Abstract
Toxoplasma gondii (T. gondii) affects around 30% of humans worldwide. Recently, it has emerged as a significant opportunistic pathogen to immunocompromised patients. Data available is still lacking about toxoplasmosis in cancer patients in Egypt. This study aimed to reveal the current trend of T. gondii in cancer patients in Sohag, Egypt. Sera from 50 cancer patients and 50 healthy controls were screened for Toxoplasma IgG and IgM. Further, buffy coats from both groups were used for detection of T. gondii B1 and RE genes via conventional and nested PCR, respectively. The overall seroprevalence of T. gondii IgG was high (58%). IgG and IgM were detected in 30% and 9% cancer patients, respectively. Patients with solid cancers exhibited a greater IgG seropositivity compared to those with hematologic tumors (77.27% and 46.43%, respectively) (P = 0.03). Concerning the molecular results, only 4 (9%) were positive regarding both PCR assays. In conclusion, T. gondii is highly prevalent in cancer patients in Sohag, Egypt. PCR is strongly recommended to complement serology to diagnose acute or reactivated toxoplasmosis in cancer patients. B1 PCR was found to be equivalent to RE PCR. Nevertheless, thorough large-scale research must be implemented.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-88680-3.
Keywords: Toxoplasma Gondii, Serology, Molecular, Cancer patients, Egypt
Subject terms: Parasitology, Parasite genomics, Parasite host response
Introduction
Toxoplasma gondii (T. gondii) is an intracellular protozoan parasite that can infect any nucleated vertebrate cells. It affects 30–65% of the human population worldwide1,2. Humans become primarily infected by ingesting raw or undercooked meat containing viable tissue cysts, or by ingesting water or food contaminated with oocysts from infected cat feces3. The primary infection in immunocompetent individuals is typically either asymptomatic or oligosymptomatic with lymphadenopathy and flu-like symptoms, depending on host susceptibility and the virulence of the strain4,5. T. gondii has recently emerged as a significant opportunistic pathogen that poses a life-threatening risk to immunocompromised patients. Cancer patients are exposed to a risk of reactivating a latent infection as a result of deficient cellular immunity or antineoplastic drugs. Cancer chemotherapy induces profound and persistent CD4 T cells depletion6. T. gondii disseminates through the host body with using the Trojan horse strategy, it can even enter immune-privileged sites resulting in toxoplasmic chorioretinitis and encephalitis following reactivation7. Thus, effective, rapid and accurate diagnosis is crucial and desirable to initiate adequate treatment and to achieve good prognosis8. The diagnosis of T. gondii infection continues to be a major challenge. Currently, serological diagnosis plays a crucial role in the diagnosis of T. gondii human infections9. Despite the innovations in serologic methods, there are still intractable limitations including the inability to confirm the existence of the parasite in immunocompromised patients10. To tackle these constraints, a variety of molecular assays such as conventional, nested, real-time PCR (qPCR) and loop-mediated isothermal amplification (LAMP) have been developed to detect T. gondii DNA in different biological samples11. Since molecular diagnosis is not influenced by the immunologic status of the patient, it is postulated to be the best option for cancer patients12. Moreover, molecular methods can efficiently detect Toxoplasma in fluids or tissues as it typically circulates at low concentrations, or inconstantly13. Very few reports are available about toxoplasmosis in cancer patients14. This study aimed to reveal the current trend of T. gondii in cancer patients utilizing serologic and molecular diagnostic methods in Sohag Governorate.
Methods
The current research was authorized by the Ethical Institutional Review Board of the Faculty of Medicine, Sohag University, Sohag, Egypt. This study adhered to the principles outlined in the Helsinki Declaration,1964. The Medical Research Ethics Committee, affiliated with the Faculty of Medicine at Sohag University, operates under the oversight of the Office for Human Research Protections (OHRP) with the assigned identification number IRB00013006. This study was registered at Clinicaltrials.gov (ID: NCT04834076). [http://clinicaltrials.gov/ct2/show/record/ “NCT04834076]). Initial Release: 04/04/2021.
Study design
This prospective case-control study was performed in Sohag University Hospitals, Faculty of Medicine, Sohag University, Sohag Governorate, Upper Egypt from January 2023 to December 2023. Fifty cancer patients on chemotherapy were referred to the Oncology Department, Faculty of Medicine, Sohag University, and 50 age and sex matched healthy controls were recruited from patients’ relatives for the study. Cancer patients were further categorized into 2 subgroups: solid organ malignancies and hematologic malignancies subgroups. Furthermore, any prior anti-Toxoplasma therapy was the exclusion criteria.
Relevant demographic data including age, and gender, were obtained for all recruited participants using a questionnaire form. Clinical data explored in patients included the type of cancer, the presence or absence of metastases, and the past history of blood transfusion. These variables were selected based on the literature. Data were obtained from the patients, physicians, and medical examination records.
Procedures were performed in the Central Research Laboratory at Sohag University and the Lab of Molecular Medical Parasitology (LMMP), Kasr Al-Ainy Faculty of Medicine, Cairo University, Egypt.
The researchers provided a comprehensive explanation of the study’s objectives and methodologies to all participants, ensuring that each participating patient provided written informed consent.
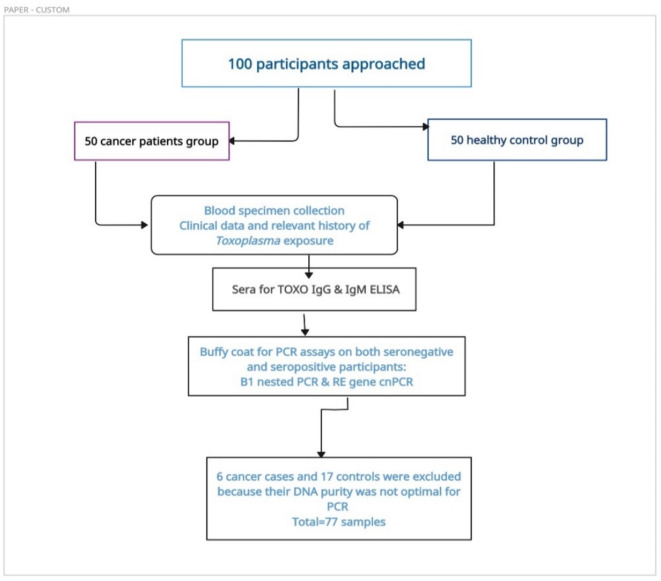
The workflow for this study is summarized as follows: (Fig. 1).
Fig. 1.
Workflow of the current study (Created by https://app.creately.com).
Sample collection
5 ml of venous blood, from each participant, was collected via venipuncture under sterile conditions and aliquoted to sterilized (Gel Clot activator vacuum tubes) and EDTA tubes. Samples were immediately transported to the Central Research Laboratory, Faculty of Medicine, Sohag University. The blood in the Gel Clot activator vacuum tubes was left for 30 min at room temperature for clotting. Sera were separated by centrifugation at 500 g for 10 min, collected in 1.5 ml Eppendorf tubes, coded, and preserved at -20º C till the time of ELISA testing.
Serologic diagnosis
Sera collected were screened for presence of Toxoplasma-specific IgG and IgM antibodies to detect their serostatus. The test was performed using the commercially available PRECHEK EIA kit Anti-TOX IgG & IgM ELISA TEST KITs (Serum/Plasma) (provided by (Precheck Bio, INC, CA, USA) for qualitative assessment of IgM and IgG. Procedural steps were performed according to the manufacturing company instructions.
Optical Densities (O.D.) were read at 450 nm using an automatic ELISA reader (THERMO ELECTRON CORPORATION Multiskan MCC 355 Microplate Reader, 51118170, Shanghai, China) within 15 min. Specimens that gave absorbances equal to or greater than the cutoff value were considered initially reactive. and positive and negative controls were included in each test.
Molecular diagnosis
Genomic DNA extraction
Genomic DNA was isolated from buffy coats using Qiagen extraction blood minikit (QIAamp® DNA Minikit, Hilden Germany) (Cat. No. 51104), following the manufacturer’s instructions. The DNA yield and the ratio of absorbance at 260 nm to 280 nm (A260/A280 ratio) were measured using the NanoDrop Q5000 UV-Vis Spectrophotometer (Quawell Technology, Inc, San Jose, CA, USA). The extracted DNA was stored at − 20 °C until the PCR was performed.
Gene targets
Two T. gondii multi-copy gene targets were chosen to perform the PCR assays: (1) The B1 gene, also referred to as B1 repeat, (GenBank accession no. AF179871) is a 2214 bp (bp) sequence with unknown function that is repeated 35 times in the genome of T. gondii. (2) The RE gene, also referred to as the 529 bp repeat, which is also of unknown function has been described in the genome of T. gondii in 200-300-fold repeats (GenBank accession no. AF146527.1).
Concerning the B1 gene, the external primers: B1-F1 and B1-R1 and the internal primers B1-F2 and B1-R2 were previously designed by Burg and his colleagues15. Refer to Table 1 which illustrates the exact sequences and the sizes of the expected amplicons.
Table 1.
Primer sequences used in this study to amplify the B1 gene.
| Primer name | Sequences | Amplicon |
|---|---|---|
| B1-F1 | 5′-GGAACTGCATCCGTTCATGAG-3′ | 194 bp |
| B1-R1 | 5′-TCTTTAAAGCGTTCGGGTC-3′ | |
| B1-F2 | 5′-TGCATAGGTTGCAGTCACTG-3′ | 100 bp |
| B1-R2 | 5′-GGCGACCAATCTGCGAATACACC-3′ |
The primer sequences for RE gene are shown below in Table 2 as first designed by Homan and his colleagues16.
Table 2.
Primer sequences used in this study to amplify the RE gene.
| Primer name | Sequences | Amplicon |
|---|---|---|
| TOX-4 | 5'CGCTGCAGGGAGGAAGACGAAAGTTG-3' | 529 bp |
| TOX-5 | 5’CGCTGCAGACACAGTGCATCTGGATT-3' |
Nested PCR for B1 gene
The PCR Reaction of the first round was performed in final volume of 25 µl including 12.5 µl DreamTaq PCR Master Mix (2X) (Thermo scientific, U.K.), 1 µl of each primer, 0.1 µl Taq polymerase (Thermo Scientific, UK), final volume of DNA template was adjusted to 10 µl using DD H2O. The amplicon of the first round was utilized as a DNA template for the second round PCR reaction. The final volume was also 25 µl, containing 12.5 µl PCR Master Mix 2X (Thermo Scientific™), 1 µl of each primer, 0.1 µl Taq polymerase (Thermo Scientific, UK), 8.4 µl dd H2O and 2 µl of DNA template.
A 96-well thermocycler (Thermo Scientific Arktik™TCA4848) was used for the PCR amplification process. The amplifications were made in two sequential steps. The conditions of the two rounds are illustrated in Table 3.
Table 3.
Thermal cycling conditions used in nested PCR assay (B1 gene).
| Step 1-B1 | Cycles no | Step 2-B1 | Cycles no | |
|---|---|---|---|---|
| Initial denaturation | 94ºC 3′ | 94ºC 3′ | ||
| Denaturation | 94ºC 60′′ | 30X | 94ºC 60′′ | 35x |
| Annealing | 55ºC 60′′ | 60ºC 60′′ | ||
| Extension | 72ºC 60′′ | 72ºC 60′′ | ||
| Final extension | 72ºC 7′ | 72ºC 7′ |
Each PCR run included a positive control (pure tachyzoites of RH strain preserved in PBS at -20º C) kindly provided by Professor Mona Mohamed Tolba, Parasitology Department, Medical Research Institute, Alexandria University, Egypt and Professor Eman El-Wakil, Parasitology Department, TRBI, Egypt. Furthermore, DNase-free water was used as a negative control to exclude contamination in the reaction.
PCR for the RE gene
The PCR Reaction was performed in 25 µl total volume containing 12.5 µl DreamTaq PCR Master Mix (2X) (Thermo scientific, U.K), 1 µl of each primer, 0.1 µl Taq polymerase (Thermo scientific, UK), final volume of DNA template was adjusted to 10 µl using DD H2O. The conditions of the optimized PCR experiment are illustrated in Table 4.
Table 4.
Thermal cycling conditions used in PCR assay (RE gene).
| RE gene | Cycle no | |
|---|---|---|
| Initial denaturation | 94ºC 5′ | |
| Denaturation | 94ºC 60′′ | 35x |
| Annealing | 65ºC 60′′ | |
| Extension | 72ºC 60′′ | |
| Final extension | 72ºC 7′ |
After thermal cycles, PCR products were electrophoresed in 1.5% agarose gel and stained with Ethidium bromide (EtBr) in horizontal gel electrophoresis. DNA fragments were visualized under ultraviolet (UV) transilluminator. The size of DNA fragments was determined on comparison with 100-bp ladder.
Statistical analyses
Data was analyzed using STATA version 17.0 (Stata Statistical Software: Release 17.0 College Station, TX: Stata Corp LP.). Data were expressed as mean ± SD and counts or percentages where appropriate. The Mann–Whitney test was used for non-parametric data and the χ2 test was used for categorical variables. The Fisher’s exact test was used when the expected cell count deviation was < 5. ROC curve was depicted to explore the diagnostic performance of RE gene PCR, analysed as area under the curve (AUC), standard error (SE) and 95% CI. Validity statistics (sensitivity, specificity, positive and negative predictive value –PPV & NPV-) were calculated. Logistic regression analysis was calculated to investigate the independent association between infection parameters and cancer (Odds Ratio -OR-, 95% confidence interval − 95% CI- and p-value-). A p-value of < 0.05 was considered statistically significant.
Results
Clinical and demographic data of the study groups
The age range of cancer patients spanned from 3 to 76 years, with a mean age of 37.46 ± 18.57 years and IQR (40.5). This was comparable to the age range observed in the healthy individuals, which was 4 to 69 years, with a mean age of 37.0 ± 14.72 years and IQR (37). Cancer patients included 28 males (56%) while the control group included 27 males (54%). Both groups were comparable and matched for age and sex (Table 5).
Table 5.
Differences in baseline characteristics among the studied groups.
| Controls (n = 50) | Cases (n = 50) | P-value | |
|---|---|---|---|
| Age | |||
| Mean ± SD | 37.00 ± 14.7 | 37.46 ± 18.6 | = 0.158* |
| Median (IQR) | 37 (23) | 40.5 (26) | |
| Sex | |||
| Female | 23 (46%) | 22 (44%) | = 0.841* |
| Male | 27 (54%) | 28 (56%) | |
*Mann Whitney U-test was used to compare the median difference between groups.
**Chi-square test was used to compare the proportion difference between groups.
Serologic findings
The overall seroprevalence of T. gondii IgG in all the study participants was high (58%). However, T. gondii IgM was detected in only 9 study participants (9%). This study compared the seropositivity of Toxoplasma IgG and IgM in cancer cases versus the control group and no differences of statistical significance was detected (p = 0.25 & 0.49, respectively) as shown in Table 6.
Table 6.
Distribution of T. Gondii IgG and IgM among cancer cases and control group.
| Cancer group | Control group | P value | OR | |||
|---|---|---|---|---|---|---|
| Seropositive | Total tested | Seropositive | Total tested | |||
| T. gondii IgG | 30 (60%) | 50 (100%) | 28 (56%) | 50 (100%) | 0.25 | 1.15 (0.52–2.57) |
| T. gondii IgM | 6 (12%) | 50 (100%) | 3 (6%) | 50 (100%) | 0.49 | 2.11 (0.50–8.97) |
*Regression model included age, sex residence as a-priori factors in addition to T. gondii IgG and IgM.
IgG seropositivity was detected in a nearly same fashion in cancer and control groups (30% and 28%, respectively). However, cancer cases showed double infection risk by Toxoplasma IgM (12% versus 6%, respectively). The results are depicted in the following bar graph (Fig. 2).
Fig. 2.

Bar graph showing the frequencies of Toxoplasma IgG and IgM among both study groups.
The cancer group was further categorized into two subgroups: hematologic and solid cancer groups. Patients diagnosed with hematologic malignancies represented (56%) while (44%) had solid cancers.
This study revealed that patients with solid organ cancers exhibited a greater rate of IgG seropositivity compared to those with hematologic tumors (77.27% and 46.43%, respectively). This difference was found to be statistically significant (p = 0.03). Results are listed in Table 7.
Table 7.
T. Gondii IgG and IgM seropositivity among hematologic and solid cancer groups.
| Hematologic cancers | Solid cancers | P value | ||
|---|---|---|---|---|
| T. gondii IgG | Negative | 15 (53.57%) | 5 (22.73%) | 0.03 |
| Positive | 13 (46.43%) | 17 (77.27%) | ||
| T. gondii IgM | Negative | 23 (82.14%) | 21 (95.45%) | 0.21** |
| Positive | 5 (17.86%) | 1 (4.55%) |
P value was calculated using the Chi square test, **P value was calculated using fisher exact test.
P value in bold is < 0.05 (statistically significant).
Table 8 illustrates the different serologic combinations of T. gondii IgG and IgM among both study groups. The highest serologic combination “IgM-, IgG+” accounted for 52% in both cancer and control groups. However, only two cancer cases and one healthy individual were “IgM+, IgG-”. No differences of statistical significance were found.
Table 8.
Distribution of the studied population by ELISA in cancer cases and controls.
| ELISA results | Cancer cases | Controls | OR (95% CI) | P value |
|---|---|---|---|---|
| IgM − , IgG − | 18 (36.00%) | 21 (42.00%) | 0.78 (0.32:1.87) | 0.54 |
| IgM − , IgG + | 26 (52.00%) | 26 (52.00%) | (0.42:2.36) | 1.00 |
| IgM + , IgG − | 2 (4.00%) | 1 (2.00%) | 2.04 (0.10:122.86) | 0.56 |
| IgM + , IgG + | 4 (8.00%) | 2 (4.00%) | 2.09 (0.28:23.94) | 0.40 |
P value was calculated using the Chi square test.
P < 0.05 is statistically significant.
*Regression model included age, sex residence as a-priori factors in addition to T. gondii IgG and IgM.
The distribution of the seroprevalence of T. gondii IgG according to the clinical diagnosis of cancer is presented in Table 9. Lung and colon cancers (100%) exhibited the highest prevalences of T. gondii followed by brain and hepatocellular carcinoma (75%). There was no statistical significance. Moreover, Hodgkin’s and non-Hodgkin’s lymphomas had (55.56%) and (71.43%) seroprevalences, respectively. However, no statistical significance was found.
Table 9.
The distribution of Toxoplasma IgG seropositivity among various hematologic and solid malignancies.
| Cancer type | IgG seropositivity | P value | |
|---|---|---|---|
| Negative | Positive | ||
| Hematologic cancers | |||
| Acute lymphoblastic leukaemia | 7 (77.78%) | 2 (22.22%) | 0.28 |
| Non-Hodgkin lymphoma | 4 (44.44%) | 5 (55.56%) | |
| Hodgkin lymphoma | 2 (28.57%) | 5 (71.43%) | |
| Chronic lymphoblastic leukaemia | 1 (50.00%) | 1 (50.00%) | |
| Acute myeloid leukaemia | 1 (100%) | 0 | |
| Solid cancers | |||
| Breast cancer | 3 (27.27%) | 8 (72.73%) | 0.90 |
| Brain cancer | 1 (25.00%) | 3 (75.00%) | |
| Hepatocellular carcinoma | 1 (25.00%) | 3 (75.00%) | |
| Lung cancer | 0 | 2 (100%) | |
| Colon cancer | 0 | 1 (100%) | |
P value was calculated using the Chi square test.
P < 0.05 is statistically significant.
As demonstrated in Table 10, Toxoplasma IgM seropositivity was reported only in one case of the solid cancers subgroup which was a case of lung cancer (50%). Moreover, there was a statistical significance when comparing the solid cancers subgroup (P = 0.03).
Table 10.
The distribution of Toxoplasma IgM seropositivity among various hematologic and solid malignancies.
| Cancer type | IgM seropositivity | P value | |
|---|---|---|---|
| Negative | Positive | ||
| Hematologic cancer | |||
| Acute lymphoblastic leukemia | 8 (88.89%) | 1 (11.11%) | |
| Non-Hodgkin lymphoma | 8 (88.89%) | 1 (11.11%) | |
| Hodgkin lymphoma | 5 (71.43%) | 2 (28.57%) | 0.60 |
| Chronic lymphoblastic leukemia | 1 (50.00%) | 1 (50.00%) | |
| Acute myeloid leukemia | 1 (100%) | 0 | |
| Solid cancer | |||
| Breast cancer | 11 (100%) | 0 | |
| Brain cancer | 4 (100%) | 0 | |
| Hepatocellular carcinoma | 4 (100%) | 0 | 0.03 |
| Lung cancer | 1 (50.00%) | 1 (50.00%) | |
| Colon cancer | 1 (100%) | 0 | |
P value was calculated using the Chi square test.
P < 0.05 is statistically significant (in bold).
Molecular findings
The DNA of the 100 samples, positive and negative for anti-Toxoplasma antibodies, was extracted. After DNA purification, out of the 100 samples, 6 samples from cancer cases and 17 samples from controls (total = 23 samples) were excluded from the molecular experiments because their DNA concentration and/or purity were not optimal. This study found that only 4 cancer cases were positive by both PCR experiments. None of the samples of the healthy controls could amplify Toxoplasma B1 nor RE genes. According to our nested-PCR assay on buffy coat samples from the cancer patients and the non-cancerous control group, only 4 (9%) out of 44 cancer patients were positive regarding T. gondii B1 gene amplification. Additionally, none out of the samples of the 33 healthy individuals showed a specific 100 bp DNA band in 1.5% agarose gel (Fig. 3).
Fig. 3.
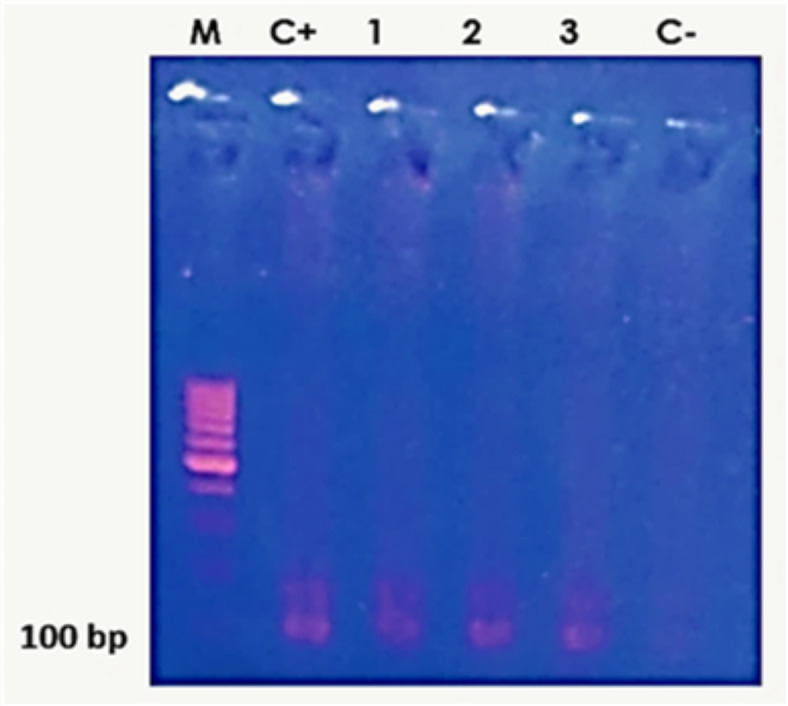
Results of the nested PCR using Toxoplasma B1 gene: Lane C + shows RH strain as a positive control. Lanes 1, 2 and 3 show the second nested PCR amplification of the B1 gene. Lane C- is the negative control. M is the 100 bp molecular weight marker.
To amplify the 529 bp fragment, PCR was carried out on 77 buffy coat samples of the study subjects regardless of the presence or absence of anti-T. gondii antibodies using the RE gene marker. From a total of 77 buffy coat samples, from the cancer patients and healthy controls, 4 (9%) samples contained T. gondii DNA and showed a 529 bp band in 1.5% agarose gel (Fig. 4). The 4 samples that successfully amplified RE genes were cancer patients and none of the healthy controls’ samples could amplify the gene.
Fig. 4.

PCR products of T. gondii from the buffy coats of 4 cancer patients. M: 100 bp molecular weight marker; C+: positive control; lanes 1,2,3 and 4: PCR products of RE gene.
The agreement between PCR and nested PCR was calculated using the Cohen kappa coefficient (κ). The analyzed results were interpreted considering the ranges published by Landis and Koch17. The analysis of the obtained results showed that the same 4 samples were positive by both PCR and nested PCR. Cohen kappa coefficient κ was 1 indicating perfect agreement.
Table 10 depicts the diagnostic parameters of PCR (RE gene) versus nested PCR assay. The obtained results showed that the RE gene-PCR had a sensitivity, specificity, PPV, and NPV of 100% (Table 11).
Table 11.
Diagnostic performance parameters of the RE gene PCR.
| Nested PCR (B1 gene) | Total | AUC | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | ||||||||
| PCR (RE gene) | Positive | 4 (100%) | 0 (0%) | 4 | 1 | 100% | 100% | 100% | 100% |
| Negative | 0 (0%) | 4 (100%) | 4 | ||||||
| Total | 4 | 4 | 8 | ||||||
The ROC curve analysis was conducted to estimate the diagnostic performance of PCR (RE gene). The area under the curve (AUC) was calculated and it was 1.0, thereby indicating a perfect test (i.e., 100% sensitive) (Fig. 5).
Fig. 5.

Area under the ROC curve of the PCR (RE gene) versus the nested PCR (B1 gene) as the gold standard diagnostic test.
Out of the four PCR-positive samples, 2 cases were solid cancers (hepatocellular carcinoma and brain cancer). In addition, 2 cases were diagnosed with HL. One HL patient was seronegative for both Toxoplasma IgG and IgM. The other 3 PCR-positive patients were “IgM-, IgG+” (Table 12).
Table 12.
The serostatus of the PCR-positive cancer cases according to their clinical diagnosis.
| PCR + ve cancer cases | IgM-, IgG- | IgM-, IgG + |
|---|---|---|
| Hodgkin’s lymphoma (HL) | 1(25%) | 1(25%) |
| Hepatocellular carcinoma (HCC) | 0 | 1(25%) |
| Brain cancer | 0 | 1(25%) |
The RE PCR and B1-nested PCR produced positive results (the presence of T. gondii DNA) for 3 of the 43 IgM-, IgG + seropositive samples. Out of 25 seronegative samples (IgM−, IgG−), one sample was successfully amplified. However, none of the (IgM+, IgG-) and (IgM+, IgG+) were positive by PCR. The positive samples were the same in the 2 different PCR techniques (Table 13).
Table 13.
Comparative results of the PCR assays on “IgM+, IgG+, 10 IgM−, IgG + and IgM−, IgG−“samples.
| PCR-negative (n = 73) | PCR-positive (n = 4) | |
|---|---|---|
| IgM − , IgG − | 24 (32.88%) | 1 (25.00%) |
| IgM − , IgG + | 40 (54.79%) | 3 (75.00%) |
| IgM + , IgG − | 3 (4.11%) | 0 |
| IgM + , IgG + | 6 (8.22%) | 0 |
Discussion
The escalating incidence of cancer cases has highlighted the significance of toxoplasmosis. It ranks as the third leading cause of mortality in immunocompromised patients following Pneumocystis and Cryptosporidium. In spite of its crucial role, it failed to receive the attention it deserves18. Clinical and epidemiological studies have shown evidence of a possible link between parasitic protozoan infections and cancer. T. gondii is associated with leukemia and lymphoma19. There is no data about the current trend of T. gondii infection in our governorate. Hence, the current case–control study is the first to determine the sero-epidemiology and molecular detection of T. gondii in cancer patients from Sohag Governorate, Upper Egypt compared to healthy controls.
The overall seroprevalence of T. gondii IgG in all the study participants was high (58%). A recent review by Rouatbi et al.20 discussed prevalence rates in North Africa and reported that the overall seroprevalence of T. gondii in in Egyptians is reaching 59.6% which is consistent with the results obtained by this study. Lower prevalence rates were reported in aborted women (35.6%) and primigravidae pregnant women from Sohag governorate (47.1%)21,22. The current study revealed that T. gondii IgG was slightly higher in cancer patients than the control group (60% and 56%, respectively). Although, the IgM prevalence was found to be two folds in cancer patients than healthy controls (12% versus 6%, respectively), it was insignificant. Yet, cancer patients are still more prone to toxoplasmosis (OR = 2). This could be justified that reactivation of infection, and the risk of reinfection are seemingly similar in all individuals. The presence of specific IgG and absence of IgM strongly points to a prior infection. However, the IgM can be occasionally evanescent or even negative23.
To monitor any possible rise in IgG levels, it is recommended to perform a second serology 3 weeks later. Stable IgG levels denote chronic toxoplasmosis. A significant increase in IgG levels prompts performing determination of IgG avidity. Reinfection or reactivation is strongly suspected in cases of high IgG avidity. Serological follow-up is unnecessary if this situation occurs in an immunocompetent subject24. It is challenging to make a definitive serologic diagnosis of acute infection from a single serum sample because IgM antibodies can last for months, and the avidity index may stay low for several months25, thus definitive diagnosis requires the sequential appearance of specific IgM and IgG antibodies in the same sample. We could not do this due to financial constraints. It is inevitable to choose the right technique at the appropriate cost. Confirmation methods are mostly inhouse, complex, or costly methods, such as dye test, IFAT, and immunoblot. Furthermore, they should be utilized exclusively in reference laboratories24. It is worth to take into consideration that seropositivity is not always indicative of latent infection, because cancer patients may frequently receive blood transfusions, that could lead to passively transferred antibodies26.
Few recent studies from Egypt reported various seropositivity rates of T. gondii in patients suffering from various malignancies. For instance, Abdel Malek et al. and Saad et al.27,28 had detected T. gondii IgG in 40%, and 20%, and IgM in 5.8%, and 4% cancer patients, respectively. This was lower than our results. Moreover, Saad et al. reported that seropositive cases for anti-Toxoplasma IgM and IgG accounted for (4.3%) & (56.5%) in cancer group29. Similar seroprevalence rates of anti-T. gondii IgG in Egyptian cancer patients from different governorates were reported (55.8%, 61.1%, 62.5%, 66.7% and 67.5%)27,29–32. Recent reports’ data, both national and regional, warrant cautious interpretation due to limited sample sizes and inadequate information on population characteristics and pertinent Toxoplasma risk factors.
Furthermore, this disparity is influenced by the various geographical and environmental factors which facilitate the transmission of infection. Additionally, there is a noticeable discrepancy in the prevalence of toxoplasmosis between past and modern studies conducted in Egypt. This variation emphasised the present escalating baseline rate of T. gondii seropositivity and indicated the heightened pace of infection transmission throughout the last two decades. In 1991, Khalil and colleagues documented the existence of anti-Toxoplasma antibodies in 36% of lung cancer patients33. Many similar studies were conducted worldwide with variable seroprevalence rates. For instance, anti-T. gondii IgG was reported in 60% in Turkish cancer patients which was consistent with our study results34. Moreover, anti-T. gondii antibodies (IgG) were detected in 82.1% of Iraqi cancer patients35. This was significantly higher than this study results. Conversely, lower prevalences of anti-T. gondii IgG antibodies were reported from China, Iran, Saudia Arabia and Jordan (35.56%, 36.6%, 30.6% and 39.5%, respectively)19,26,36,37.
The occurrence of IgM antibodies solely (IgM positive/IgG negative) is seldom observed because of the brief interval between their appearance. This coincides with the current results, which indicate that IgM was identified exclusively in 3% of the samples, while IgM and IgG were detected concurrently in 6% of the samples. This study revealed that patients with solid organ malignancies showed a larger proportion of IgG seropositivity compared to those with hematologic tumours (77.27% and 46.43%, respectively). This difference was determined to be statistically significant (P = 0.03). The findings also demonstrated that Toxoplasma IgM had a prevalence of (17.86%) in haematological malignancies and (4.55%) in solid organ cancers. This difference was determined to be statistically non-significant. This agreed with Abdel Malek et al.28 who stated that patients with solid organ tumors had a two times higher prevalence rate of toxoplasmosis (24%) compared to hematological malignancies (12%), and this difference was statistically insignificant (P = 0.06). Conversely, Ali et al.31 reported that patients with hematological malignancies had greater IgG seropositivity compared to those with solid organ tumours (40% and 26.7% respectively), with a statistically significant difference (P = 0.002). Furthermore, a recent Iranian study revealed that a greater occurrence of anti-Toxoplasma IgG antibodies in patients diagnosed with lymphoma and leukaemia (P = 0.001) compared to those with other solid cancerous masses18. Nevertheless, two explanations can elucidate our findings. Firstly, the majority of patients with solid tumours have compromised cellular immunity. Their classification is that of high-risk neutropenic patients. Secondly, corticosteroids are the cornerstone in the majority of chemotherapy agents prescribed to patients with haematological malignancies. The greatest frequency of T. gondii IgG was found in 100% of lung and colon malignancies, followed by brain and hepatocellular carcinomas (75%). This was relatively in concordance with an earlier lung cancer patients had the greatest rate of T. gondii infection (60.94%), followed by cervical cancer patients (50.00%) and brain cancer patients (42.31%)36. Inconsistently, Iraqi breast and colon cancer patients had higher anti-T. gondii IgG seropositivity (77.50%, 77.42%) than the control group (20.54%)38. However,31 reported that all (100%) stomach and bone cancers were positive for Toxoplasma IgG. This is somewhat consistent with the findings of Mostafa et al.27, who found that the highest IgG positivity was found in breast cancer (96.1%), followed by bone carcinoma (80%), brain tumour (69.2%), and liver tumour (35%). A recent meta-analysis was conducted to assess the potential the association between T. gondii infection/exposure and the likelihood of the brain tumours development. The pooled analysis revealed an overall (OR) of 1.96 (95% CI, 1.37–2.80), suggesting a substantial higher risk of brain tumours linked to T. gondii infection /exposure39.
The current research found that Toxoplasma IgM seropositivity was the highest equally in lung cancer and chronic lymphoblastic leukemia (50%), followed by Hodgkin’s lymphoma (28%). The high seroprevalence in leukemic patients agreed with the results of an evidence based meta-analysis (OR = 3.05; 95%CI = 1.83–5.08)40. Contrary to this, the authors of a Chinese study reported that the positivity of Toxoplasma-IgM antibodies was the highest in intracranial malignancies (7.69%), followed by lymphoma (4.76%), hepatocellular carcinoma (3.64) and lastly, breast cancer (3.57)41. A relatively lower prevalence was reported. (28.9%) of CCL patients were positive for anti-T. gondii IgM antibodies in a case–control study conducted on leukemic Chinese children42. Furthermore, Kalantari and his colleagues19 stated that anti–T. gondii IgM was not detected in paediatric hematologic malignancies. In the current study, it was found that the highest serologic combination was “IgM-, IgG + ” which accounted for 52% in both cancer and control groups. However, only two cancer cases and one healthy individual were “IgM + , IgG-”. No differences of statistical significance were found. This can be clarified that most Egyptians; cancer patients and the healthy individuals had a past infection. In other words, they are chronically infected, and they are at risk of reactivation.
Whilst the objective of the search for Toxoplasma IgM antibodies was to confirm the acute infection, it soon became evident that IgM can persist for several months or even years. A recent excellent review provided a comprehensive analysis of multiple mechanisms that may enhance our comprehension of the “chronic IgM” phenomenon. One of them is that micro-reactivation (excystment)– (encystment) cycles of T. gondii can consistently produce low levels of IgM for extended durations. Tested by both non reference laboratories (NRLs) and reference laboratories, Toxoplasma IgM alone is unable to differentiate between a freshly contracted infection and a chronic one43. A complete serologic panel should be performed at the reference laboratory as mandated by the FDA for an accurate interpretation for all Toxoplasma IgM specimens identified as positive by NRLs44. Occasionally, serology may not be sufficient to detect active infection in individuals with impaired immunity. Multiple factors may hinder the generation of specific anti-Toxoplasma antibodies when undergoing antineoplastic therapy. This may result in a reduction of the titre to negligible levels. In other circumstances, the antibody response may be delayed for a duration beyond 4 weeks. These parameters may account for the lack or delay of a recent increase in IgM production in cancer patients recruited in the current study45.
Innovations in molecular diagnostic techniques during the past 30 years have completely transformed the diagnosis of parasite diseases. Owing to their exceptional sensitivity and specificity, they are increasingly recommended as a crucial diagnostic tool for congenital toxoplasmosis, toxoplasmosis in immunocompromised persons, and ocular toxoplasmosis46. They are independent of the immune response, so they may facilitate more accurate diagnosis. Hence, it is highly advised to use PCR assays to detect T. gondii genomic DNA in blood or bodily fluids as a complementary diagnostic approach, particularly in immunocompromised individuals47. Even so, in developing nations, including Egypt, molecular techniques are not frequently used in clinical settings for routine diagnosis and monitoring due to their high cost and time-consuming nature. The B1 gene (30–35 copies/genome) and the Rep529 repetitive elements (200–300 copies/genome) are multi-copy, highly conserved genes in the T. gondii genome estimated to have higher analytical sensitivity than single copy genes for detecting T. gondii DNA48. Therefore, in the present study, PCR assays relying on the T. gondii B1 gene and the RE 529-bp region were chosen in particular because to their established trustworthiness in recent literature. These are some examples49–56. The current study performed molecular detection of Toxoplasma DNA on buffy coat extracts from 44 cancer cases and 33 controls regardless of their serostatus. Two PCR techniques were performed: nested PCR and PCR for detection of the B1 gene and the rep-529 fragment (RE gene), respectively.
Our results revealed that only 4 samples of cancer patients (5.19%) could successfully amplify both the B1 gene and the 529 bp fragment. However, all the controls were Toxoplasma-PCR negative. Generally, the detection of T. gondii in clinical samples confirms its existence which can be due to initial, reactivated, or persistent infection1. Despite finding of Toxoplasma DNA in blood indicates parasitemia, its clinical value for persons with compromised immune systems remains questionable57. Numerous studies have shown the inadequate sensitivity of PCR techniques using peripheral blood, presumably due to low parasite concentrations58. The present study suggested that sensitivity of the RE-based PCR experiment was equivalent to the B1 gene PCR. This was unexpectedly different from a large body of evidence which supports the superiority and accuracy of the RE segment over the B1 gene. This can be clarified by the fact that the B1 PCR is more specific but less sensitive than the RE PCR due to the difference in the number of their copies in T. gondii genome. When the sensitivity of the PCR targeting the B1 gene was enhanced by performing nested PCR, both had the same sensitivity and specificity. Nevertheless, participants exhibited a low positivity rate that did not correlate substantially to their serological profile.
In 2010, it was reported that the rep 529 element was absent in 4.8% of human T. gondii-positive samples. The data supported that the B1 gene may be the target of choice59. Thereafter, similar results were stated60. Despite this, a recent work has shown that the effectiveness of Rep 529 qPCR is not influenced by the genotype of T. gondii isolates and truly, it outperforms B1 qPCR accordingly60. The low PCR yield in the present work was similar to what was found by55,61–63 (8.3%, 10%, 11% and 11.1%, respectively). Our results strongly go in agreement with the results reported previously in 2021. The authors stated that (5.43%) samples contained T. gondii DNA and showed the expected 529 bp band18. Additionally, a 3-year retrospective study was carried out in 15 reference laboratories from the network of the French National Reference Centre for Toxoplasmosis. The predominant source of positive samples was blood, accounting for 55% of the cases. This was followed by CSF (17%), AF (16%), BAL (5%), and different tissue biopsy samples (7%). T. gondii DNA detection in blood was whether DNA extraction was performed using whole blood or the leukocyte fraction49. On the contrary, lower detection rates (3.55%) and (1%) were previously reported in China and Iran41,64. The diversity in outcomes may be attributed to different genotypes of T. gondii, particularly highly virulent strains along with variations of parasitemia. In Egypt, a paucity of studies has been performed to molecularly diagnose toxoplasmosis in cancer patients.
Recently, Toxoplasma DNA was revealed in 32.5% of children with hematologic malignancies which was higher than our result32. As a matter of fact, this tremendous heterogenicity in Toxoplasma PCR detection rates could be attributed to the lack of standardization. In other words, the utilization of different DNA purification methods and Kits, gene targets, primer sets, PCR protocols, sample types, sample size and lastly different study subjects. The RE-PCR and B1-nested PCR produced positive results for 3 of the 43 (IgM-, IgG +) seropositive samples. Out of 25 seronegative samples (IgM − , IgG −), one sample was successfully amplified. However, none of the (IgM + , IgG-) and (IgM + , IgG +) were positive by PCR. In other words, the present study revealed that 3 (75%) out of the 4 cancer cases positive by PCR were (IgG + , IgM-), and one case (25%) was (IgM-, IgG-) seronegative. The presence of a positive PCR in a patient with negative serology is a crucial indication that a seronegative result obtained by ELISA does not provide a definitive negative result for toxoplasmosis. Therefore, it is necessary to conduct more sensitive supplemental tests52. The presence of a positive PCR in a patient with negative serology is a crucial indication that a seronegative result obtained by ELISA does not provide a definitive negative result for toxoplasmosis. Therefore, it is necessary to conduct more sensitive supplemental tests. Inability to find Toxoplasma DNA in a significant proportion of seropositive patients was documented in various population cohorts32. This may be elucidated by the limited quantity of DNA found in blood samples65. The successful amplification of T. gondii DNA in blood samples appears to be influenced by the timing of sampling following infection due to the transitory nature of parasitaemia45.
Further variables contributing to negative PCR results are the limited sample size used for DNA extraction in relation to the overall blood volume in the human body, and the presence of certain PCR inhibitors, namely heme, haemoglobin, and lactoferrin66. In addition, the analysis should be performed in multiplicate65. Due to the restricted financial resources of this study, the PCR assays were performed in uniplicates. This may be a factor of getting this low PCR detection rate. Prior studies have indicated that PCR sensitivity varies between 12 and 100% for CSF and between 15 and 80% for blood. The specificity and PPV of PCR can reach up to 100%. A negative PCR result does not, however, formally rule out reactivation67. Diagnostics of toxoplasmosis in individuals with impaired immune systems should be conducted via algorithms including a combination of molecular, serologic, clinical, and radiologic findings68. The IgG-positive result indicates that these three patients are possibly experiencing chronic toxoplasmosis. PCR-positivity suggested that treatment with immunosuppressive drugs rendered them more prone to reactivation. The 4th patient tested positive for PCR and obtained negative results for anti-Toxoplasma IgM and IgG antibody tests. The above findings imply evidence of a novel infection. The negative ELISA test could be due to the immunosuppressive treatment. The diminished detection rate cannot definitively rule out recent infection and could be attributed to a limited quantity of parasites in the bloodstream, a low concentration of parasite DNA in clinical samples relative to the whole blood volume in the human body, or a brief duration of parasitemia. The seronegative cases, in which the B1 gene was definitively detected by PCR, could indicate the existence of circulating Toxoplasma tachyzoites that are not correlated with antibodies. The hypothesised interpretation is that the infection may have occurred very recently during serological testing, resulting in a minimal production of detectable antibodies by serology69. In accordance with our findings, PCR indicated that 5.05% of cancer patients tested positive for the B1 gene of T. gondii. The anti-Toxoplasma IgM antibody test yielded negative results for all five positive cases. Out of the five positive cases, four contained anti-Toxoplasma IgG antibodies, while one tested negative for ELISA IgG antibodies45. By contrast, Toxoplasma DNA was found in 9 out of 9 samples of IgM + /IgG + and 10 out of 255 samples of IgM − /IgG + 18. Furthermore, PCR screening revealed negative results for 19 out of the 30 patients with haematologic malignancies who were ELISA positive. Nevertheless, there was no significant correlation between the PCR positivity rate and the specific type of cancer32. It was plausible that several limitations could have influenced the results obtained, the first was the limited sample size and the financial constraints. The second was that we did not use the avidity test or T. gondii IgA. The authors did not use the "area of residence" as a matching criteria between the groups. General limitations of the molecular work like shortages of reagents due to high cost are considered. Our study was limited by being a single centre institutional study including patients attending Sohag University Hospitals which could be the reason that there were relatively few Toxoplasma DNA detection rates. Very few reports have been published concerning the sero-molecular epidemiology and genotypic characterization of Toxoplasma strains in cancer patients in Egypt.
Conclusions
T. gondii is highly prevalent in cancer patients undergoing chemotherapy and the healthy controls as well in Sohag, Upper Egypt. This work provides a baseline information on T. gondii sero-molecular epidemiology. Further large-scale research is indispensable. PCR is strongly recommended to complement serology in the diagnosis of acute or reactivated T. gondii infection in cancer patients. B1 nested PCR was found to be equivalent to RE PCR. Nevertheless, further thorough research must be implemented.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to extend their sincere appreciation Professor Dominique SOLDATI-FAVRE, full Professor at the Department of Microbiology and Molecular Medicine, Vice-Dean of the Faculty of medicine, University of Geneva, Switzerland for her kind providence of the primers used in the molecular experiments.
Author contributions
E.F.F., M.E.M.T., A.M.A. and H.A.E. contributed to the conceptualization and design of the work. E.F.F. conducted the laboratory experiments. E.F.F., M.E.M.T., A.M.A. and H.A.E. substantially contributed to the interpretation of the data and the writing of the paper. Furthermore, M.E.M.T. and E.F.F. thoroughly reviewed the manuscript, endorsed the final version that was submitted. E.F.F., M.E.M.T., A.M.A. and H.A.E. accepted the responsibility to handle any inquiries regarding the correctness or integrity of any component of the work.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
No funds were received to conduct the current study.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information.
Declarations
Competing interests
The authors declare no competing interests.
Consent to participate
The participants/ their legal guardians gave informed consents for participation in the current study.
Consent for publication
All authors agreed to the publication of the manuscript.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Q Liu ZD Wang SY Huang XQ Zhu 2015 Diagnosis of toxoplasmosis and typing of Toxoplasma gondii Parasit. Vectors 8 292 10.1186/s13071-015-0902-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.C Yoon YS Ham WJ Gil CS Yang 2024 Exploring the potential of Toxoplasma gondii in drug development and as a delivery system Exp. Mol. Med. 56 2 289 300 10.1038/s12276-024-01165-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ZD Wang HH Liu ZX Ma HY Ma ZY Li ZB Yang XQ Zhu B Xu F Wei Q Liu 2017 Toxoplasma gondii infection in immunocompromised patients: A systematic review and meta-analysis Front. Microbiol 8 389 10.3389/fmicb.2017.00389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D Andreou 2024 Toxoplasma gondii infection associated with inflammasome activation and neuronal injury Sci. Rep. 14 1 5327 10.1038/s41598-024-55887-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.C Dard H Fricker-Hidalgo MP Brenier-Pinchart H Pelloux 2016 Relevance of and new developments in serology for toxoplasmosis Trends Parasitol. 32 6 492 506 10.1016/j.pt.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 6.S Scerra H Coignard-Biehler F Lanternier 2013 Disseminated toxoplasmosis in non-allografted patients with hematologic malignancies: report of two cases and literature review Eur. J. Clin. Microbiol. Infect. Dis. 32 1259 1268 10.1007/s10096-013-1879-8 [DOI] [PubMed] [Google Scholar]
- 7.J Flegr 2015 Neurological and neuropsychiatric consequences of chronic Toxoplasma infection Curr. Clin. Micro. Rep. 2 163 172 10.1007/s40588-015-0024-0 [Google Scholar]
- 8.MK Hegazy SI Awad NE Saleh MM Hegazy 2020 Loop mediated isothermal amplification (LAMP) of toxoplasma DNA from dried blood spots Exp. Parasitol. 211 2 10786910.1016/j.exppara.2020.107869 [DOI] [PubMed] [Google Scholar]
- 9.RHD Ybañez AP Ybañez Y Nishikawa 2020 Review on the current trends of toxoplasmosis serodiagnosis in humans Front. Cell Infect. Microbiol. 10 5 1 18 10.3389/fcimb.2020.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.GSG Zeedan 2022 Rapid diagnosis of Toxoplasma gondii using loop-mediated isothermal amplification assay in camels and small ruminants Beni-Suef Univ. J. Basic Appl. Sci. 11 1 10.1186/s43088-021-00184-x [Google Scholar]
- 11.A Rostami P Karanis S Fallahi 2018 Advances in serological, imaging techniques and molecular diagnosis of toxoplasma gondii infection Infect 46 3 303 315 10.1007/s15010017-1111-3 [DOI] [PubMed] [Google Scholar]
- 12.P Mousavi 2018 Detection of Toxoplasma gondi in acute and chronic phases of infection in immunocompromised patients and pregnant women with real-time PCR assay using TaqMan fluorescent probe Iran. J. Parasitol. 13 3 373 381 [PMC free article] [PubMed] [Google Scholar]
- 13.F Robert-Gangneux S Belaz 2016 Molecular diagnosis of toxoplasmosis in immunocompromised patients Curr. Opin. Infect. Dis. 29 4 330 339 10.1097/QCO.0000000000000275 [DOI] [PubMed] [Google Scholar]
- 14.J Bajnok 2019 High frequency of infection of lung cancer patients with the parasite Toxoplasma gondii ERJ. Open Res. 5 2 00143 02018 10.1183/23120541.00143-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.JL Burg CM Grover P Pouletty JC Boothroyd 1989 Direct and sensitive detection of a pathogenic protozoan Toxoplasma gondii, by polymerase chain reaction J. Clin. Microbiol. 27 8 1787 1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WL Homan M Vercammen J Braekeleer De H Verschueren 2000 Identification of a 200- to 300-fold repetitive 529 bp DNA fragment in Toxoplasma gondii, and its use for diagnostic and quantitative PCR Int. J. Parasitol. 30 1 69 75 10.1016/s0020-7519(99)00170-8 [DOI] [PubMed] [Google Scholar]
- 17.JR Landis GG Koch 1977 The measurement of observer agreement for categorical data Biometrics 1 159 174 10.2307/2529310 [PubMed] [Google Scholar]
- 18.SA Hosseini 2021 Toxoplasmosis among cancer patients undergoing chemotherapy: A population study based on the serological, molecular, and epidemiological aspects Trans. R. Soc. Trop. Med. Hyg. 115 6 677 686 10.1093/trstmh/traa112 [DOI] [PubMed] [Google Scholar]
- 19.N Kalantari 2018 Association between Toxoplasma gondii exposure and paediatrics haematological malignancies: A case-control study Epidemiol. Infect. 146 15 1896 1902 10.1017/S0950268818002194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.M Rouatbi 2019 Toxoplasma gondii infection and toxoplasmosis in North Africa: A review. Infection par Toxoplasma gondii et toxoplasmose en Afrique du Nord: synthèse Parasite 10.1051/parasite/2019006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NM Abdel-Aziz AA Hassanien MI Arafa 2020 Detection of Toxoplasma gondii in aborted women and meat of slaughtered sheep and cattle in Sohag city, upper Egypt Adv. Anim. Vet. Sci. 8 6 680 686 10.17582/journal.aavs/2020/8.6.680.686 [Google Scholar]
- 22.S Hussien M Mousa MMA El-mawgod A Sobhy 2017 Seroprevalence of Toxoplasma gondii among primigravida women J. Egypt. Soc. Parasitol. 47 2 381 388 10.21608/jesp.2017.77791 [Google Scholar]
- 23.H Fricker-Hidalgo 2013 Toxoplasma seroconversion with negative or transient immunoglobulin M in pregnant women: myth or reality? A French multicenter retrospective study J. Clin. Microbiol. 51 7 2103 2111 10.1128/JCM.00169-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O Villard 2016 Serological diagnosis of Toxoplasma gondii infection recommendations from the French national reference center for toxoplasmosis Diagn. Microbiol. Infect. Dis. 84 22 33 10.1016/j.diagmicrobio.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 25.F Peyron M Wallon F Kieffer G Graweg 2016 Toxoplasmosis JS Remington JO Klein CB Wilson V Nizet YA Maldonado Eds Infectious Diseases of the Fetus and Newborn Infant Elsevier Saunders Philadelphia, USA 949 1042 [Google Scholar]
- 26.A Imam 2017 Serologic evidence of Toxoplasma gondii infection among cancer patients. A prospective study from Qassim region Saudi Arabia Saudi Med. J. 38 3 319 321 10.15537/smj.2017.3.18535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NES Mostafa 2018 The relationship between toxoplasmosis and different types of human tumors J. Infect. Dev. Ctries. 12 2 137 141 10.3855/jidc.9672 [DOI] [PubMed] [Google Scholar]
- 28.R Abdel Malek 2018 Toxoplasmosis an overlooked disease: Seroprevalence in cancer patients Asian Pac. J. Cancer Prev. 19 7 1987 1991 10.22034/APJCP.2018.19.7.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.A Saad 2020 Role of IgG avidity test in diagnosis of Toxoplasmosis among immunocompromised patients Egypt J. Med. Microbiol. 29 2 25 31 10.21608/ejmm.2020.250012 [Google Scholar]
- 30.AA Elblihy SM Alhusseiny SA Elmabood SN El-beshbishi 2023 Toxoplasma gondii seropositivity among Egyptian children with haematological malignancies Parasitol. United J. 16 1 57 63 10.21608/puj.2023.199269.1204 [Google Scholar]
- 31.MI Ali WM Abd El Wahab DA Hamdy A Hassan 2019 Toxoplasma gondii in cancer patients receiving chemotherapy: Seroprevalence and interferon gamma level J. Parasit. Dis. 43 3 464 471 10.1007/s12639019-01111-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.TM Shawa HA El-taweel MA El-gammal SS Khalil S Heba 2023 Toxoplasmosis in adult patients with haematologic malignancy: Seroprevalence of anti- Toxoplasma antibodies and molecular diagnosis Parasitol. United J. 16 1 79 86 10.21608/puj.2023.203903.1209 [Google Scholar]
- 33.HM Khalil 1991 Opportunistic parasitic infections in immunocompromised hosts J. Egypt Soc. Parasitol. 21 3 657 668 [PubMed] [Google Scholar]
- 34.M Alim S Ozcelik N Ozpinar 2018 Seroprevalence of anti-Toxoplasma gondii antibodies in patients receiving cancer treatment Cumhuriyet Med. J. 40 1 19 24 10.7197/223.vi.387046 [Google Scholar]
- 35.IM Al-Tameemi B Hashim Abdullah S Jabbar Raisan 2019 Seroprevalence of Toxoplasma gondii among cancer patients in Basrah province/Iraq World J. Pharm. Res. 8 1 193 199 10.20959/wjpr20191-13808 [Google Scholar]
- 36.W Cong 2015 Toxoplasma gondii infection in cancer patients: Prevalence, risk factors, genotypes and association with clinical diagnosis Cancer Lett. 359 2 307 313 10.1016/j.canlet.2015.01.036 [DOI] [PubMed] [Google Scholar]
- 37.NS Hijjawi 2018 Seroprevalence of Toxoplasma gondii in cancer patients admitted to hospitals of the royal medical services in Jordan Jordan J. Biol. Sci. 11 5 537 540 [Google Scholar]
- 38.DF Ahmed EJ Saheb 2017 The association of Toxoplasma gondii infection in breast and colorectal cancer patients Int. J. Clin. Oncol. 2 4 86 92 10.11648/j.ijcocr.20170204.13 [Google Scholar]
- 39.A Abdollahi 2022 Toxoplasma gondii infection / exposure and the risk of brain tumors: A systematic review and meta-analysis Cancer Epidemiol. 77 10211910.1016/j.canep.2022.102119 [DOI] [PubMed] [Google Scholar]
- 40.Y Huang 2016 Is Toxoplasma gondii infection a risk factor for leukemia? An evidence-based meta-analysis Med. Sci. Monit. 22 1547 1552 10.12659/msm.897155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.L Wang 2015 Seroprevalence and genetic characterization of Toxoplasma gondii in cancer patients in Anhui Province, Eastern China Parasit. Vectors 10.1186/s13071-015-0778-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.N Zhou 2019 Seroprevalence and risk factors of Toxoplasma gondii infection in children with leukemia in Shandong Province, Eastern China: A case-control prospective study PeerJ 7 e6604 10.7717/peerj.6604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.JA Vargas-Villavicencio I Cañedo-Solares D Correa 2022 Anti Toxoplasma gondii IgM long persistence: What are the underlying mechanisms? Microorganisms 10.3390/microorganisms10081659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.R Dhakal 2015 Significance of a positive toxoplasma immunoglobulin M test result in the United States J. Clin. Microbiol. 53 11 3601 3605 10.1128/JCM.01663-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MN Khabaz L Elkhateeb J Al-Alami 2011 Reactivation of latent Toxoplasma gondii in immunocompromised cancer patients Comp. Clin. Path. 20 2 183 186 10.1007/s00580-010-0975-8 [Google Scholar]
- 46.A Gashout 2016 Molecular diagnosis of Toxoplasma gondii infection in Libya BMC Infect. Dis. 16 157 10.1186/s12879016-1491-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.F Robert-Gangneux 2015 Molecular diagnosis of toxoplasmosis in immunocompromised patients: A 3-year multicenter retrospective study J. Clin. Microbiol. 53 5 1677 1684 10.1128/JCM.03282-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.C Gomez 2019 Dual-target, real-time PCR for the diagnosis of intraocular Toxoplasma gondii infections Br. J. Ophthalmol. 103 4 569 572 10.1136/bjoph.lmol-2018-313064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.K Sardarian 2019 Detection of Toxoplasma gondii B1 gene in placenta does not prove congenital toxoplasmosis Hum. Antibodies 27 1 31 35 10.3233/HAB-180346 [DOI] [PubMed] [Google Scholar]
- 50.HK Valian 2020 Comparison of the RE-529 sequence and B1 gene for Toxoplasma gondii detection in blood samples of the at-risk seropositive cases using uracil DNA glycosylase supplemented loop-mediated isothermal amplification (UDG-LAMP) assay Microb. Pathog. 140 10393810.1016/j.micpath.2019.103938 [DOI] [PubMed] [Google Scholar]
- 51.JM Costa 2013 Direct genotyping of Toxoplasma gondii from amniotic fluids based on B1 gene polymorphism using minisequencing analysis BMC Infect. Dis. 13 552 10.1186/1471-2334-13-552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.S Fallahi 2014 Comparison of the RE and B1 gene for detection of Toxoplasma gondii infection in children with cancer Parasitol. Int. 63 1 37 41 10.1016/j.parint.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 53.F Veronesi 2017 Comparison of PCR assays targeting the multi-copy targets B1 gene and 529 bp repetitive element for detection of Toxoplasma gondii in swine muscle Food Microbiol. 63 213 216 10.1016/j.fm.2016.11.022 [DOI] [PubMed] [Google Scholar]
- 54.HEM Eldeek 2017 Toxoplasma genotyping in congenital toxoplasmosis in Upper Egypt: Evidence of type I strain Parasitol. Res. 116 9 2393 2406 10.1007/s00436-017-5541-8 [DOI] [PubMed] [Google Scholar]
- 55.EF Botein 2019 Serological and molecular screening of umbilical cord blood for Toxoplasma gondii infection Transpl. Infect. Dis. 21 4 e1311710.1111/tid.13117 [DOI] [PubMed] [Google Scholar]
- 56.MP Brenier-Pinchart 2015 Molecular diagnosis of toxoplasmosis: Value of the buffy coat for the detection of circulating Toxoplasma gondii Diagn. Microbiol. Infect. Dis. 82 4 289 291 10.1016/j.diagmicrobio.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 57.R Gutierrez-Loli 2019 Development of a novel protocol based on blood clot to improve the sensitivity of qPCR detection of toxoplasma gondii in peripheral blood specimens Am. J. Trop. Med. Hyg. 100 1 83 89 10.4269/ajtmh.17-0920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Edvinsson, B., Lappalainen, M., Evengård, B., ESCMID Study Group for Toxoplasmosis 2006 Real-time PCR targeting a 529-bp repeat element for diagnosis of toxoplasmosis Clin. Microbiol. Infect. 12 2 131 136 10.1111/j.1469-0691.2005.01332.x [DOI] [PubMed] [Google Scholar]
- 59.T Wahab B Edvinsson D Palm J Lindh 2010 Comparison of the AF146527 and B1 repeated elements, two real-time PCR targets used for detection of Toxoplasma gondii J. Clin. Microbiol. 48 2 591 592 10.1128/JCM.01113-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.C Pomares 2020 Is real-time PCR targeting rep 529 suitable for diagnosis of toxoplasmosis in patients infected with non-type II strains in North America? J. Clin. Microbiol. 58 2 e01223 e1319 10.1128/JCM.01223-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.HA Al-toban 2019 Molecular detection of Toxoplasma gondii in a sample of Iraqi patients with acute leukemia and stem cell transplantation Iraqi J. Hematol. 8 38 44 10.4103/ijh.ijh [Google Scholar]
- 62.S Abolghasemi 2022 The prevalence of early toxoplasmosis after bone marrow transplantation using PCR: A study in Iranian patients Infect. Disord. Drug Targets 22 7 62 66 10.2174/1871526522666220622102543 [DOI] [PubMed] [Google Scholar]
- 63.SM Elzeky N Nabih AA Abdel-Magied DS Abdelmagid AE Handoussa MM Hamouda 2022 Seroprevalence and genetic characterization of Toxoplasma gondii among children with neurodevelopmental disorders in Egypt J. Trop. Med. 2022 2343679 10.1155/2022/2343679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.N Arefkhah 2019 Seroprevalence and risk factors of Toxoplasma gondii infection among cancer and hemodialysis patients in southwest Iran Clin. Epidemiol. Glob. Health. 7 4 596 599 10.1016/j.cegh.2019.01.007 [Google Scholar]
- 65.S Boughattas 2010 Direct genotypic characterization of Toxoplasma gondii strains associated with congenital toxoplasmosis in Tunisia (North Africa) Am. J. Trop. Med. Hyg. 82 6 1041 1046 10.4269/ajtmh.2010.09-0691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ivovic, V., Vujanic, M., Zivkovic, T., Klun, I., Djurkovic-Djakovic, O. Molecular detection and genotyping of Toxoplasma gondii from clinical samples. In: Toxoplasmosis - Recent Advances, (Eds Djurković Djaković. O.) 103–120 (InTech, Rijek, Croatia, 2012). 10.5772/50830.
- 67.F Robert-Gangneux ML Dardé 2012 Epidemiology of and diagnostic strategies for toxoplasmosis Clin. Microbiol. Rev. 25 2 264 296 10.1128/CMR.05013-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.F Nakashima 2020 Serum IgG anti-Toxoplasma gondii antibody concentrations do not correlate nested PCR results in blood donors Front. Cell Infect. Microbiol. 9 461 10.3389/fcimb.2019.00461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O El-Geddawi M El-Sayad N Sadek N Hussien M Ahmed 2016 Detection of T. gondii infection in blood donors in Alexandria, Egypt, using serological and molecular strategies Parasitol. United J. 9 1 24 10.4103/1687-7942.192992 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the paper and its Supplementary Information.