Abstract
We describe the effects of polyethylene glycol-conjugated adenosine deaminase (ADA) replacement therapy on lymphocyte counts, activation, apoptosis, proliferation, and cytokine secretion in a 14-month-old girl with “delayed-onset” ADA deficiency and marked immunodysregulation. Pretreatment lymphopenia affected T cells (CD4, 150/μl; CD8, 459/μl), B cells (16/μl), and NK cells (55/μl). T cells were uniformly activated and largely apoptotic (CD4, 59%; CD8, 82%); and T-cell-dependent cytokine levels in plasma were elevated, including the levels of interleukin 2 (IL-2; 26 pg/ml), IL-4 (81 pg/ml), IL-5 (46 pg/ml), gamma interferon (1,430 pg/ml), tumor necrosis factor alpha (210 pg/ml), and IL-10 (168 pg/ml). Mitogen-stimulated peripheral blood mononuclear cells show reduced IL-2 secretion and proliferation. During the first 5 months of therapy there was clinical improvement and partial immune reconstitution, with nearly normal lymphocyte subset numbers, reduced T-cell activation and CD4-cell apoptosis, and decreased plasma cytokine levels. In parallel, IL-2 secretion and the lymphocyte mitogenic response improved. Between 4 and 7 months, immunoglobulin G antibodies to bovine ADA developed and resulted in the complete reversal of immune recovery.
Adenosine deaminase (ADA) deficiency is an autosomal recessive disorder of purine metabolism which presents as severe combined immunodeficiency of infancy in 85 to 90% of patients and as delayed- or late (adult)-onset combined immunodeficiency in 10 to 15% of patients (13, 15). ADA is a “housekeeping enzyme” in all tissues, resides predominantly in the cytoplasm, and is expressed at 800- to 1,000-fold higher levels in lymphoid cells than in erythrocytes. The absence of ADA causes an accumulation of toxic metabolites that impairs lymphocyte differentiation, viability, and function (17, 18). Clinically significant hepatic and neurological abnormalities also occur in some subjects.
ADA-deficient patients who are not considered suitable for bone marrow or stem cell transplantation can be treated by enzyme replacement with polyethylene glycol (PEG)-conjugated bovine ADA (PEG-ADA) (16). By correcting metabolic abnormalities, PEG-ADA permits variable improvements in lymphocyte counts and immune function (17). However, in most reports of patients receiving PEG-ADA, the course of immune reconstitution has not been well characterized. We have monitored in detail the effects of PEG-ADA therapy on lymphopenia, the level of naive CD4 cells, T-cell activation, T-cell apoptosis, and the cytokine profile in a patient with a delayed-onset phenotype who manifested marked immune dysregulation as well as immunodeficiency. Immune function improved in this patient, until she developed a neutralizing antibody to PEG-ADA.
CASE REPORT
We describe a 10-month-old girl who presented with recurrent infections (bronchopneumonia, viral infections, persistent otitis media); hepatopathy with elevated transaminase levels, reduced cholinesterase levels, and hepatosplenomegaly; hypoplasia of the thymus gland; skin rash; hemolytic anemia; and thyroid antibodies. A diagnosis of ADA deficiency was made by demonstrating the absence of ADA activity in erythrocytes and lymphocytes and the accumulation of toxic metabolites (urine deoxyadenosine and total deoxyadenosine nucleotides [dAXP] in erythrocytes) (4). Homozygosity for a previously reported (3) missense mutation, Val129Met (V129M) in exon 5, was demonstrated by sequencing of the cDNA and genomic DNA prepared from skin fibroblasts. Immunological analysis at the time of diagnosis showed eosinophilia, an elevated immunoglobulin E (IgE) level (3,770 IU/ml; normal level, <60 IU/ml) as well as an elevated IgG level (1,700 mg/dl; normal range, 500 to 1,300 mg/dl) by immune nephelometry, lymphopenia, and impaired lymphocyte function (Table 1). The results of skin testing for delayed hypersensitivity (candida, tetanus toxoid, diphtheria toxoid, tuberculin, proteus, trichophyton, and streptococcus antigens), blood group isoagglutinins, and antibody response to vaccination antigens (tetanus and diphtheria titer) were negative. In vitro lymphocyte mitogen and recall antigen responses were attenuated.
TABLE 1.
Metabolic and immunological parameters before and after PEG-ADA treatment
| Parameter | Controls | Patient at the following times (wk)
|
||
|---|---|---|---|---|
| 0 | 13-18 | 23-28 | ||
| Metabolic parameters | ||||
| ADA activity (nmol/h/mg) | 40.1a | 1.07 | 101 | 1.3 |
| % dAXP | 0 | 12.3 | 0 | 15.8 |
| Lymphocyte subsets (no. of cells/μl [%]) | ||||
| T (CD4) | 1,000-4,600 (31-54)b | 150 (19) | 512 (32) | 192 (24) |
| T (CD8) | 400-2,100 (12-28) | 464 (58) | 720 (45) | 448 (56) |
| B (CD20) | 600-2,700 (15-39) | 16 (2) | 144 (9) | 10 (1.2) |
| NK (CD56+/CD3−) | 200-1,200 (3-17) | 55 (7) | 192 (12) | 40 (5) |
| T-cell subsets (% of total CD4 or CD8 counts) | ||||
| CD4/CD45RA | 16 | 50 | 17 | |
| CD4/CD45RO | 100 | 69 | 100 | |
| CD4/CD28 | <100c | 100 | 100 | 88 |
| CD8/CD28 | 50-68 | 10 | 20 | 18 |
| CD4/CD95 | 7-20 | 100 | 100 | 100 |
| CD8/CD95 | 12-16 | 100 | 82 | 100 |
| Apoptosis (%) | ||||
| Annexin V CD4 | 8d | 59 | 32 | 59 |
| Annexin V CD8 | 11 | 82 | 83 | 96 |
| Cytokine concn (pg/ml) | ||||
| IL-2 | <3a | 26 | 3 | 24 |
| IL-4 | <3 | 81 | 6 | 51 |
| IL-5 | <3 | 46 | 10 | 57 |
| IL-10 | <10 | 168 | 38 | 284 |
| IFN-γ | <10 | 1,430 | 493 | 1,959 |
| TNF-α | <10 | 210 | 17 | 221 |
| IL-2 concn secretion upon stimulation (pg/ml) | ||||
| PMA-ionomycine | 5,000a | 140 | 2824 | 27 |
| PHA | 190 | 2 | 20 | 20 |
| Lymphocyte proliferation (cpm) | ||||
| PHA (medium) | >90,000a (>500) | 10,000 (1,000) | 69,000 (400) | 2,300 (100) |
| Anti-CD3 | >50,000 | 18,000 | 53,000 | 300 |
For the measurements of metabolic parameters, plasma cytokines, cytokines secretion, and lymphocyte proliferation, we used healthy adults as a daily control.
Values for healthy controls were obtained from reference 10; 70 samples from a group of 9- to 15-month-old healthy children were analyzed.
Values for healthy controls were obtained from reference 21; samples from 20 healthy children (age, 5.3 ± 2.7 years) were analyzed.
Values for healthy controls were obtained from reference 20; samples from 10 healthy children (age, 3.5 ± 2.9 years) were investigated.
Because no suitable donor for bone marrow transplantation was available, replacement therapy with PEG-ADA (ADAGEN, Orphan Europe, Paris, France; ENZON, Inc., Bridgewater, NJ) was started at age 14 months at a dose of two intramuscular injections of 30 U/kg of body weight per week. After 7 months of PEG-ADA treatment, the patient had catheter sepsis and cytomegalovirus reactivation. At about the same time, circulating PEG-ADA activity declined, toxic metabolites reappeared, and IgG antibody to bovine ADA was detected by enzyme-linked immunosorbent assay (ELISA) and by an assay for direct inhibition of ADA catalytic activity. Subsequently, the levels of the immunological parameters returned to pretreatment levels within 4 weeks. High-dose intravenous immunoglobulins and prednisone therapy aimed at suppressing the inhibitory antibody (7, 9) were not successful. PEG-ADA therapy was discontinued, and an HLA-identical bone marrow transplantation from a matched unrelated donor was carried out 1 year after the start of PEG-ADA supplementation. The patient died 4 months after transplantation from an overwhelming viral infection.
MATERIALS AND METHODS
Metabolic and immunological parameters were evaluated at monthly intervals before and after the start of PEG-ADA therapy.
Metabolic parameters.
The level of circulating PEG-ADA was assessed by measuring the ADA activity present in frozen plasma or in extracts of dried blood spot filter cards (prepared from heparinized whole blood). Total adenosine and deoxyadenosine nucleotides (AXP and dAXP, respectively) were measured both in frozen washed red blood cells and in extracts of blood spot cards. These determinations were performed by the laboratory of M. S. Hershfield by using methods described previously (4). In brief, ADA activity was determined by radiochemical assay. AXP and dAXP were measured by high-performance liquid chromatography quantification of the adenosine and deoxyadenosine produced upon enzymatic dephosphorylation of extracted nucleotides. The “percent dAXP,” that is, [amount of dAXP/(amount of AXP + amount of dAXP)] × 100, was used to assess dAXP elevation (4).
IgG anti-ADA antibody.
Plasma levels of IgG antibody to unmodified bovine ADA were measured by ELISA, as described previously (7). In order to confirm antibody specificity, the ELISA was performed in duplicate, without and with prior addition to the plasma samples of purified bovine ADA (20 μg) as a competing antigen (7) (Fig. 1B). Inhibitory (neutralizing) antibody to ADA was assessed by measuring the ability of the plasma samples to inhibit ADA enzymatic activity, as described previously (7).
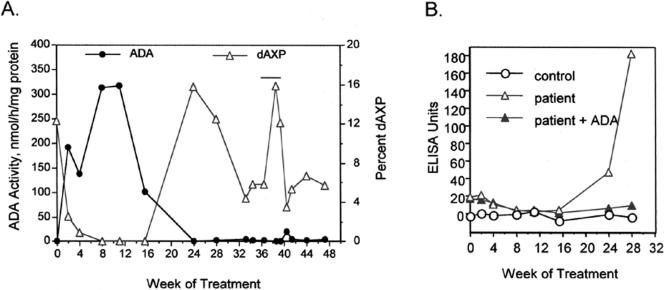
FIG. 1.
(A) Levels of ADA and dAXP activity in eluates of dried blood spots from the patient. PEG-ADA therapy was initiated at week 0 at a dose of 30 U/kg twice weekly; at week 12 the dose was decreased to 30 U/kg once a week. The twice-weekly injection schedule was resumed after week 24, except for the period between weeks 36 and 40, when PEG-ADA therapy was interrupted (horizontal bar). For reference, the mean ADA activity in eluates of six blood spots from healthy controls prepared and analyzed simultaneously was 32.3 nmol/h/mg protein. (B) ELISA data showing the evolution of the IgG antibody response to bovine ADA (triangles, patient; circles, control). The antigen immobilized on the ELISA plate was purified bovine ADA. In order to confirm the antibody specificity, as described previously (7), the ELISA for the patient samples was performed in duplicate, without (open triangles) and with (solid triangles) prior addition to the plasma samples of purified bovine ADA (20 μg) as a competing antigen. In other studies (data not shown), patient plasma from week 28 of treatment (but not pretreatment patient plasma or control plasma) directly inhibited the catalytic activity of PEG-ADA (7).
Immunophenotyping.
Cell isolation and immunofluorescence analysis were performed as described previously (20, 21). Blood was collected by venipuncture into tubes with EDTA anticoagulant and was processed within <2 h. T-cell (CD4, CD8), NK-cell (CD56+/CD3−), B-cell (CD20), and T-cell subsets (CD28, CD95, CD45RA, CD45RO) were measured by flow cytometry with a fluorescence-activated cell sorter (FACS Calibur; Becton Dickinson [BD]) with dye-conjugated monoclonal antibodies that recognize the CD antigens present on the cells. Two-color immunofluorescence was performed with the following antibodies: anti-CD45RA fluorescein isothiocyanate (FITC) and anti-CD4 phycoerythrin (PE), anti-CD45RO PE and anti-CD4 FITC, and anti-CD3 FITC and anti-CD56 PE. Three-color immunofluorescence was used for anti-CD8 FITC, anti-CD4 PE, and anti-CD3 peridinin chlorophyll protein (PerCP); anti-CD28 FITC, anti-CD4 PE, and anti-CD8 PerCP; and anti-CD4 FITC, anti-CD95 PE, anti-CD8 PerCP (the antibody manufacturers were Immunotech [Hamburg, Germany] for CD28, Pharmingen for CD95, and Becton Dickinson [Heidelberg, Germany] for the other antibodies). Based on the typical forward-side-scatter pattern, an electronic gate was applied to the lymphocyte population, and at least 2,000 events were analyzed for the expression of the lymphocyte surface markers. Data were collected by using CELLQuest 3.3 software.
T-cell apoptosis.
Ex vivo T-cell apoptosis was measured as described previously (20). Apoptosis was determined by flow cytometric quantitation with fluorescinated annexin V staining and propidium iodide (PI; Immunotech, Hamburg, Germany) exclusion. Blood was collected by venipuncture into heparinized tubes and was processed within <2 h. The peripheral blood mononuclear cells (PBMCs) in patient and control samples were isolated by Ficoll-Hypaque density gradient centrifugation. Samples were labeled with PE-conjugated monoclonal antibodies directed against CD4 or CD8 (Becton Dickinson) and were incubated for 10 min with monoclonal antibodies and with FITC-conjugated annexin V and PI. Annexin binding buffer (Pharmingen) was used for all washes and incubation steps. Afterwards, analyses were performed with the FACS Calibur flow cytometer by gating on CD8 or CD4 cells with bright fluorescence. Thereby, monocytes which express CD4 at a low intensity were excluded. Apoptotic CD4 and CD8 cells were then measured by analyzing the green (annexin V FITCs labeled) versus the red (PI) fluorescence.
Lymphocyte mitogen-induced proliferation assay.
A total of 1 × 105 PBMCs of patient and control samples were incubated with medium containing the T-cell mitogens phytohemagglutinin (PHA; 2 μg/ml; Murex Biotech Limited, Dartford, United Kingdom), OKT3 (anti-CD3; 5 and 0.5 ng/ml; PeliCluster, Amsterdam, The Netherlands), or medium alone (RPMI 1640 plus fetal calf serum [10%], glutamine [2 mM], and penicillin-streptomycin [100 IU/ml]). The maximum rate of DNA synthesis was reached after 72 h of incubation with the mitogen. Afterwards, the cells were pulsed with [3H]thymidine (0.5 μCi/well; Amersham Corp., Braunschweig, Germany) and harvested after 4 h. The proliferation of PBMCs was measured after they were harvested onto nitrocellulose filters with a cell harvester (Inotech, Dottikon, Switzerland). After measurement in a scintillation counter (TRI-CARB 2100 TR; Packard, Böblingen, Germany), the results were expressed in counts per minute.
Cytokines.
Ex vivo plasma cytokines were determined by a Becton Dickinson cytometric bead array (CBA) assay after 24 h for PHA (2 μg/ml) and IP (phorbol-12- myristate-13-acetate; 20 ng/ml [Sigma, Deisenhof, Germany] and ionomycine [0.3 μM; Calbiochem, Darmstadt, Germany]) stimulation or in medium alone for interleukin 2 (IL-2), IL-4, IL-5, IL-10, tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ) (8). The CBA assay consisted of a mixture of six types of beads that were uniform in size but that contained a red-emitting dye with different fluorescence intensities. A different capture antibody against one of six cytokines was covalently coupled to each type of bead. The cytokines that bound to these antibodies were detected by the use of antibodies labeled with PE (incubation time, 10 min). The fluorescence intensity measured with PE was proportional to the cytokine concentration in the sample and was quantified from a calibration curve. Two-color flow cytometric analysis was performed with a FACS Calibur flow cytometer. Data were acquired and analyzed with the Becton Dickinson CBA assay software.
RESULTS
Immunological findings before enzyme substitution.
Immunological investigations showed marked lymphopenia (792/μl) that affected T, B, and NK cells; a relative increase in the CD8-cell subset; and an inverted CD4/CD8 ratio of 0.33 (Table 1). Only 16% of CD4 cells expressed a naive CD4+/CD45RA+ phenotype. Both CD4 and CD8 T cells were maximally activated: memory CD4+/CD45RO+ phenotype, 100% (of total CD4); CD4+/CD95+ phenotype, 100% (of total CD4); and CD8+/CD95+ phenotype, 100% (of total CD8). All CD4 cells bore the T-cell coreceptor CD28, but the level of CD28 expression on CD8 cells was significantly decreased at 10%. In addition, a high percentage of T cells were apoptotic (CD4, 59%; CD8, 82%). The concentrations in plasma of immunostimulatory and growth-promoting cytokines (IL-2, IL-4, IL-5, IL-10, IFN-γ) and of the proinflammatory cytokine TNF-α were increased. Upon stimulation the patient's PBMCs showed reduced levels of IL-2 secretion and lymphocyte proliferation was attenuated.
Immunological findings after enzyme substitution.
Maintenance of high plasma ADA activity led to normalization of toxic metabolites (dAXP) in erythrocytes after the start of PEG-ADA treatment (weeks 1 to 16) (Fig. 1A). Treatment was associated with marked clinical improvement, with a reduction of hepatosplenomegaly; normalization of liver enzyme levels; normal eosinophil counts; an increase in weight; and resolution of hemolysis, thyroid antibodies, and dermatitis.
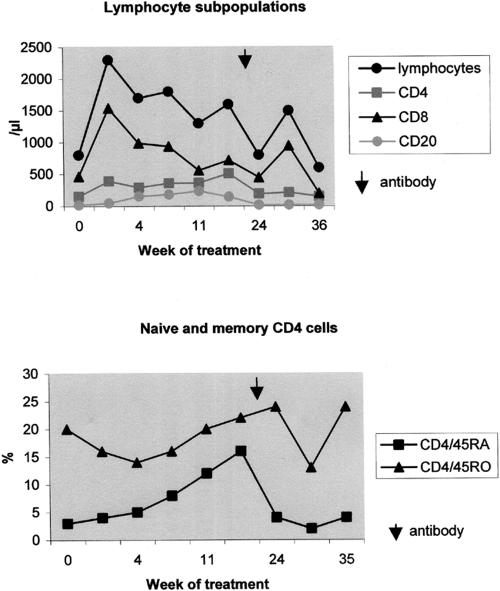
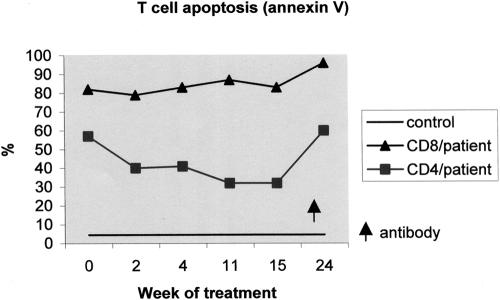
Within 4 to 5 months, the patient's total lymphocyte counts increased to 1,000 to 2,000/μl and lymphocyte subsets reached nearly normal values (Table 1; Fig. 2). The CD4/CD8 ratio increased to 0.7. Fifty percent of the CD4 cells showed a naive CD4+/CD45RA+ phenotype, but T-cell activation continued to be elevated: memory CD4+/CD45RO+ phenotype, 69% (of total CD4); CD4+/CD95+ phenotype, 100% (of total CD4); and CD8+/CD95+ phenotype, 82% (of total CD8) (Fig. 2). The CD28 expression on CD8 cells increased to 20%. CD4-cell apoptosis decreased markedly from 59% to 32%, but there was no change in CD8-cell apoptosis (82% and 83%) during treatment (Fig. 3).
FIG. 2.
Pattern of immune reconstitution in the patient after PEG-ADA therapy starting at week 0 and changes because of the appearance of an IgG antibody to bovine ADA (arrow). The kinetics of total lymphocyte counts and lymphocyte subsets (CD4, CD8, and CD20 cells) (upper panel) and the course of naive and memory CD4 cells (CD4+/CD45RA+ and CD4+/CD45RO+) (lower panel) are shown.
FIG. 3.
Changes in T-cell apoptosis in the patient after PEG-ADA therapy starting at week 0 and after the appearance of an IgG antibody to bovine ADA (arrow). The kinetics of ex vivo T-cell apoptosis (annexin V assay) for CD4 and CD8 cells in comparison to the standard are shown.
T-cell-dependent plasma cytokine (IL-2, IL-4, IL-5) levels decreased slowly to normal levels ≤10 pg/ml, and the levels of the proinflammatory plasma cytokine TNF-α normalized. However, IL-10 and IFN-γ levels remained elevated in plasma. The capacity of PBMCs to secrete IL-2 after IP stimulation increased, and the lymphocyte mitogenic response in vitro also improved significantly.
IgG antibody to bovine ADA.
IgG antibody to bovine ADA became detectable by ELISA between the 16th and the 24th week of treatment (Fig. 1B). This was associated with a marked decline in circulating PEG-ADA activity and a concomitant increase in the level of dAXP, measured both after elution from dried blood spots (Fig. 1B) and in the corresponding plasma and washed erythrocyte samples (data not shown). This was followed by a decline in lymphocyte subsets (T, B, and NK cells), and the activation and ex vivo apoptosis of T cells returned to pretreatment levels (Table 1). There was also an increase in plasma cytokine levels and an almost complete loss of the capacity of PBMCs to secrete IL-2 and to proliferate in response to mitogens. Thus, neutralizing antibody to PEG-ADA resulted in a complete reversal of the partial immune recovery within approximately 4 weeks.
DISCUSSION
PEG-ADA treatment resulted in clinical improvement and partial immune reconstitution (15). In our patient, the clinical status and correction of the metabolic and hepatic abnormalities improved within 4 weeks. T-lymphocyte counts and the CD4/CD8 ratio increased, but there was relatively less improvement in B-cell counts. This is in contrast to the response in some other patients, in whom B-cell counts have increased sharply in the first month or so of PEG-ADA therapy, preceding the appearance of T cells (6). The reason for this variability among patients and its significance is unclear. Early in vitro studies with ADA-inhibited B- and T-lymphoblastoid cell lines suggested that the T-cell populations in ADA deficiency may be more sensitive than B cells to the toxic ADA substrate 2′-deoxyadenosine (dAdo), but later studies with untransformed T and B cells found less of a difference (17). Most studies indicate that ADA deficiency arrests T-cell development in the thymus. Consistent with this, the pattern of T-cell surface antigen expression following the initiation of PEG-ADA therapy suggests maturation of T lymphocytes from early thymic T-cell progenitors (25). In ADA-deficient mice, dATP accumulation appears to exert a greater deleterious effect on B-cell maturation in peripheral lymphoid organs than in the bone marrow (1). The B-cell ontogeny has not been studied following initiation of PEG-ADA therapy, but the recovery of B-cell function is found to be more complete and more rapid in ADA-deficient patients after treatment with PEG-ADA than after successful bone marrow transplantation (22).
By eliminating ADA substrates, PEG-ADA therapy may influence the course and the extent of immune reconstitution in several ways. First, this could provide a protective effect on immature T-cell progenitors, allowing thymopoiesis to recover and resulting within several weeks to a few months in the appearance of mature T cells (19, 25). Second, by eliminating dAdo, PEG-ADA therapy might protect both thymocytes and mature T cells from apoptosis (24). In ADA deficiency, dATP pool expansion induces excessive apoptosis by a p53- and caspase-dependent pathway. dAdo-induced apoptosis appears to occur at the transition between the double-negative (CD4−CD8−) and the double-positive (CD4+CD8+) stages of thymocyte differentiation, and dATP accumulation also induces apoptosis in mature T lymphocytes (5, 12, 23).
The extremely elevated frequency of apoptosis measured ex vivo in the peripheral T cells of our patient (32 to 96%) has not been described before in other ADA-deficient patients. The significant decrease in CD4-cell apoptosis observed after PEG-ADA treatment may have contributed to CD4 reconstitution. Alternatively, the increased apoptosis may have been due to the marked degree of T-cell activation that we observed, with a high prevalence of CD4+/CD45RO+ memory, CD4+/CD95+, CD8+CD28−, and CD8+/CD95+ T-cell subsets (69 to 100%). It is unclear whether this activation was due to the immune dysfunction caused by ADA deficiency or to an infection. During immune reconstitution during PEG-ADA therapy, there were no clinical or laboratory signs of active infection, although latent subclinical infections (e.g., cytomegalovirus infection) might have caused continuous T-cell activation.
Apart from lymphocyte reconstitution, we were interested in the effects of PEG-ADA on cytokine synthesis, as measured by the cytometric bead array assay (8). Interestingly, in our patient lymphocyte and PBMC alterations before treatment were associated with high levels of plasma cytokines (IL-2, IL-4, IL-5, IL-10, IFN-γ, TNF-α). The predominance of the IL-4, IL-5, and IL-10 cytokines suggested a TH2-type pattern. This may explain some of the clinical and laboratory findings, such as eosinophilia, elevated IgE levels, and skin rash. However, these data have to be interpreted with caution. Plasma cytokines have short half-lives, and their primary effects occur in the lymphoid microenvironment rather than in the periphery. The production of cytokines by PBMCs after in vitro stimulation provides more relevant information. In our patient the ex vivo IL-2 secretion after stimulation was markedly reduced before the start of enzyme replacement and improved after the start of enzyme replacement. IL-2 is important for T-cell growth and activates effector T and NK cells. dAdo at higher concentrations is known to block IL-2 production and IL-2 receptor expression (17). ADA deficiency may cause the failure of a T-cell subset to produce IL-2 and the failure of the CD8 population to respond to IL-2 (11).
Neutralizing anti-ADA antibody has developed in about 10% of patients treated with PEG-ADA (7, 14, 15). In several of these cases, as in our patient, neutralizing antibody has reduced circulating PEG-ADA activity to a degree that allowed toxic metabolites to reaccumulate, abrogating immune recovery. A review of this phenomenon suggests possible predisposing factors in our patient (15). First, she was homozygous for a missense mutation, V129M, that results in an ADA protein with greatly diminished, but not absent, catalytic activity, which confers a delayed-onset phenotype (2, 3). Patients with this phenotype often manifest immune dysregulation and autoimmune phenomena, which in our patient included eosinophilia, elevated IgE levels, excessive T-cell activation, hemolytic anemia, and thyroid antibodies at the time of diagnosis. Neutralizing anti-ADA antibody has also developed in some other patients with a delayed-onset phenotype (7). Second, the appearance of anti-ADA antibody coincided with an episode of central catheter sepsis, which was also associated with the reemergence of hemolysis. The same sequence of events has occurred in another patient who developed hemolytic anemia and neutralizing antibody to PEG-ADA following central catheter sepsis (15).
In conclusion, enzyme substitution therapy with PEG-ADA in a girl with delayed-onset ADA deficiency resulted in a partial but impressive immune reconstitution in vivo, as well as recovery of lymphocyte subset numbers, reduction of T-cell activation and CD4-cell apoptosis, near normalization of plasma cytokine concentrations, and improvement in IL-2 production and lymphocyte proliferation in vitro. Unfortunately, the development of an inhibitory IgG antibody to bovine ADA resulted in a complete reversal of the immune recovery.
Acknowledgments
E. Lainka thanks Oliver Weiergraeber (Research Centre Juelich, Germany) for critical reading of the manuscript. The continuous participation of the patient and her parents is greatly acknowledged. We are sorry for the fatal outcome for this young child.
M. S. Hershfield acknowledges the support of grant DK-20902 from the National Institutes of Health and a grant from Enzon, Inc., the manufacturer of PEG-ADA. T. Niehues acknowledges the research support from Orphan Europe GmbH (Germany).
REFERENCES
- 1.Aldrich, M. D., W. Chen, M. R. Blackburn, H. Martinez-Valdez, S. K. Datta, and R. E. Kellems. 2003. Impaired germinal center maturation in adenosine deaminase deficiency. J. Immunol. 171:5562-5570. [DOI] [PubMed] [Google Scholar]
- 2.Arredondo-Vega, F. X., I. Santisteban, S. Daniels, S. Toutain, and M. S. Hershfield. 1998. Adenosine deaminase deficiency: genotype-phenotype correlations based on expressed activity of 29 mutant alleles. Am. J. Hum. Genet. 63:1049-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arrendondo-Vega, F. X., I. Santisteban, L. D. Notarangelo, J. El Dahr, R. Buckley, C. Roifman, et al. 1998. Seven novel mutations in the adenosine deaminase (ADA) gene in patients with severe and delayed onset combined immunodeficiency: G74C, V129M, G140E, R149W, Q199P, 462delG, and E337del. Mutations in brief no. 142. Hum. Mut. 11:482. [Online.] [DOI] [PubMed] [Google Scholar]
- 4.Arredondo-Vega, F. X., I. Santisteban, E. Richard, P. Bali, M. Koleilat, M. Loubser, et al. 2002. Adenosine deaminase deficiency with mosaicism for a “second-site suppressor” of a splicing mutation: decline in revertant T lymphocytes during enzyme replacement therapy. Blood 99:1005-1013. [DOI] [PubMed] [Google Scholar]
- 5.Benveniste, P., and A. Cohen. 1995. p53 expression is required for thymocyte apoptosis induced by adenosine deaminase deficiency. Proc. Natl. Acad. Sci. USA 92:8373-8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bollinger, M. E., F. X. Arredondo-Vega, I. Santisteban, K. Schwarz, M. S. Hershfield, and H. M. Lederman. 1996. Hepatic dysfunction as a complication of adenosine deaminase deficiency. N. Engl. J. Med. 334:1367-1371. [DOI] [PubMed] [Google Scholar]
- 7.Chaffee, S., A. Mary, E. R. Stiehm, D. Girault, A. Fischer, and M. S. Hershfield. 1992. IgG antibody response to polyethylene glycol-modified adenosine deaminase in patients with adenosine deaminase deficiency. J. Clin. Investig. 89:1643-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, R., L. Lowe, J. D. Wilson, E. Crowther, K. Tzeggai, J. E. Bishop, et al. 1999. Simultaneous quantification of six human cytokines in a single sample using microparticle-based flow cytometric technology. Clin. Chem. 45:1693-1694. [PubMed] [Google Scholar]
- 9.Chun, J. D., N. Lee, R. H. Kobayashi, S. Chaffee, M. S. Hershfield, and E. R. Stiehm. 1993. Suppression of an antibody to adenosine deaminase (ADA) in an ADA-deficient patient receiving polyethylene glycol modified adenosine deaminase. Ann. Allergy 70:462-466. [PubMed] [Google Scholar]
- 10.Comans-Bitter, W. M., R. de Groot, R. van den Beemd, H. J. Neijens, W. C. J. Hop, K. Groeneveld, et al. 1997. Immunophenotyping of blood lymphocytes in childhood, reference values for lymphocyte subpopulations. J. Pediatr. 130:388-393. [DOI] [PubMed] [Google Scholar]
- 11.Cowan, M. J., W. Smith, and A. J. Ammann. 1989. Interleukin 2 responsive lymphocytes in patients with adenosine deaminase deficiency. Clin. Immunol. Immunopathol. 53:59-67. [DOI] [PubMed] [Google Scholar]
- 12.Genini, D., I. Budihardjo, W. Plunkett, X. Wang, C. J. Carrera, H. B. Cottam, et al. 2000. Nucleotide requirements for the in vitro activation of the apoptosis protein-activating factor-1-mediated caspase pathway. J. Biol. Chem. 275:29-34. [DOI] [PubMed] [Google Scholar]
- 13.Giblett, E. R., J. E. Anderson, F. Cohen, B. Pollara, and H. J. Meuwissen. 1972. Adenosine deaminase deficiency in two patients with severely impaired immunity. Lancet ii:1067-1069. [DOI] [PubMed] [Google Scholar]
- 14.Hershfield, M. S. 1997. Biochemistry and immunology of poly(ethylene glycol)-modified adenosine deaminase (PEG-ADA), p. 145-154. In J. M. Harris and S Zalipsky (ed.), Poly(ethylene glycol) chemistry and biological applications. American Chemical Society, Washington, D.C.
- 15.Hershfield, M. S. 2004. Combined immune deficiencies due to purine enzyme defects, p. 480-504. In E. R. Stiehm, H. D. Ochs, and J. Winkelstein (ed.), Immunologic disorders in infants and children, 5th ed. The W. B. Saunders Co., Philadelphia, Pa.
- 16.Hershfield, M. S., R. H. Buckley, M. L. Greenberg, A. L. Melton, R. Schiff, C. Hatem, et al. 1987. Treatment of adenosine deaminase deficiency with polyethylene glycol-modified adenosine deaminase. N. Engl. J. Med. 316:589-596. [DOI] [PubMed] [Google Scholar]
- 17.Hershfield, M. S., and B. S. Mitchell. 2001. Immunodeficiency diseases caused by adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency, p. 2585-2611. In C. R. Scriver et al. (ed.), Metabolic and molecular bases of inherited disease, vol. II, 8th ed. McGraw-Hill Book Co., New York, N.Y.
- 18.Hirschhorn, R. 1993. Overview of biochemical abnormalities and molecular genetics of adenosine deaminase deficiency. Pediatr. Res. 33:S35-S41. [DOI] [PubMed] [Google Scholar]
- 19.Hirschhorn, R. 1999. Immunodeficiency disease due to deficiency of adenosine deaminase, p. 121-139. In H. D. Ochs et al. (ed.), Primary immunodeficiency diseases, a molecular and genetic approach. Oxford University Press, Oxford, United Kingdom.
- 20.Niehues, T., T. W. McCloskey, J. Ndagijimana, G. Horneff, V. Wahn, and S. Pahwa. 2001. Apoptosis in T-lymphocyte subsets in human immunodeficiency virus-infected children measured immediately ex vivo and following in vitro activation. Clin. Diagn. Lab. Immunol. 8:74-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niehues, T., J. Ndagijimana, G. Horneff, and V. Wahn. 1998. CD28 expression in pediatric human immunodeficiency virus infection. Pediatr. Res. 44:265-268. [DOI] [PubMed] [Google Scholar]
- 22.Ochs, H. D., R. H. Buckley, R. H. Kobayashi, A. L. Kobayashi, R. U. Sorensen, S. D. Douglas, et al. 1992. Antibody responses to bacteriophage φX174 in patients with adenosine deaminase deficiency. Blood 80:1163-1171. [PubMed] [Google Scholar]
- 23.Thompson, L. F., C. J. Van De Wiele, A. B. Laurent, S. W. Hooker, J. G. Vaughan, H. Jiang, et al. 2000. Metabolites from apoptotic thymocytes inhibit thymopoiesis in adenosine deaminase-deficient fetal thymic organ cultures. J. Clin. Investig. 106:1149-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe-Fukunaga, R., C. I. Brannan, N. G. Copeland, N. A. Jenkins, and S. Nagata. 1992. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 356:314-317. [DOI] [PubMed] [Google Scholar]
- 25.Weinberg, K., M. S. Hershfield, J. Bastian, D. Kohn, L. Sender, R. Parkman, et al. 1993. T lymphocyte ontogeny in adenosine deaminase-deficient severe combined immune deficiency after treatment with polyethylene glycol-modified adenosine deaminase. J. Clin. Investig. 92:596-602. [DOI] [PMC free article] [PubMed] [Google Scholar]