Abstract
Four proteomic biomarkers (human neutrophil peptide 1 [HNP1], HNP2 [defensins], calgranulin C [Cal-C], and Cal-A) characterize the fingerprint of intra-amniotic inflammation (IAI). We compared proteomic technology using surfaced-enhanced laser desorption-ionization-time of flight (SELDI-TOF) mass spectrometry to enzyme-linked immunosorbent assay (ELISA) for detection of these biomarkers. Amniocentesis was performed on 48 women enrolled in two groups: those with intact membranes (n = 27; gestational age [GA], 26.0 ± 0.8 weeks) and those with preterm premature rupture of the membranes (PPROM; n = 21; GA, 28.4 ± 0.9 weeks). Paired abdominal amniotic fluids (aAFs)-vaginal AFs (vAFs) were analyzed in PPROM women. Quantitative aspects of HNP1-3, Cal-C, Cal-A, and calprotectin (a complex of Cal-A with Cal-B) were assessed by ELISA. SELDI-TOF mass spectrometry tracings from 16/48 (33.3%) aAFs and 13/17 (88.2%) vAFs were consistent with IAI (three or four biomarkers present). IAI (by SELDI-TOF mass spectrometry) was associated with increased HNP1-3 and Cal-C measured by ELISA. However, immunoassays detected Cal-A in only 4 of the AFs even though its specific SELDI-TOF mass spectrometry peak was identified in 19/48 AFs. Calprotectin immunoreactivity was decreased in AFs retrieved from women with IAI (P = 0.01). In conclusion, IAI is associated with increased HNP1-3 levels. In the absence of isoform-specific ELISAs, mass spectrometry remains the only way to discriminate the HNP biomarker isoforms. Monomeric Cal-A is not reliably estimated by specific ELISA as it binds to Cal-B to form the calprotectin complex. Cal-C was reliably measured by SELDI-TOF mass spectrometry or specific ELISA.
Premature delivery (PTD; birth before 37 weeks in humans) is a significant public health problem. While complicating 7 to 12% of deliveries, prematurity is associated with 75% of infant mortality and 50% of long-term neurological handicaps, including blindness, deafness, developmental delay, cerebral palsy, and chronic lung disease (4, 10, 18, 22). The etiologies of most preterm births remain unknown (2, 5).
Clinically, PTD presents as either preterm labor with intact membranes or with preterm premature rupture of the membranes (PPROM). Several distinct pathophysiological pathways are implicated as triggers for PTD (21): excessive myometrial stretching, decidual hemorrhage, maternal and fetal stress, and inflammation (9). Inflammation may be initiated along various pathways of which infection is but one. Microbial invasion of the amniotic cavity, as evidenced by a positive amniotic fluid (AF) culture, occurs in 10% of the patients with preterm labor and intact membranes and in 38% of the patients with PPROM (25). We previously demonstrated that it is not necessarily the intrauterine infection that causes poor outcomes but rather the resulting intrauterine inflammation which can damage the fetus long before triggering labor (7). Thus, specific and sensitive tests for intrauterine inflammation rather than for bacterial infection may be more important in the search for targeted treatments.
Our group recently identified specific AF fingerprints for two of the four recognized pathways leading to preterm delivery (inflammation and hemorrhage) using proteomic technology (SELDI-TOF [surface-enhanced laser desorption-ionization-time-of-flight] mass spectrometry) (6, 8, 31). The relevant protein peaks of the final proteomic descriptor were extracted using a logical stepwise algorithm, which we named mass-restricted (MR) analysis. We found that four protein peaks (3,377, 3,448, 10,443, and 10,834 Da) were sufficient for the diagnosis of intra-amniotic inflammation (IAI; defined as an AF white blood cell [WBC] count of >100 cells/mm3) with 100% sensitivity and specificity (8). These biomarkers were identified as two defensins, human neutrophil peptide 1 (HNP1) and HNP2, and S100 proteins calgranulin C (Cal-C; S100A12) and Cal-A (S100A8, MRP-8), respectively. We used a categorical descriptor (the MR score) to define IAI in place of individual receiver-operator curves obtained from analyzing continuous variables. We subsequently confirmed the original findings of several studies (6, 8). However, many investigators have relied on enzyme-linked immunosorbent assays (ELISAs) for their studies and the relationships between the quantitative and qualitative aspects (presence or absence) of the protein biomarkers composing the MR score are untested. The purpose of the present investigation was to determine whether the available ELISAs can be used to identify and quantitate these biomarkers of IAI.
MATERIALS AND METHODS
Patients and samples.
AF was retrieved by transabdominal amniocentesis (abdominal AF [aAF]) from 48 women with symptoms of premature birth and either (i) intact membranes (n = 27) or (ii) PPROM (n = 21). GA was based on an early (prior to 20 weeks of GA) ultrasonographic examination. All clinical procedures were performed for clinical indications to rule out IAI and/or infection. Membrane rupture was confirmed by the direct visualization of AF “pooling” through the cervical os at speculum examination and positive “nitrazine” and “ferning” tests. An amnio-dye test was performed and confirmed a diagnosis of PPROM in four women with absent vaginal pooling. Paired aAF and vaginal AF (vAF) samples were also successfully obtained from 17 women with PPROM at the time of their admission by aspirating the fluid collected in the posterior vaginal fornix. Cultures for aerobic and anaerobic bacteria and genital mycoplasmas, Gram staining, and counting of WBC and red blood cells were performed immediately after amniocentesis as part of the clinical workup. Excess specimen was centrifuged at 700 × g and 4°C for 10 min, aliquoted, and stored at −80°C until analysis. The Institutional Boards of Yale University and Wayne State University approved this research study. Written informed consent was obtained from all participants prior to the procedure. Subjects were enrolled prospectively based on the availability of one of the investigators (C.S.B.) for consent procedures.
SELDI-TOF mass spectrometry analysis.
The SELDI-TOF mass spectrometry analysis technique has been previously detailed (8). Briefly, 5 μl of AF diluted 10-fold in phosphate-buffered saline (PBS) was placed on spots of duplicate H4 arrays (eight-spot H4 array; Ciphergen Biosystems, Fremont, CA). Some spots on some arrays were covered with PBS alone (control). After a 1-h incubation in a humidified chamber, the samples were aspirated and the spots washed individually with 25% aqueous acetonitrile solution, air dried, and covered with matrix solution (containing an energy-absorbing molecule) diluted in 0.5% trifluoroacetic acid-50% acetonitrile. The matrix consisted of either 1 μl of a 20% saturated solution of α-cyano-4-hydroxycinnamic acid (CHCA) on one of the arrays or two sequential applications of 1 μl of a 50% saturated solution of sinnapinic acid (SPA) on the other. The arrays were read in a ProteinChip Reader (model PBS IIC; Ciphergen Biosystems) using the ProteinChip Software 3.1.1.
Peaks composing the MR score were identified by their conspicuous aspect at or in proximity of their known respective masses, 3,377.0 and 3,448.1 Da on the CHCA tracing (corresponding to HNP2 and -1, respectively) and 10,443.8 and 10,834.5 Da (corresponding to Cal-C and Cal-A, respectively) on the SPA tracings as previously described (6, 8, 31). Briefly, the regions of interest were manually detected using the centroid peak detection tool built into the software. The centroid is defined as the point on the m/z (mass versus charge of the analyte) at which a line divides a peak into two parts having equal integrated intensities. After baseline subtraction, quantitative peak parameters (intensity [peak height at centroid], signal-to-noise ratio, and area under the peak) are exported to an Excel spreadsheet for manual analysis. The mass [m/z at centroid − 1] is analyzed as an attribute of peak identity. For qualitative analysis, peak presence or peak absence is estimated objectively using an algorithm that evaluates peak presence relative the signal-to-noise (S/N) ratio from the spots covered with PBS at the corresponding mass value (8). A peak is considered present and assigned arbitrarily a value of 1 if its S/N ratio exceeds the threshold of 2 × (average + standard deviation) of the S/N ratio from PBS spots at the corresponding mass. If a peak is absent on some tracings but present in others, the selection takes into calculation the relative background noise in that respective spectrum. We note that the value of the threshold varies with each type of instrument (PBS II or PBS IIC) but, once established, can be used for all subsequent analyses of tracings obtained from that type of instrument.
The MR score was derived through a nonhierarchical analysis of SELDI-TOF mass spectrometry tracings of AF from patients with and without IAI and infection using a data-mining algorithm based on the sequential application of Boolean logic criteria. An additional (nonbiomarker) peak, β2-microglobulin (β2MG), which is present in all AF samples in the 10- to 14-kDa area of the SPA SELDI-TOF mass spectrometry tracings, provides for quick orientation to the mass/charge axis of the spectra and was designated the R (reference) peak by us (6, 31). The peak corresponding to HNP3 (3,492 Da; not a biomarker for IAI) was determined on the CHCA tracings in a similar fashion as the peaks comprising the MR score.
The MR score ranges from 0 when all four protein biomarker peaks are absent to 4 when all four protein peaks are present. An MR score of 3 or 4 indicates IAI and is highly predictive of imminent preterm delivery (8). There was no overlap between the patients included in the present study and in our prior studies using this methodology (6, 8, 31).
Immunoassay procedures.
Immunoassays for human HNP1-3, calgranulin A (MRP-8, Cal-A), and calprotectin (MRP8/14) were performed according to the manufacturer's instructions (HyCult Biotechnology, Uden, The Netherlands, or Chemicon International, Temecula CA). Appropriate sample dilutions were determined in pilot experiments, and some AF samples were assayed at several dilutions (1:1 to 1:1,000) to ensure correct interpolation. ELISAs for Cal-A and calprotectin were repeated twice to ensure consistency. β2MG (R peak) was measured using a specific ELISA (ICN Pharmaceuticals, Orangeburg, NY) as a control. All assays used a sandwich format with microtiter plates coated with the capture antibody. The plates were read using a Spectramax microtiter plate reader equipped with SoftmaxPro v.311 software (Molecular Devices, Sunnyvale, CA). Intra- and interassay coefficients of variation for each assay were within the ranges reported by manufacturers. Cal-C ELISAs were performed as previously described (16, 17, 20).
Statistical analysis included normality testing using the Kolmogorov-Smirnov test and Student t tests or Mann-Whitney tests as appropriate. Relationships between variables (correlations) were explored using Pearson product moment or Spearman rank order correlations. Comparison between correlations was achieved based on the z statistic (15) as computed by MedCalc Software (Broekstraat, Belgium). Normally distributed data are presented as means and 95% confidence intervals (CIs) or standard errors. Skewed data are presented as medians and ranges. A P value of less than 0.05 was considered statistically significant.
RESULTS
Patient characteristics.
The demographic characteristics of the subjects are illustrated in Table 1. Women with PPROM were older (P = 0.018) and of higher parity (P = 0.013) and gravidity (P = 0.023). The difference in GA between groups at amniocentesis (P = 0.055) failed to achieve significance. Women with PPROM delivered earlier (P = 0.043) after shorter amniocentesis-to-delivery (latency) intervals (P < 0.001) compared to women with intact membranes at enrollment.
TABLE 1.
Patient, AF, and outcome characteristics
| Variable | All patients (n = 48) | Patients with:
|
P value | |
|---|---|---|---|---|
| Intact membranes (n = 27) | PPROM (n = 21) | |||
| Clinical characteristics at amniocentesis | ||||
| Mean age yr (95% CI) | 27.5 (25.6-29.3) | 25.5 (23.3-27.8) | 30.0 (27.2-32.8) | 0.018a |
| Mean gestational age, wk (95% CI) | 27.0 (25.7-28.3) | 26.0 (24.2-27.7) | 28.4 (26.7-30.2) | 0.057a |
| Median gravidity (range) | 2.5 (1-11) | 2 (1-9) | 4 (1-11) | 0.023b |
| Median parity (range) | 1 (0-8) | 0 (0-8) | 2 (0-7) | 0.013b |
| AF laboratory analyses | ||||
| Mean glucose concn, g/dl (95% CI) | 23.8 (19.6-28.0) | 27.7 (22.8-32.6) | 19.0 (12.2-25.8) | 0.046a |
| No. with glucose concn of <15 g/dl (%) | 15 (31%) | 4 (15) | 11 (52) | 0.129c |
| Mean WBC count, cells/mm3 (95% CI) | 15.0 (0-33,500) | 11.0 (0-1,370) | 36.0 (0-33,500) | 0.220b |
| No. with WBC count of >50 cells/mm3 (%) | 15 (31) | 5 (19) | 10 (48) | 0.058c |
| No. with WBC count of >100 cells/mm3 (%) | 12 (25) | 3 (11) | 9 (43) | 0.018c |
| No. with positive culture result (%) | 12 (25) | 3 (11) | 9 (43) | 0.185c |
| No. with positive Gram stain (%) | 7 (15) | 3 (11) | 4 (19) | 1.000c |
| Delivery and outcome measurements | ||||
| Mean gestational age at delivery, wk (95% CI) | 30.7 (28.7-32.6) | 32.4 (29.5-35.4) | 28.4 (26.5-30.2) | 0.043a |
| Median latency, days (range) | 5.5 (0-151) | 23.0 (1-151) | 1.0 (0-13) | <0.001b |
Student t test.
Mann-Whitney test.
Fisher's exact test.
The prevalence of a glucose concentration of <15g/dl for the whole study group was 31% (15/48), that of a WBC count of >100 cells/mm3 was 25% (12/48), that of a positive Gram stain result was 15% (7/48), and that of a positive culture result was 25% (12/48). The PPROM group had a lower glucose concentration compared to women with intact membranes (P = 0.046). More women with PPROM had a WBC count of >100 cells/mm3 (PPROM, 43% [9/21]; intact, 11% [3/27] [P = 0.018]). There were no significant differences in median WBC counts or the prevalence of a positive Gram stain or culture result between groups.
SELDI-TOF mass spectrometry results.
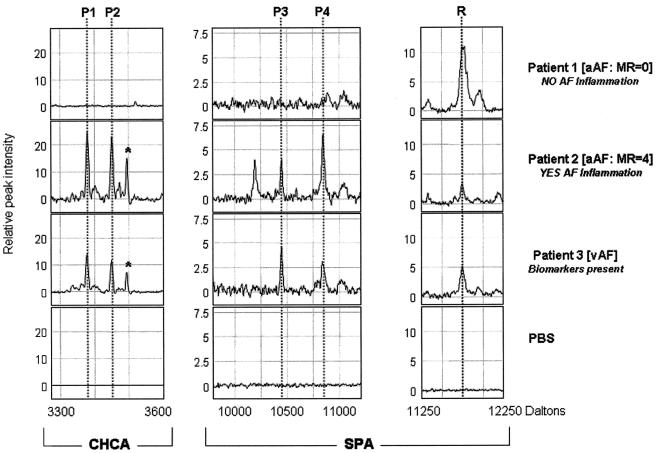
The presence of three or four of the peaks of the MR analysis score is diagnostic of IAI (8). Representative SELDI-TOF mass spectrometry tracings of AF from a patient without (MR score = 0, patient 1) and one with IAI (MR score = 4, patient 2) are shown in Fig. 1. A representative SELDI-TOF mass spectrometry profile of the vAF from a woman with PPROM is also displayed (patient 3) in Fig. 1. The peaks (P1 [HNP2], P2 [HNP1], P3 [Cal-C], and P4 [Cal-A]) corresponding to the inflammatory biomarkers of the MR score with their experimental masses are indicated in Table 2 (average and 95% CI).
FIG. 1.
Representative SELDI-TOF mass spectrometry profiles of AF retrieved from three patients undergoing transabdominal amniocentesis. Patient 1 (no AF inflammation) and patient 2 (AF inflammation) had intact membranes. A representative vAF SELDI-TOF mass spectrometry profile is displayed for patient 3 (PPROM). Dotted lines indicate the biomarker peaks composing the MR score (P1, HNP2; P2, HNP1; P3, Cal-C; P4, Cal-A) or the reference peak (R; fragment of β2MG). The x axis of the tracings represents the molecular mass in daltons; the y axis represents normalized peak intensity. The asterisk in the tracing of each of the first two patients indicates the position of the peak corresponding to HNP3.
TABLE 2.
Presence and experimental masses of the biomarker peak components of the MR score
| Biomarker | Protein identity | Calculated mass (Da)a | Observed mass, Da (95% CI) | No. of samples with peak present (%)
|
|
|---|---|---|---|---|---|
| Amniocentesis (n = 48) | Vaginal pool (n = 17) | ||||
| Individual biomarkers | |||||
| P1 | HNP-2 | 3,377.0 | 3,378.0 (3,377.7-3,378.3) | 24 (50) | 16 (94) |
| P2 | HNP-1 | 3,448.1 | 3,448.2 (3,447.6-3,448.8) | 22 (46) | 17 (100) |
| P3 | Cal-C (S100A12) | 10,443.8 | 10,446.6 (10,444.4-10,448.7) | 14 (29) | 14 (82) |
| P4 | Cal-A (S100A8) | 10,834.5 | 10,837.2 (10,832.6-10,841.9) | 16 (33) | 15 (88) |
| MR scores | |||||
| MR 0-2 (at least 2 of the P1-P4 peaks present) | 32 (66) | 2 (12) | |||
| MR 3-4 (3 or 4 of the P1-P4 peaks present) | 16 (33) | 15 (88) | |||
The calculated mass is the theoretical mass reported by the Swiss-Prot database (www.expasy.ch) of the protein without posttranslational modifications.
The MR score was consistent with IAI (3 or 4) in one-third of aAF samples (16/48) (Table 2). The proteomic profile of the vAF samples had all four biomarkers present in 88% (15/17) of patients with PPROM. As previously mentioned, we analyzed “matched” samples of aAF versus vAF in 17 women with PPROM. Only 5 women out 17 had an MR score of 3 or 4 in their aAF, yet 15 of the vAF samples showed all proteomic biomarker peaks present.
ELISA measurements for the biomarkers of the MR score.
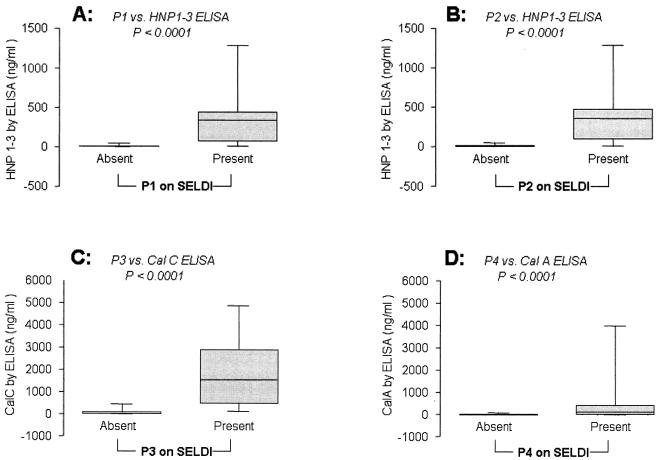
Median concentrations of the inflammatory biomarkers measured by ELISAs were compared to the MR analysis score (presence given a Boolean indicator of 1 and absence given a Boolean indicator of 0) of the respective or related peak(s) (Fig. 2).
FIG. 2.
ELISA levels of HNP1 to -3 (A and B), Cal-C (C), and monomeric Cal-A (D) in samples where the objective SELDI-TOF mass spectrometry analysis estimated as present the corresponding biomarker peaks: A, P1 (HNP2); B, P2 (HNP1); C, P3 (Cal-C); D, P4 (Cal-A). Graphs show the median, quartile (limits of the box), and range (limits of the bars). Statistical comparisons were performed using Mann-Whitney tests.
The ELISAs for HNP failed to discriminate among the three isoforms corresponding to HNP1, HNP2, and HNP3. There was higher HNP1-3 immunoreactivity in samples where P1 (HNP2) was detected by SELDI-TOF mass spectrometry (HNP1-3 median [range]: P1 absent, 8.9 [7.1 to 47.3] ng/ml; P1 present, 341.2 [14.0 to 1,285.8] ng/ml; Mann-Whitney P < 0.0001) (Fig. 2A). The findings were similar in samples where P2 (HNP1) was detected by SELDI-TOF mass spectrometry (HNP1-3 median [range]: P2 absent, 9.7 [7.1 to 47.3] ng/ml; P2 present, 356.9 [8.6 to 1,285.8] ng/ml; Mann-Whitney P < 0.0001) (Fig. 2B). Cal-C immunoreactivity was elevated in AF samples where P3 was detected by SELDI-TOF mass spectrometry (Cal-C median [range]: P3 absent, 24.2 [8.7 to 443.2] ng/ml; P3 present, 1,536.4 [122.6 to 4,866.7] ng/ml; Mann-Whitney P < 0.0001) (Fig. 2C). The specific ELISA concentration of monomeric Cal-A was also higher in samples where P4 was detected by SELDI-TOF mass spectrometry (Cal-A median [range]: P4 absent, 0.0 [0.0 to 78.6] ng/ml; P4 present, 104.8 [0.0 to 3,980.4] ng/ml; Mann-Whitney P < 0.0001) (Fig. 2D).
To determine the strength of association between the quantitative measurement obtained using ELISA and the objective presence of a peak detected by SELDI-TOF mass spectrometry, correlation coefficients were calculated for each of the peaks of the MR score (0 = peak absent, 1 = peak present) and the respective analytes measured by ELISA. The Spearman rank order correlation coefficients were as follows: P1, 0.819 (P < 0.001); P2, 0.782 (P < 0.001); P3, 0.828 (P < 0.001); P4, 0.517 (P < 0.001). The correlation was weaker for P4 (Cal-A) than for the other biomarker peaks (P4 versus P1 z statistic = 3.0 [P = 0.003]; P4 versus P2 z statistic = 2.5 [P = 0.013]; P4 versus P3 z statistic = 2.4 [P = 0.016]). In fact, in 48% (15/31) of the samples where P4 was detected by SELDI-TOF mass spectrometry, monomeric Cal-A was undetectable (i.e., below 0 standard) using ELISA.
ELISA versus SELDI-TOF mass spectrometry for assessment of neutrophil defensins as biomarkers of IAI.
The ELISAs measure immunoreactivity nondiscriminately corresponding to HNP1, -2 and -3, while mass spectrometry identifies the specific peaks for HNP1 and HNP2. Twenty-three samples obtained either by amniocentesis or from the vaginal pool were negative by SELDI-TOF mass spectrometry for all three defensin isoform peaks. Sixteen amniotic samples were positive by SELDI-TOF mass spectrometry for HNP1 and/or HNP2, while the remaining 26 samples showed SELDI-TOF mass spectrometry peaks for all three defensin isoforms. In no instance was the SELDI-TOF mass spectrometry peak corresponding to HNP3 present alone. HNP3 was present in only 62% (26/42) of the samples with at least one defensin isoform present (Fisher's exact P < 0.001).
ELISA measurements for Cal-A versus calprotectin.
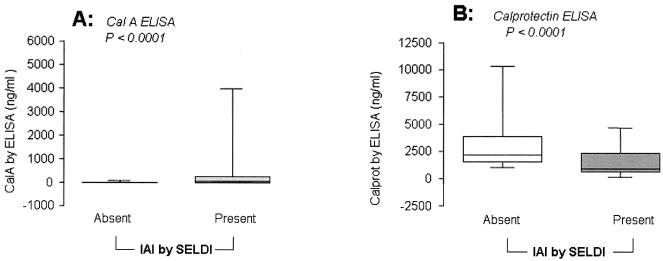
To investigate the discrepancy between the detection of the P4 biomarker (Cal-A) peak by SELDI-TOF mass spectrometry and monomeric Cal-A measured by ELISA, the levels of calprotectin, a complex formed by the combination of Cal-A with another calgranulin, Cal-B (12.4 kDa), was measured. The patients were clustered based on the presence of IAI (MR analysis score, >2). There was an inverse relationship between AF levels of monomeric Cal-A and calprotectin. Patients without IAI (MR analysis score = 0 to 2) had significantly lower median levels of Cal-A compared with patients with IAI, i.e., those with an MR analysis score of 3 or 4 (Cal-A median [range]: IAI absent, 0.0 [0.0 to 78.6] ng/ml; IAI present, 28.0 [0.0 to 3,980.4] ng/ml; Mann-Whitney P < 0.0001) (Fig. 3A). Calprotectin levels were significantly decreased in samples with IAI (calprotectin median [range]: IAI absent, 2,164.4 [1,013.4 to 1,0323.0] ng/ml; IAI present, 898.3 [158.8 to 4,661.0] ng/ml; Mann-Whitney P < 0.0001) (Fig. 3B), suggesting perhaps altered binding under conditions of inflammation. There was an inverse correlation between Cal-A and calprotectin as measured by ELISA (Pearson's r = −0.336; P = 0.022). The levels of AF calprotectin did not correlate with either the ELISA levels of HNP1-3 (Pearson's r = −0.134; P = 0.445) or Cal-C (Pearson's r = −0.042; P = 0.876). For example, for the tracings shown in Fig. 1, ELISA measured for patient 1 (MR score = 0) 0 ng/ml Cal-A and 2,203.1 ng/ml calprotectin and for patient 2 (MR analysis score = 4) 123.8 ng/ml Cal-A and 581.5 ng/ml calprotectin, while in the vAF of patient 3 levels were 89.6 ng/ml Cal-A and 644.2 ng/ml calprotectin.
FIG. 3.
ELISA levels of monomeric Cal-A (A) and calprotectin (B) in samples where the objective SELDI-TOF mass spectrometry analysis evaluated IAI as present (MR score = 3 or 4) or absent (MR score = 0 to 2). Graphs show the median, quartile (limits of the box), and range (limits of the bars). Statistical comparisons were performed using Mann-Whitney tests.
ELISA measurements for β2MG.
β2MG is present in all AF samples and corresponds to the R peak on the SPA tracings when using SELDI-TOF mass spectrometry (Fig. 1). The R peak is useful for the quick orientation to the m/z axis of the SPA tracings because of its close proximity to the P3 and P4 biomarker peaks. The ELISA for β2MG confirms there is no relationship between the R peak or β2MG and inflammation (β2MG average [95% CI]: IAI absent, 5,799.7 [4,224.8 to 10,024.5] ng/ml; IAI present, 5,160.9 [3,247.7 to 8,408.7] ng/ml; t test P = 0.554). β2MG did not correlate with either Cal-A levels (Pearson's r = −0.010; P = 0.955) or calprotectin (Pearson's r = −0.021; P = 0.931).
DISCUSSION
The novel findings of the present study include the following. (i) Immunoreactive levels of biomarkers identified using the MR score are differentially expressed in human aAF and vAF samples. (ii) Cal-A (biomarker at 10.8 kDa) cannot be reliably assessed by ELISA probably because it binds to Cal-B (13.2 kDa) to form calprotectin (not a biomarker for IAI). (iii) Cal-C can reliably be measured by specific immunoassay probably because it does not form a calprotectin-like complex. As a result, ELISAs for these biomarkers are poor investigative and clinical tools while mass spectrometry in general and SELDI-TOF mass spectrometry in particular remain the only methods to discriminate between defensin isoforms as biomarkers in the absence of ELISAs specific for defensins 1, 2, and 3.
Though basic research has made great progress dissecting the molecular mechanisms for many human diseases (14), the translation of that knowledge into clinical diagnostic and therapeutic tools remains challenging. A direct analysis of the AF is the most accurate way to detect IAI whether accompanied by infection or not (3, 32). Clinically used tests for inflammation include WBC and neutrophil counts, while intra-amniotic infection is sought by glucose concentration, Gram staining, and microbial cultures. Predictive values using ELISA for interleukin-6 and some antimicrobial peptides (defensins, calprotectin, and bacterial permeability-increasing protein) have also been examined (11, 12, 13). However, the combination of the current clinical tests to diagnose IAI have proven poorly predictive compared to the MR score as they rely on nonadditive receiver-operator curves generated based on quantitative immunoassays (12, 26). In support of this conclusion, a surrogate composite score reported by Espinosa et al. based on addition of immunoassay studies had much lower sensitivity and specificity than the 100% accuracy of the MR score (8, 12).
Biomarkers sensitive and specific for the detection of early-onset disease are critical to understanding complex true biological phenomena. These markers have characteristics that make them central to the development and application of new laboratory techniques, new clinical investigations, and drug design. It is reasonable to assume that once the identities of pertinent biomarkers are established and a sensitive antibody is available, a translational immunoassay can be used for quantification of the biomarker and application in clinical practice. However, the present investigation demonstrates that this assumption is in some scenarios flawed and illustrates the danger of using ELISAs as a sole determinant in the search for biomarkers.
We find disparity between biomarkers for IAI using mass spectrometry platforms (SELDI-TOF mass spectrometry in the present investigation) and immunological assays (ELISA). While AF samples with MR analysis score peaks also have higher median ELISA levels for that biomarker, there are clear discrepancies between the two modalities, in particular for assessment of defensins and Cal-A. Only the SELDI-TOF mass spectrometry peaks corresponding to the HNP1 and HNP2 isoforms are inflammatory biomarkers, but the available immunoassays cannot discriminate between them. Thirty-eight percent of AFs with either HNP1 or HNP2 present by SELDI-TOF mass spectrometry were missing HNP3. In almost half (48%) of the samples where the SELDI-TOF mass spectrometry biomarker Cal-A was present, the corresponding specific ELISA was unable to measure any Cal-A.
Many promising biomarkers and surrogate biomarkers never make it into clinical practice. Thus, it is critical to understand the basis for their discriminatory powers. In humans, alpha-defensins (HNPs) comprise 30 to 50% of the azurophil granule protein of neutrophils (24). Three of the human peptides, HNP1, HNP2, and HNP3, are almost identical in sequence. HNP1 and HNP3 are 30 amino acids long and differ only at the first amino acid. As a consequence of their similarity, no antibody generated to date discriminates among the three isoforms. However, only two of the isoforms (HNP1 and HNP2) are discriminative biomarkers of IAI. In the present investigation, 38% of the samples showing HNP1 and HNP2 peaks lacked HNP3. Mass spectrometry easily discriminates between isoforms differing in one amino acid and can allow independent estimation of the peaks with biomarker value.
The remaining two biomarkers belong to the large and heterogeneous family of S100 proteins, characterized by the presence of two EF hand calcium binding domains (23). Because Cal-A (S100A8) and Cal-B (S100A9) differ in molecular weight, cellular source, and function (27), one might expect differentiation by ELISA to be feasible. Granulocytes, monocytes/macrophages, neutrophils, and keratinocytes each express Cal-A and Cal-B (28). Cal-A and Cal-B combine to form a noncovalent heterodimeric complex in a calcium-dependent manner (calprotectin). Calprotectin (but not Cal-A or Cal-B in monomeric form) has antimicrobial properties (19, 29). Although the initial consensus was that the function of monomeric forms was solely associated with that of the calprotectin complex, other research supports an independent function for the monomeric forms (33). Nevertheless, we have previously demonstrated a decrease in Cal-B (S100A9) gene expression in the human myometrium during normal labor (1). We also first noted the diagnostic relevance of Cal-A in IAI associated with preterm delivery (8) and suggested that IAI resulting in preterm birth may be a chronic rather than an acute phenomenon. In the present study, we demonstrate that the quantity of the heterodimeric complex calprotectin is inversely correlated with the abundance of the monomeric form (Cal-A) and the presence of IAI as defined by an MR analysis score of 3 or 4. This strongly supports the idea that the calprotectin complex measured by ELISA cannot have diagnostic value for IAI.
Calgranulin C (Cal-C, S100A12) is a newer member of the S100 family. It shares the two EF hand domains that bind zinc in addition to calcium. We previously identified the presence of Cal-C in AF and used it as a biomarker for IAI. Cal-C expression is specifically restricted to granulocytes, and it is one of the major calcium-binding proteins in these cells (30). Cal-A, Cal-B, and Cal-C translocate simultaneously from the cytosol to cytoskeletal and membrane structures in a calcium-dependent manner. However, the fact that no evidence for direct protein-protein interactions of S100A12 with either Cal-A, Cal-B, or the heterodimer is found strongly suggests that Cal-C acts independently of Cal-A, Cal-B, and the heterodimer calprotectin in inflammatory processes during calcium-dependent signaling (30). This probably explains why in the present study Cal-A assessment by SELDI-TOF mass spectrometry analysis correlated far better than quantitation by ELISA while Cal-C was well estimated by both.
Proteomic mass spectrometry techniques such as SELDI-TOF mass spectrometry provide for the identification of novel biomarkers. Monomeric Cal-A (SELDI-TOF mass spectrometry biomarker at 10.8 kDa) is not reliably estimated by ELISA, probably since it binds in biological fluids to Cal-B (13.2 kDa) to form the calprotectin complex, which is not an IAI biomarker. Thus, at present, multiple ELISAs cannot substitute for the techniques we have described using SELDI-TOF mass spectrometry. However, the choice of customized antibodies targeted to different epitopes may in the future overcome this limitation.
Acknowledgments
This work was supported by Public Health Service grants from the National Institute of Child and Human Development (RO1 HD 047321-01 to I.A.B.) and the Heart Lung and Blood Institute (RO1 HL 49041-12 to C.P.W.).
We thank Jiro Hitomi, Department of Anatomy, Iwate Medical University School of Medicine, for assisting in this project.
REFERENCES
- 1.Aguan, K., J. A. Carvajal, L. P. Thompson, and C. P. Weiner. 2000. Application of a functional genomics approach to identify differentially expressed genes in human myometrium during pregnancy and labour. Mol. Hum. Reprod. 6:1141-1145. [DOI] [PubMed] [Google Scholar]
- 2.Ananth, C. V., D. P. Misra, K. Demissie, and J. C. Smulian. 2001. Rates of preterm delivery among black women and white women in the United States over two decades: an age-period-cohort analysis. Am. J. Epidemiol. 154:657-665. [DOI] [PubMed] [Google Scholar]
- 3.Angus, S. R., S. Y. Segel, C. D. Hsu, G. J. Locksmith, P. Clark, M. D. Sammel, G. A. Macones, J. F. Strauss III, and S. Parry. 2001. Amniotic fluid matrix metalloproteinase-8 indicates intra-amniotic infection. Am. J. Obstet. Gynecol. 185:1232-1238. [DOI] [PubMed] [Google Scholar]
- 4.Berkowitz, G. S., and E. Papiernik. 1993. Epidemiology of preterm birth. Epidemiol. Rev. 15:414-443. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein, S., R. Heimler, and P. Sasidharan. 1998. Approaching the management of the neonatal intensive care unit graduate through history and physical assessment. Pediatr. Clin. N. Am. 45:79-105. [DOI] [PubMed] [Google Scholar]
- 6.Buhimschi, I. A., C. S. Buhimschi, R. Christner, and C. P. Weiner. 2005. Proteomics technology for the accurate diagnosis of inflammation in twin pregnancies. Br. J. Obstet. Gynaecol. 112:250-255. [DOI] [PubMed] [Google Scholar]
- 7.Buhimschi, I. A., C. S. Buhimschi, and C. P. Weiner. 2003. Protective effect of N-acetylcysteine against fetal death and preterm labor induced by maternal inflammation. Am. J. Obstet. Gynecol. 188:203-208. [DOI] [PubMed] [Google Scholar]
- 8.Buhimschi, I. A., R. Christner, and C. S. Buhimschi. 2005. Proteomic biomarker analysis of amniotic fluid for identification of intra-amniotic inflammation. Br. J. Obstet. Gynaecol. 112:173-181. [DOI] [PubMed] [Google Scholar]
- 9.Buhimschi, I. A., W. B. Kramer, C. S. Buhimschi, L. P. Thompson, and C. P. Weiner. 2000. Reduction-oxidation state (REDOX) regulation of matrix-metalloprotease activity in human fetal membranes. Am. J. Obstet. Gynecol. 182:458-464. [DOI] [PubMed] [Google Scholar]
- 10.Creasy, R. K. 1993. Preterm birth prevention: where are we? Am. J. Obstet. Gynecol. 168:1223-1230. [DOI] [PubMed] [Google Scholar]
- 11.Diamond, D. L., J. R. Kimball, S. Krisanaprakornkit, T. Ganz, and B. A. Dale. 2001. Detection of beta-defensins secreted by human oral epithelial cells. J. Immunol. Methods 256:65-76. [DOI] [PubMed] [Google Scholar]
- 12.Espinoza, J., T. Chaiworapongsa, R. Romero, S. Edwin, C. Rathnasabapathy, R. Gomez, E. Bujold, N. Camacho, Y. M. Kim, S. Hassan, S. Blackwell, J. Whitty, S. Berman, M. Redman, B. H. Yoon, and Y. Sorokin. 2003. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J. Matern. Fetal Neonatal Med. 13:2-21. [DOI] [PubMed] [Google Scholar]
- 13.Heine, R. P., H. Wiesenfeld, L. Mortimer, and P. C. Greig. 1998. Amniotic fluid defensins: potential markers of subclinical intrauterine infection. Clin. Infect. Dis. 27:513-518. [DOI] [PubMed] [Google Scholar]
- 14.Hewitt, S. M., J. Dear, and R. A. Star. 2004. Discovery of protein biomarkers for renal diseases. J. Am. Soc. Nephrol. 15:1677-1689. [DOI] [PubMed] [Google Scholar]
- 15.Hinkle, D. E., W. Wiersma., and S. G. Jurs. 1988. Applied statistics for the behavioral sciences, 2nd ed. Houghton Mifflin Company, Boston, Mass.
- 16.Hitomi, J., T. Kimura, E. Kusumi, S. Nakagawa, S. Kuwabara, K. Hatakeyama, and K. Yamaguchi. 1998. Novel S100 proteins in human esophageal epithelial cells: CAAF1 expression is associated with cell growth arrest. Arch. Histol. Cytol. 61:163-178. [DOI] [PubMed] [Google Scholar]
- 17.Hitomi, J., K. Yamaguchi, Y. Kikuchi, T. Kimura, K. Maruyama, and K. Nagasaki. 1996. Related A novel calcium-binding protein in amniotic fluid, CAAF1: its molecular cloning and tissue distribution. J. Cell Sci. 109:805-815. [DOI] [PubMed] [Google Scholar]
- 18.Iams, J. D. 1995. Preterm labor. Clin. Obstet. Gynecol. 38:673-810. [Google Scholar]
- 19.Kerkhoff, C., M. Klempt, V. Kaever, and C. Sorg. 1999. The two calcium-binding proteins, S100A8 and S100A9, are involved in the metabolism of arachidonic acid in human neutrophils. J. Biol. Chem. 274:32672-32679. [DOI] [PubMed] [Google Scholar]
- 20.Kosaki, A., T. Hasegawa, T. Kimura, K. Iida, J. Hitomi, H. Matsubara, Y. Mori, M. Okigaki, N. Toyoda, H. Masaki, M. Inoue-Shibata, M. Nishikawa, and T. Iwasaka. 2004. Increased plasma S100A12 (EN-RAGE) levels in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 89:5423-5428. [DOI] [PubMed] [Google Scholar]
- 21.Lockwood, C. J., and E. Kuczynski. 1999. Markers of risk for preterm delivery. J. Perinat. Med. 27:5-20. [DOI] [PubMed] [Google Scholar]
- 22.Nelson, K. B., and J. K. Grether. 1999. Causes of cerebral palsy. Curr. Opin. Pediatr. 11:487-491. [DOI] [PubMed] [Google Scholar]
- 23.Odink, K., N. Cerletti, J. Bruggen, R. G. Clerc, L. Tarcsay, G. Zwadlo, G. Gerhards, R. Schlegel, and C. Sorg. 1987. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature 330:80-82. [DOI] [PubMed] [Google Scholar]
- 24.Rice, W. G., T. Ganz, J. M. Kinkade, Jr., M. E. Selsted, R. I. Lehrer, and R. T. Parmley. 1987. Defensin rich dense granules of human neutrophils. Blood 70:757-765. [PubMed] [Google Scholar]
- 25.Romero, R., C. Avila, C. A. Brekus, and M. Mazor. 1990. The role of systemic and intrauterine infection in preterm parturition, p. 319-354. In R. E. Garfield (ed.), Uterine contractility. Serono Symposia, USA, Inc., Norwell, Mass.
- 26.Romero, R., B. H. Yoon, M. Mazor, R. Gomez, M. P. Diamond, J. S. Kenney, M. Ramirez, P. L. Fidel, Y. Sorokin, and D. Cotton. 1993. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and Gram stain in patients with preterm labor and intact membranes. Am. J. Obstet. Gynecol. 169:805-816. [DOI] [PubMed] [Google Scholar]
- 27.Roth, J., M. Goebeler, C. van den Bos, and C. Sorg. 1993. Expression of calcium-binding proteins MRP8 and MRP14 is associated with distinct monocytic differentiation pathways in HL-60 cells. Biochem. Biophys. Res. Commun. 191:565-570. [DOI] [PubMed] [Google Scholar]
- 28.Sorg, C. 1992. The calcium binding proteins MRP8 and MRP14 in acute and chronic inflammation. Behring Inst. Mitt. 91:126-137. [PubMed] [Google Scholar]
- 29.Steinbakk, M., C. F. Naess-Andresen, E. Lingaas, I. Dale, P. Brandtzaeg, and M. K. Fagerhol. 1990. Antimicrobial actions of calcium binding leucocyte L1 protein, calprotectin. Lancet 336:763-765. [DOI] [PubMed] [Google Scholar]
- 30.Vogl, T., C. Propper, M. Hartmann, A. Strey, K. Strupat, C. van den Bos, C. Sorg, and J. Roth. 1999. S100A12 is expressed exclusively by granulocytes and acts independently from MRP8 and MRP14. J. Biol. Chem. 274:25291-25296. [DOI] [PubMed] [Google Scholar]
- 31.Weiner, C. P., K.-Y. Lee, C. S. Buhimschi, R. Cristner, and I. A. Buhimschi. 2005. Proteomic biomarkers predictive of the success of rescue cerclage. Am. J. Obstet. Gynecol. 192:710-718. [DOI] [PubMed]
- 32.Yoon, B. H., R. Romero, C. J. Kim, J. K. Jun, R. Gomez, J. H. Choi, and H. C. Syn. 1995. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am. J. Obstet. Gynecol. 172:960-970. [DOI] [PubMed] [Google Scholar]
- 33.Zwadlo, G., J. Bruggen, G. Gerhards, R. Schlegel, and C. Sorg. 1988. Two calcium-binding proteins associated with specific stages of myeloid cell differentiation are expressed by subsets of macrophages in inflammatory tissues. Clin. Exp. Immunol. 72:510-515. [PMC free article] [PubMed] [Google Scholar]