10 titer of the second run was regressed on the log10 titer of the first run. Additionally, complement is known to be added in excess to the reaction mixture, even when the test sera are heat inactivated. NZ ESR and MC HPA retested the study sera only once more, using serum complement bridged to the plasma supplied by NIPH, which introduced an added variable to the testing procedure. Chiron did not implement changes, and its original data set was used for the repeat analysis.
Statistical analysis.
MC HPA, NZ ESR, and NIPH used a starting dilution in the SBA assay of 1:2, while Chiron commenced with a dilution of 1:4. For the analyses presented here, samples with recorded titers below 4 were set to a titer of 2 for statistical analyses. For all analyses, within-subject-visit geometric means were used as the titers for NIPH (two assay runs) and Chiron (three assay runs), and single measurement determinations were used as the titers for HPA and NZ ESR. Thus, for the repeat study, the variances associated with the subject-blood draw data are theoretically different among the four laboratories. As a result, the differences in the number of replicate measurements between labs have an effect on the precision of the reported titers and thus on the comparison of results between labs. All data manipulation and statistical analyses were conducted using SAS for Windows version 9.1.3.
Pearson's chi-square test was used to determine whether the proportions of fourfold increases in SBA between the different laboratories were significantly different. This chi-square test does not account for the “paired” nature of the data (in that each of the four laboratories was testing the same subject samples). To account for the “paired” nature of the data, an analysis of variance (ANOVA) model was used to test whether the mean n-folds differed significantly by laboratory after adjusting for differences among subjects.
Geometric mean titers (GMTs) and concentrations with 95% confidence intervals (CIs) were calculated per blood draw for each laboratory. Sample means and 95% CIs were calculated using the log10 titer data. These statistics were transformed to the titer scale as GMTs and 95% CIs. Separately, for each blood draw (prevaccination, post-first dose, post-second dose, and post-third dose), an ANOVA model was used to test differences in the means of the log10 titers after adjusting for differences in means among subjects, model that blocked for subject. When the test showed significant differences between the means of the log10 titers, Dunnett's posttest procedure was conducted for pair-wise comparisons between the results from MC HPA and those from each of the other three laboratories (three pair-wise comparisons). The statistics from these comparisons were transformed to the titer scale.
Regression statistics, including the Pearson correlation coefficient (r), were calculated by using log10-transformed titer data. Least-squares regression was used to estimate the slope, intercept, and correlation coefficient separately for each of the NZ ESR, NIPH, and Chiron log10 titers regressed on MC HPA log10 titers.
RESULTS
Initial interlaboratory study.
A total of 78 sera were assayed by each laboratory, comprising prevaccination (n = 21), post-first dose (n = 21), post-second dose (n = 20), and post-third dose (n = 16) sera. Prevaccination and post-third-dose vaccination data were available for all laboratories for 15 of the 21 subjects. Table 1 shows that the percentages of subjects with SBA titers of ≥4 were discrepant between laboratories in the initial study across all four blood draws (with the exception of post-first and post-third dose data between NZ ESR and NIPH).
TABLE 1.
Percentages of subjects from initial study with SBA titers of ≥4 by time of blood draw and laboratory
| Time of blood draw | % of subjects (no. positive/total no. tested) with SBA titers of ≥4
|
|||
|---|---|---|---|---|
| MC HPA | NZ ESR | NIPH | Chiron | |
| Prevaccination | 10 (2/21) | 62 (13/21) | 90 (19/21) | 29 (6/21) |
| Post-first dose | 38 (8/21) | 95 (20/21) | 95 (20/21) | 81 (17/21) |
| Post-second dose | 45 (9/20) | 95 (19/20) | 90 (18/20) | 80 (16/20) |
| Post-third dose | 42 (8/19) | 100 (17/17) | 100 (17/17) | 88 (14/16) |
Initial bridging investigations highlighted differences between laboratory protocols. Differences in culture media, bactericidal buffers used, and microtiter plate brands used had no effect on the assays (data not shown). Differences in the handling and maintenance of strain 44/76-SL were shown to be of importance. On average, fourfold-higher titers for the same sera were measured at NZ ESR when the original target strain rather than the replacement target strain was used. In contrast, 2.2-fold-lower titers were observed at MC HPA when the original strain, tested with stationary incubation during the reaction, was compared to the replacement strain with mixing. At MC HPA, using a subset of samples, the effect on titers of rocking the reaction mixture was tested, and the results are shown in Table 2. For 23 of 32 samples of the sample subset, an increase of 1 or more titer steps was measured when the complement reaction mixture was mixed rather than subjected to a stationary incubation. Differences in titers were also observed when the target strain was processed using a single colony selection rather than maintained as a mixed population (Table 2). Maintenance of a mixed population resulted in an increase of 1 or more titer steps for 33 of 49 of the samples assayed.
TABLE 2.
SBA titer step differences by reaction mixture motion and population type
| Comparators | No. of samples with a titer step difference of:
|
||||
|---|---|---|---|---|---|
| −1 | 0 | 1 | 2 | 3 | |
| Stationary vs raking reaction mixturea | 1 | 8 | 18 | 4 | 1 |
| Single-colony vs mixed populationb | 2 | 14 | 21 | 11 | 1 |
The difference in results with stationary reaction mixtures and those subjected to rocking at 65 rpm was determined by subtracting stationary reaction results from rocking reaction results. The average increase in titer step using rocking vs stationary reaction mixture was 0.88 titer step after deleting results for samples with negative titers for both reaction types.
The difference in results with single-colony and mixed-population samples was determined by subtracting single-colony results from mixed-population results. The average increase in titer step using mixed population instead of single colony was 0.90 titer step after deleting results for samples with negative titers for both population types.
With careful bridging, use of plasma as opposed to serum human complement was determined to have no effect on the resulting titers (data not shown).
Following these investigations, rocking of the reaction mixture was incorporated into the MC HPA protocol, and harmonization of the assay was completed by the resending of the target strain to all laboratories with instructions on maintenance of a mixed population. The subject samples were reassayed using the replacement target strain.
Repeat interlaboratory study.
With the implementation of methodological changes, the sera were reassayed, and the data across all four labs were found to be more consistent. The percentages of subjects with SBA titers of ≥4 were found to be similar across all laboratories for all blood draws per laboratory (Table 3).
TABLE 3.
Percentages of subjects from repeat study with SBA titers of ≥4 by time of blood draw and laboratory
| Time of blood draw | % of subjects (no. positive/total no. tested) with SBA titers of ≥4
|
|||
|---|---|---|---|---|
| MC HPA (1 run) | NZ ESR (1 run) | NIPH (2 runs) | Chiron (3 runs) | |
| Prevaccination | 37 (7/19) | 37 (7/19) | 37 (7/19) | 32 (6/19) |
| Post-first dose | 76 (16/21) | 81 (17/21) | 81 (17/21) | 81 (17/21) |
| Post-second dose | 85 (17/20) | 85 (17/20) | 80 (16/20) | 80 (16/20) |
| Post-third dose | 88 (14/16) | 88 (14/16) | 94 (15/16) | 88 (14/16) |
Among the 15 subjects for whom samples from both prevaccination and post-third-dose vaccination were available and assayed at each laboratory, fourfold increases in SBA titers occurred for 11/15, 11/15, 9/15, and 9/15 subjects for MC HPA, NZ ESR, NIPH, and Chiron, respectively (not a significant difference between laboratories; P = 0.753 for chi-square test). An additional ANOVA, tested in a model that accounted for subject, showed that the average increase in titer (within a subject) from prevaccination to post-third-dose vaccination did vary from laboratory to laboratory (P = 0.0213).
SBA GMTs with 95% CIs are shown in Table 4. SBA GMTs between MC HPA and NZ ESR, NIPH, and Chiron each were compared separately for prevaccination, post-first dose, post-second dose, and post-third dose samples in an ANOVA model. For post-third dose samples, Chiron's GMTs were higher (at a 5% level of significance) than the MC HPA GMTs. No other significant differences were found (Table 5).
TABLE 4.
SBA GMTs with 95% CIs per time of blood draw for each laboratory
| Time of blood draw | No. of serum samples | SBA GMT (95% CI)
|
|||
|---|---|---|---|---|---|
| MC HPA (1 run) | NZ ESR (1 run) | NIPH (2 runs) | Chiron (3 runs) | ||
| Prevaccination | 19 | 3.3 (2.0-5.5) | 3.7 (2.2-6.4) | 3.5 (2.2-5.5) | 3.4 (2.2-5.2) |
| Post-first dose | 21 | 8.5 (4.5-16.2) | 9.1 (5.0-16.6) | 8.8 (5.2-15.0) | 10.3 (6.0-17.7) |
| Post-second dose | 20 | 8.6 (5.0-14.8) | 8.9 (5.2-15.1) | 8.7 (5.4-14.1) | 9.6 (5.7-16.4) |
| Post-third dose | 16 | 13.5 (6.2-29.0) | 12.9 (6.3-26.5) | 15.0 (7.9-28.4) | 20.2 (10.2-39.9) |
TABLE 5.
ANOVA results from repeat study comparing mean log10 titers between laboratories within visits
| Visit (overall P value for lab effect on GMT) | Labs compared | Estimated difference in log10 titer scalea | 95% CI for difference in mean log10 titera |
|---|---|---|---|
| Prevaccination (0.0246) | Chiron vs MC HPA | 0.005 (1.01) | −0.087, 0.097 (0.82, 1.25) |
| NZ ESR vs MC HPA | 0.048 (1.12) | −0.045, 0.140 (0.90, 1.38) | |
| NIPH vs MC HPA | 0.024 (1.06) | −0.068, 0.116 (0.86, 1.31) | |
| Post-first dose (0.0080) | Chiron vs MC HPA | 0.081 (1.21) | −0.004, 0.167 (0.99, 1.47) |
| NZ ESR vs MC HPA | 0.029 (1.07) | −0.057, 0.114 (0.88, 1.30) | |
| NIPH vs MC HPA | 0.014 (1.03) | −0.071, 0.100 (0.85, 1.26) | |
| Post-second dose (0.1417) | Chiron vs MC HPA | 0.050 (1.12) | −0.040, 0.141 (0.91, 1.38) |
| NZ ESR vs MC HPA | 0.015 (1.04) | −0.076, 0.106 (0.84, 1.28) | |
| NIPH vs MC HPA | 0.008 (1.02) | −0.083, 0.098 (0.83, 1.25) | |
| Post-third dose (0.0025) | Chiron vs MC HPA | 0.176 (1.50) | 0.036, 0.315* (1.09, 2.07) |
| NZ ESR vs MC HPA | −0.019 (0.96) | −0.158, 0.120 (0.70, 1.32) | |
| NIPH vs MC HPA | 0.047 (1.11) | −0.092, 0.186 (0.81, 1.53) |
Titer scale values calculated from nonrounded log10 titer values. Values in parentheses show the 95% confidence interval transformed to the titer scale. *, significance at 5% level.
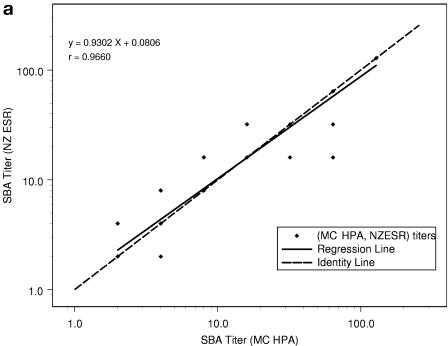
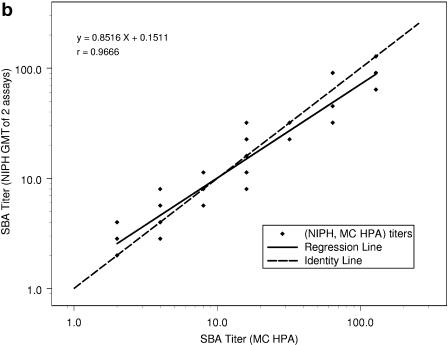
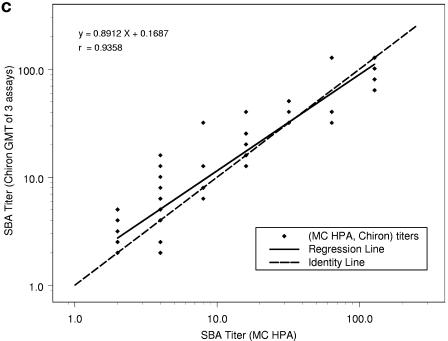
When the SBA log10 titer results from the repeat study were regressed on the MC HPA log10 titers, the results were as follows for NZ ESR, NIPH, and Chiron, respectively: correlations (r) were determined to be 0.966, 0.967, and 0.936; intercepts were 0.08, 0.15, and 0.17; and slopes (with 95% CIs) were 0.93 (0.87 to 0.99), 0.85 (0.80 to 0.97), and 0.89 (0.81 to 0.97). In Fig. 1, the MC HPA titers are represented on the horizontal axis in each plot and are from one assay for each subject-visit. Note that there are 76 data points for each of the three plots but that the number of apparent data points varies between plots because of differences in the number of replicate measurements. The NZ ESR titers are from one run for each subject-visit, the NIPH titers are the means of two replicates for each subject-visit, and the Chiron titers are the GMTs of three replicates for each subject-visit. Thus, NZ ESR titers take on dilution step values of 2, 4, 8, 16, 32, etc., resulting in a high percentage of overlapping points. When the calculated means of multiple runs are used, i.e., two multiple runs for NIPH and three for Chiron, titers can take on these same values plus intermediate dilution step values, creating the appearance of more data points.
FIG. 1.
Comparison of SBA titers from repeat study (n = 76) between NZ ESR and MC HPA (a), NIPH and MC HPA (b), and Chiron and MC HPA (c), with regression of y equivalent to log10(titer from first laboratory in the pair) on x equivalent to log10(titer from second laboratory in the pair).
The SBA titer step difference is shown in Table 6. When MC HPA is used as the comparator, it can be seen that NIPH had no samples with a titer step difference outside of ±2. When results from MC HPA were compared to those from Chiron, two samples (3%) showed a titer step difference of +2 or greater (with Chiron's results higher for both of these samples). When results from MC HPA were compared to those from NZ ESR, one sample (1%) showed a titer step difference of ≥2, with the MC HPA titer higher than the NZ ESR titer. Compared to results from MC HPA, 79%, 83%, and 75% of the titers showed step differences of less than ±1 with NZ ESR, NIPH, and Chiron, respectively.
TABLE 6.
Repeat study interlaboratory titer step differences for SBA titers, compared to results from MC HPA (1 run)
| Laboratory (no. of runs) | No. of samples with serum bactericidal titer step difference ofa:
|
||||||
|---|---|---|---|---|---|---|---|
| −2.99 to −2.00 | −1.99 to −1.00 | −0.99 to −0.01 | 0 | 0.01 to 0.99 | 1.00 to 1.99 | 2.00 to 2.99 | |
| NZ ESR (1) | 1 | 4 | 0 | 60 | 0 | 11 | 0 |
| NIPH (2) | 0 | 6 | 11 | 32 | 20 | 7 | 0 |
| Chiron (3) | 0 | 5 | 10 | 28 | 19 | 12 | 2 |
Compared to MC HPA results.
DISCUSSION
Standardization of the serogroup B SBA assay is of importance to allow interlaboratory comparisons of functional immune responses to vaccines designed to protect against meningococcal serogroup B disease. To date, SBAs have proved to be a useful surrogate of protection, and there is evidence that protection against serogroup B disease correlates with the presence of SBAs against noncapsular antigens (5). The proportion of vaccinees with at least fourfold increases in SBA titers has been used as a correlate with clinical efficacy in adults and older children (3, 15). Standardized SBA assays for both serogroups A and C have been previously described (10), but this is the first published report on the standardization of the SBA assay for serogroup B. Perkins et al. (12) compared the immunogenicities of two OMV vaccines among young adults in Iceland, with all sera being tested in three laboratories: the Centers for Disease Control and Prevention (Atlanta, GA), NIPH (Norway), and Finlay Institute (Cuba). However, comparative SBA data generated by the different laboratories were not published.
In the present study, we evaluated and optimized assay parameters for interlaboratory reproducibility. The major factor that explained discrepant results between laboratories was associated with the handling of the target strain. Following the initial distribution of the strain from NIPH, different laboratories processed the strain in different ways. It was found that taking a sweep of colonies, as opposed to picking a single colony, resulted in marked differences in measured SBA titers between labs. Maintaining a mixed population of meningococci in the SBA assay more closely mimics the natural situation than does using a highly selected population. We recommend using similar sweeps of viable colonies when preparing frozen cultures for long-term storage of strains. When strain 44/76-SL was originally prepared for the SBA assay (7), about 10 to 13% of the CFU expressed the Opc++ phenotype on colony blots. Opc-positive and Opc-negative isolates can be picked from this 44/76-SL inoculum, and these have been proven to be stable (14). SBA activity has been demonstrated against meningococci that express large amounts of Opc but not against meningococci expressing smaller amounts (13); thus, the maintenance of an inoculum with a consistent proportion of CFU strongly expressing Opc is of paramount importance.
The introduction of the gentle-rocking step in the MC HPA SBA assay during the incubation of the reaction mixture was shown to increase the SBA titer compared to that of a stationary incubation. This step was already incorporated by Chiron. NZ ESR and NIPH both rely on thorough mixing of reagents prior to incubation, followed by occasional tapping of 96-well plates containing the reaction mixture during the incubation period. This step is necessary to keep the reaction mixture thoroughly mixed.
Although the results obtained in the four laboratories were comparable, an uncontrolled variable in the repeat study was the impact of differences in the number of replicate measurements, which varied between the four laboratories. Parametric statistical methods often depend on the assumption that populations being compared all have the same variance. A population of data collected as a mean of three observations is expected to have a smaller variance than a population of single point determinations. Additional replicates of each sample would be necessary when comparing the MC HPA and NZ ESR data sets with the NIPH and Chiron data sets to correct for different variances in the data sets from the four different laboratories in the repeat study and to improve the precision of the interlaboratory comparison.
To summarize, we evaluated various parameters, including medium, bactericidal buffer, microtiter plate brand, unknown serum starting dilution, mixing of the complement reaction, and handling of strain 44/76-SL, that may affect the measurement of SBA in human sera. The standardized SBA serogroup B assay has important differences from the previously standardized serogroup A and C assays (10) due to the use of human complement and maintenance of mixed populations of target strains with comparable, noncapsular antigen expression. The standardized SBA assay gave good agreement between the four laboratories on the percentages of subjects with SBA titers of ≥4, the GMTs across all time points tested, and the proportions of preimmune and postimmunization titers showing fourfold or greater changes.
Acknowledgments
The work performed by Meningococcal Reference Unit, HPA North West Laboratory, was supported by United Kingdom Meningococcal Research Foundation grant number 16/00 entitled “Investigation of immunological effect of candidate meningococcal B vaccines in adolescents.”
We thank Joanne Southern and Elizabeth Miller (Immunization Department, Centre for Infections, Health Protection Agency, United Kingdom) for organization of study MNB1, from which the serum samples in this study were collected. We also thank Lynne Joslin for actual blood collections.
REFERENCES
- 1.Andrews, N., R. Borrow, and E. Miller. 2003. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin. Diagn. Lab. Immunol. 10:780-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjune, G., E. A. Høiby, J. K. Grønnesby, O. Arnesen, J. H. Fredriksen, A. Halstensen, E. Holten, A. K. Lindbak, H. Nøkleby, E. Rosenqvist, L. K. Solberg, O. Closs, J. Eng, L. O. Frøholm, A. Lystad, L. S. Bakketeig, and B. Hareide. 1991. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 338:1093-1096. [DOI] [PubMed] [Google Scholar]
- 3.Boslego, J., J. Garcia, C. Cruz, W. Zollinger, B. Brandt, S. Ruiz, M. Martinez, J. Arthur, P. Underwood, W. Silva, E. Moran, W. Hankins, J. Gilly, J. Mays, and the Chilean National Committee for Meningococcal Disease. 1995. Efficacy, safety, and immunogenicity of a meningococcal group B (15:P1.3) outer membrane protein vaccine in Iquique, Chile. Vaccine 13:821-829. [DOI] [PubMed] [Google Scholar]
- 4.Cartwright, K., R. Morris, H. Rümke, A. Fox, R. Borrow, N. Begg, P. Richmond, and J. Poolman. 1999. Immunogenicity and reactogenicity in UK infants of a novel meningococcal vesicle vaccine containing multiple class 1 (PorA) outer membrane proteins. Vaccine 17:2612-2619. [DOI] [PubMed] [Google Scholar]
- 5.Gold, R., I. Goldschneider, M. L. Lepow, T. F. Draper, and M. Randolph. 1978. Carriage of Neisseria meningitidis and Neisseria lactamica in infants and children. J. Infect. Dis. 137:112-121. [DOI] [PubMed] [Google Scholar]
- 6.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Høiby, E. A., E. Rosenqvist, L. O. Frøholm, G. Bjune, B. Feiring, H. Nøkleby, and E. Ronnild. 1991. Bactericidal antibodies after vaccination with the Norwegian meningococcal serogroup B outer membrane vesicle vaccine: a brief survey. NIPH Ann. 14:147-156. [PubMed] [Google Scholar]
- 8.Holst, J., B. Feiring, J. E. Fuglesang, E. A. Høiby, H. Nøkleby, I. S. Aaberge, and E. Rosenqvist. 2003. Serum bactericidal activity correlates with the vaccine efficacy of outer membrane vesicle vaccines against Neisseria meningitidis serogroup B disease. Vaccine 21:734-737. [DOI] [PubMed] [Google Scholar]
- 9.Martin, D. R., S. J. Walker, M. G. Baker, and D. R. Lennon. 1998. New Zealand epidemic of meningococcal disease identified by a strain with phenotype B:4:P1.4. J. Infect. Dis. 177:497-500. [DOI] [PubMed] [Google Scholar]
- 10.Maslanka, S. E., L. L. Gheesling, D. E. Libutti, K. B. J. Donaldson, H. S. Harakeh, J. K. Dykes, F. F. Arhin, S. J. N. Devi, C. E. Frasch, J. C. Huang, P. Kriz-Kuzemenska, R. D. Lemmon, M. Lorange, C. C. A. M. Peeters, S. Quataert, J. Y. Tai, G. M. Carlone, and the Multilaboratory Study Group. 1997. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin. Diagn. Lab. Immunol. 4:156-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milagres, L. G., S. R. Ramos, C. T. Sacchi, C. E. A. Melles, V. S. D. Vieira, H. Sato, G. S. Brito, J. C. Moraes, and C. E. Frasch. 1994. Immune response of Brazilian children to a Neisseria meningitidis serogroup B outer membrane protein vaccine: comparison with efficacy. Infect. Immun. 62:4419-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perkins, B. A., K. Jonsdottir, H. Briem, E. Griffiths, B. D. Plikaytis, E. A Høiby, E. Rosenqvist, J. Holst, H. Nøkleby, F. Sotolongo, G. Sierra, H. C. Campa, G. M. Carlone, D. Williams, J. Dykes, D. Kapczynski, E. Tikhomirov, J. D. Wenger, and C. V. Broome. 1998. Immunogenicity of two efficacious outer membrane protein-based serogroup B meningococcal vaccines among young adults in Iceland. J. Infect. Dis. 177:683-691. [DOI] [PubMed] [Google Scholar]
- 13.Rosenqvist, E., E. A. Høiby, E. Wedege, B. Kusecek, and M. Achtman. 1993. The 5C protein of Neisseria meningitidis is highly immunogenic in humans and induces bactericidal antibodies. J. Infect. Dis. 167:1065-1073. [DOI] [PubMed] [Google Scholar]
- 14.Rosenqvist, E., E. A. Høiby, E. Wedege, K. Bryn, J. Kolberg, A. Klem, E. Rønnild, G. Bjune, and H. Nøkleby. 1995. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect. Immun. 63:4642-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sierra, G. V., H. C. Campa, N. M. Varcacel, I. L. Garcia, P. L. Izquierdo, P. F. Sotolongo, G. V. Casanueva, C. O. Rico, C. R. Rodriguez, and M. H. Terry. 1991. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 14:195-207. [PubMed] [Google Scholar]
- 16.Tappero, J. W., R. Lagos, A. M. Ballesteros, B. Plikaytis, D. Williams, J. Dykes, L. L. Gheesling, G. M. Carlone, E. A. Høiby, J. Holst, H. Nøkleby, E. Rosenqvist, G. Sierra, C. Campa, F. Sotolongo, J. Vega, J. Garcia, P. Herrera, J. T. Poolman, and B. A. Perkins. 1999. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA 281:1520-1527. [DOI] [PubMed] [Google Scholar]
- 17.Trotter, C., R. Borrow, N. Andrews, and E. Miller. 2003. Seroprevalence of meningococcal serogroup C bactericidal antibody in England and Wales in the pre-vaccination era. Vaccine 21:1094-1098. [DOI] [PubMed] [Google Scholar]