Abstract
Leucine-rich alpha 2 glycoprotein (LRG) is one of the serum biomarkers for disease activity of ulcerative colitis (UC). We focused on the correlation between the changes of LRG and the changes of endoscopic and histologic activity of UC, in comparison to the changes of fecal calprotectin (Fcal), fecal immunochemical test (FIT), and C-reactive protein (CRP). Seventy-nine patients with two or more colonoscopies were enrolled, and 123 paired colonoscopies and 121 paired biopsies were examined. With regard to the change of endoscopic/histologic activity between the preceding and subsequent colonoscopy, there was improvement (n = 29/45), unchanging (n = 63/36), and worsening (n = 31/40). The correlations between the changes of marker levels and endoscopic/histologic activity were Fcal; r = 0.50/0.39 and FIT; r = 0.41/0.40, LRG; r = 0.42/0.40 and CRP; r = 0.22/0.17. Furthermore, when the correlation between the changes of LRG levels and the changes of endoscopic/histological activity was compared with those of other markers, the correlation of LRG tended to be superior to those of CRP (CRP vs. LRG; p = 0.08/0.01). LRG is equivalent to fecal markers and superior to CRP, when inferring changes in disease activity of UC based on changes in its level.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-89615-8.
Keywords: Ulcerative colitis, Leucine-rich alpha 2 glycoprotein, Biomarker
Subject terms: Predictive markers, Prognostic markers, Colonoscopy, Gastrointestinal diseases
Introduction
Ulcerative colitis (UC) is a chronic, refractory inflammatory bowel disease (IBD) of unknown cause and can be diagnosed based on clinical evaluation and a combination of hematological, endoscopic, histological, or imaging-based investigations1. Clinical remission has been the primary target of treatment; however, endoscopic remission, which means that endoscopy shows no mucosal inflammation, has recently been recognized as more appropriate2. For this purpose, endoscopic evaluation would be desirable to accurately evaluate disease activity. However, the significant physical and economic burdens make it difficult to perform frequent endoscopic examinations. As an alternative, serum and/or stool markers, such as serum C-reactive protein (CRP), fecal calprotectin (Fcal) and fecal immunochemical test (FIT), have been studied as inflammatory markers for inferring the disease activity3–5. Although stool testing is useful, compliance is hampered in some patients6–8. In this point, blood tests are simpler examinations for all patients than fecal tests. However, because serum CRP levels are not always informative in patients with UC9, the monitoring of serum CRP is possible to evaluate disease activity of UC patients with moderate to severe active phase, but it is difficult to judge mild active phase or mucosal healing. Therefore, there is a strong need for new reliable serum markers for inferring the disease activity of UC.
Serum leucine-rich alpha 2 glycoprotein (LRG) is a substance obtained by proteomic screening from patients with rheumatoid arthritis (RA)10. Unlike CRP, LRG is induced not only by IL-6 but also by other proinflammatory cytokines10,11, and is expressed not only in liver but also in local inflammatory sites, produced by neutrophils, macrophages, hepatocytes, and intestinal epithelial cell12–14. Indeed, serum LRG was reported to be more strongly correlated with the disease activity of RA in comparison to serum CRP15.
In the case of IBD, the serum LRG levels are higher in patients with active-phase UC15. Therefore, serum LRG can be a good candidate as a novel serum marker for evaluating the disease activity of UC16–18. However, there are some reports that the correlation between LRG values and endoscopic/histologic activity was better than CRP, but inferior to fecal markers such as Fcal and FIT16,17. On the other hand, two prospective studies have reported that systematic measurement of biomarkers from the start of induction therapy correlates with treatment efficacy and can predict future endoscopic improvemen18,19, and that LRG levels after induction therapy can predict earlier endoscopic improvement than Fcal levels19. In contrast, the best way to use this marker to evaluate the disease activity of UC during the chronic course with or without treatment intervention in the clinical setting has not been determined.
In some biomarkers, because the baseline levels may differ among individuals, focusing on the changes in the levels during the course of chronic diseases may be more useful than focusing on the absolute value at one analysis point. In fact, the changes in the level of Fcal more precisely reflect the changes in endoscopic activity than those in FIT; this was revealed by focusing on the changes in values during the disease course20. Although previous report had shown that the changes of LRG were strongly associated with the changes of clinical activity scores21, an objective assessment using endoscopic or histological examination is still lacking. Therefore, in this study, we focused on the changes in biomarker levels between paired colonoscopy tests during the management of patients with UC, to determine how these biomarkers, including the serum LRG level, can be better used.
Methods
Patients
Since November 2015, we have continuously asked patients with UC who were scheduled for colonoscopy at Okayama University Hospital to provide fecal and serum samples at the same time as the colonoscopy for the purpose of analyzing correlations between biomarkers and endoscopic/histologic activity. Building on our previous study20, this study aimed to analyze the correlation between changes in colonic mucosal activity and changes in fecal and serum marker levels between two colonoscopies. Therefore, patients who underwent two or more colonoscopies and provided samples between November 2015 and November 2021 were included. In the patients who underwent more than three colonoscopies during the study period, data between two consequent colonoscopy tests were compared.
The exclusion criteria were insufficient stool collection or the failure to achieve full endoscopic observation of the patients’ lesions. Patients with other diseases that could affect the serum levels of LRG and CRP (i.e., extraintestinal complications, infectious disease, collagen disease, primary biliary cholangitis, heart failure, or malignancy) at the time of endoscopy were excluded.
Assessment of the endoscopic disease activity
Colonic mucosal activity was assessed by endoscopic disease activity and pathologic activity of tissue obtained at the time of colonoscopy. All patients in this study received bowel preparation with a polyethylene glycol-based or magnesium citrate-based electrolyte solution for colonoscopy according to the standard protocol in our hospital. According to the results of colonoscopy, disease activity was evaluated by assigning the Mayo Endoscopic Subscore (MES) to the sites with the most severe mucosal inflammation and Ulcerative Colitis Endoscopic Index of Severity (UCEIS). The MES was evaluated using a four-point scale (0–3). An MES 0–1 was defined as endoscopic improvement, while MES 2–3 was defined as endoscopic active-phase disease22,23. Change from endoscopic improvement (MES 0–1) to endoscopic active-phase disease (MES 2–3) was defined as endoscopic relapse. The UCEIS was determined based on three components: bleeding, ulcers/erosions, and mucosal pattern. Each component was scored four-point scale (0–3), resulting in a total score ranging from 0 to 824. Without referring to the medical records and results of the fecal and serum markers at the time of colonoscopy, the MES and UCEIS was assessed by two independent endoscopy specialists.
Pathologic scoring
Pathological activity was scored by gastrointestinal pathologists using the Geboes score25. The Geboes score is classified into 6 grades from grade 0 to grade 5. Histological remission is defined as Geboes score < 2.1. The biopsy specimen used for the evaluation of histopathological activity was obtained from the site with maximum endoscopic activity, while for remission cases, a biopsy specimen from the rectum was analyzed. When one or more biopsy specimens were evaluated in each patient, the highest score was used for the analyses.
Serum sampling and analyses
Blood samples were collected from all patients for the determination of the serum LRG and CRP levels on the day of endoscopy. Serum samples for LRG measurement were stored at -80 °C until use. LRG was measured in bulk later at our institution using a NANOPIA LRG kit (SEKISUI MEDICAL, Tokyo, Japan)16. Serum CRP was measured at our institution using a NANOPIA CRP kit (SEKISUI MEDICAL, Tokyo, Japan) on the day of colonoscopy. The measurement range of this high-sensitivity kit was from 0.01 mg/dL to 42 mg/dL.
Fecal sampling and analyses
Patients were requested to prepare two fecal samples within 2 days before colonoscopy for the examination of calprotectin and FIT. Fecal samples collected by the patients were stored at − 30 °C until shipment to the BML (Tokyo, Japan), where the level of calprotectin in the fecal specimen (Fcal) was measured with a fluorescence enzyme immunoassay using Phadia EliA Calprotectin 2 (Thermo Fisher Scientific, Phadia AB, Uppsala, Sweden). The FIT was performed at our institution using an OC-Sensor DIANA or PLEDIA system (Eiken Chemical, Tokyo, Japan) with stool samples collected with a Hemodia sampling probe (Eiken Chemical)16. Because the FIT is not accurate for measuring hemoglobin concentrations of < 50 ng/mL, the specimens with a hemoglobin concentration within this range (0–50 ng/mL) were all treated as 50 ng/mL in this study. Although different stool containers were used, the measurements for FIT and Fcal were conducted using same stool samples in principle.
Statistical analyses
The characteristics of patients were described with numbers and percentages for categorical variables, indicating the mean standard deviation or median (range) for continuous variables according to the distribution. Spearman’s rank correlation test was used for determining the correlations between the levels of serum/fecal markers and the MES or Geboes scores. The difference between the correlation coefficients was analyzed using Fisher’s z-transformation. Receiver operating characteristic (ROC) analyses were used to assess the cut-off values of serum/fecal markers between the endoscopic status of MES 0–1 and MES 2–3 and the histological status of Geboes score < 2.1 and Geboes score ≥ 2.1. The results were expressed as the area under the curve (AUC), with sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy with 95% confidence intervals. Two-sided p values of < 0.05 were considered to indicate statistical significance.
Ethical considerations
This study was approved by the Institutional Review Board of Okayama University Graduate School of Medicine (IRB number: 1904-035) and conducted in accordance with the Declaration of Helsinki. All research was performed in accordance with the relevant guidelines/regulations. Informed consent was obtained from each patient and/or their legal guardians.
Results
Clinical characteristics of the patients
Five hundred sixty-three patients with UC were underwent during the study period. 229 patients were registered and 352 scheduled total colonoscopies were performed during the observation period. Seventy-nine of the registered patients underwent colonoscopy tests more than once and provided fecal/serum samples (Supplemental Fig. 1). The characteristics of the 79 enrolled patients, the colonoscopy findings, and the values of biomarkers are summarized in Table 1 (Table 1). Forty male and thirty-nine female patients with a median (interquartile range [IQR]) age of 48.0 years (37.5–57.6 years) were included in the study. The median (IQR) age at the onset of the disease was 36.4 years (25.3–45.2 years old), and the median (IQR) disease duration was 9.8 years (3.8–16.8). There were 52 cases of extensive colitis (65.8%), 22 cases of left-sided colitis (27.8%), 4 cases of proctitis (5.1%), and 1 case of right-sided colitis (1.3%). For the analysis of the changes of endoscopic activity and biomarkers, the maximum MES for the colorectum, as determined based on the precedent findings of 123 paired colonoscopic, was as follows: MES 0, n = 51 (41.4%); MES 1, n = 35 (28.5%); MES 2, n = 28 (22.8%); and MES 3, n = 9 (7.3%). The interval between the two consecutive colonoscopy tests that were used for the analyses of data was 15.4 months (11.7–25.6 months).
Table 1.
Characteristics of the enrolled patients, colonoscopy findings, and biomarker values.
| Patients | n = 79 |
|---|---|
| Sex, n (%): male / female | 40 (50.6)/ 39 (49.4) |
|
Median (IQR) age at precedent colonoscopy, years Median (IQR) age at the onset of the disease, years |
48.0 (37.5–57.6) 36.4 (25.3–45.2) |
| Median (IQR) disease duration at precedent colonoscopy, years | 9.8 (3.8–16.8) |
| Disease location, n (%) | |
| Extensive/ Left-sided/ Proctitis/ Right-sided | 52 (65.8)/22 (27.8)/4 (5.1)/1 (1.3) |
| Analyzed colonoscopic pairs, 1/ 2/ 3/ 4 | 51/ 17/ 6/ 5 |
| Colonoscopies (paired) | n = 123 |
|---|---|
| Activity assessment at the precedent colonoscopy | |
| Mayo endoscopic subscore, n (%): 0/ 1/ 2/ 3 | 51 (41.4)/35 (28.5)/28 (22.8)/9 (7.3) |
|
Ulcerative Colitis Endoscopic Index of Severity, n (%): 0/1/2/3/4/5/6/7/8 |
55 (44.7)/21 (17.1)/26 (21.1)/7 (5.7)/ 7 (5.7)/2 (1.6)/4 (3.3)/1 (0.8) |
| *Geboes score, n (%): 0/1/2/3/4/5 |
17 (14.0)/38 (31.4)/12 (9.9)/ 16 (13.2)/17 (14.0)/21 (17.5) |
| Values of biomarkers, median (IQR) at the precedent colonoscopy | |
| Fecal calprotectin, µg/g | 119 (31–447) |
| Fecal immunochemical test, ng/mL | 50 (50–315) |
| Leucine-rich alpha 2 glycoprotein, µg/mL | 12.6 (10.1–14.9) |
| C-reactive protein, mg/dL | 0.08 (0.04–0.19) |
|
Median (IQR) interval between the precedent and subsequent colonoscopy, months |
15.4 (11.7–25.6) |
IQR, inter quartile range.
*Two colonoscopies, no biopsies were taken.
The treatments of the 79 patients are shown in Table 2 (Table 2). Of 123 paired cases, medication was changed between the precedent and subsequent colonoscopic examinations in 48 pairs (39.0%). Immunosuppressive medication was introduced for the 14 pairs (11.4%) and interrupted in 19 pairs (15.4%) (Supplemental Table 1). The intervals between the preceding and the subsequent endoscopy and the timing of clinical events (the discontinuation of medication, the initiation of therapy, and clinical relapse) were shown in supplemental Fig. 2.
Table 2.
Medication at the precedent colonoscopy.
| Concomitant medication at the precedent colonoscopy, n (%) | |
|---|---|
| 5-aminosalicylic acid, oral agent / suppository | 67 (84.8) / 17 (21.5) |
| Steroid, oral agent / suppository | 7 (8.9) / 3 (3.8) |
| Immunomodulator | 35 (44.3) |
| Anti-tumor necrosis factor-α agents | 9 (11.4) |
| Vedolizumab | 3 (3.8) |
| Janus kinase inhibitor | 1 (1.3) |
| Tacrolimus | 4 (5.1) |
| Indigo naturalis | 5 (6.3) |
Correlation between the changes in the MES and biomarker levels
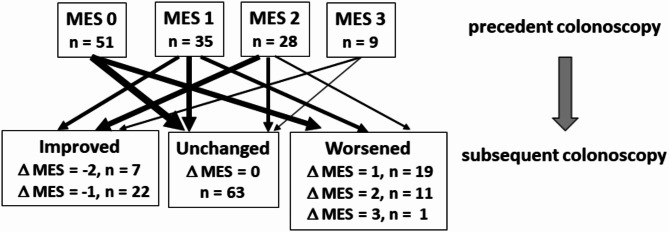
The changes in the endoscopic activity scores in 123 intervals from the consecutive colonoscopic examination are shown in Fig. 1. During the intervals of the two consecutive examinations, 29 showed improved MES values, 63 showed unchanged MES values, and 31 showed worsened MES values (Fig. 1).
Fig. 1.
Flowchart for analyses of comparison between the changes in fecal/serum marker levels and the change in MES. MES Mayo Endoscopic Subscore. The numbers of the cases with each endoscopic activity level (MES 0–3) at the precedent colonoscopy test and the numbers of cases with improved, unchanged, and worsened activity in the subsequent colonoscopy tests are indicated.
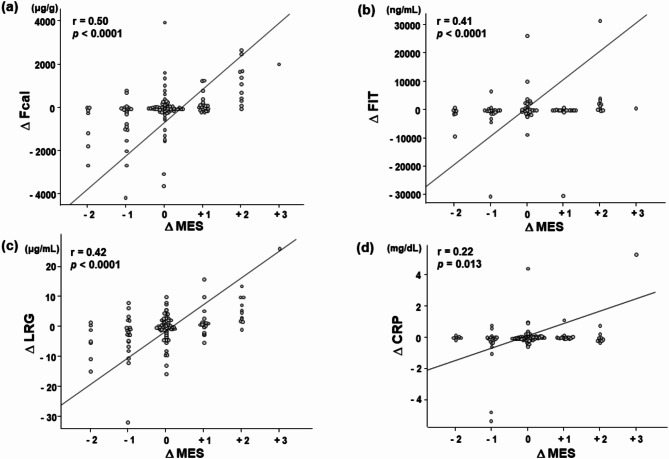
The changes of the fecal and serum marker levels in the improved, unchanged, and worsened groups, respectively, were as follows: Fcal. -167 (-1054–0) µg/g, 0 (0–64.5) µg/g, 69 (0–766.5) µg/g; FIT, -150.4 (-911 - -13.7) ng/mL, -10.9 (-112.6–64.0) ng/mL, 158.9 (-20.0–1132) ng/mL; LRG, -2.1 (-5.1 - -0.11) µg/mL, -0.08 (-1.4–2.2) µg/mL, 1.5 (0.28–5.4) µg/mL; and CRP, -0.035 (-0.18–0.038) mg/dL, 0.005 (-0.038–0.07) mg/dL, 0.01 (-0.02–0.07) mg/dL. Spearman’s rank correlations between the changes of MES and the changes of fecal/serum marker levels were as follows; Fcal, r = 0.50, p < 0.0001; FIT, r = 0.41, p < 0.0001; LRG, r = 0.42, p < 0.0001; and CRP, r = 0.22, p = 0.013 (Fig. 2). The difference in correlation coefficients with LRG was compared with Fcal, FIT, and CRP, resulting in the following p-values: Fcal, p = 0.43; FIT, p = 0.93; CRP, p = 0.08, respectively. These results suggested that the changes in LRG were significantly more strongly correlated with the changes in endoscopic activity than the changes in CRP levels. In addition, the cut-off values of change of each biomarker for endoscopic relapse were examined based on 86 cases of MES 0–1 at the first colonoscopy. Of these, 66 cases remained MES 0–1, while 20 cases showed relapse (MES 2–3) at the second colonoscopy. The cut-off values for fecal and serum markers were shown the supplemental material (Supplemental Table 2). On the other hands, the cut-off values of change of each biomarker for endoscopic improvement from MES 2–3 to MES 0–1 were also examined based on 37 cases of MES 2–3 at the first colonoscopy. As these biomarkers decreased significantly when MES 3 improved to MES 2, it was difficult to predict changes from MES 2–3 to MES 0–1 using the change of each biomarker values. (Data not shown).
Fig. 2.
Correlation between the changes in fecal/serum marker levels and the change in MES. Correlation between the change in MES and the changes in Fcal (a), FIT (b), serum LRG (c), and serum CRP (d) levels. MES, Mayo Endoscopic Subscore; Fcal, fecal calprotectin; FIT, fecal immunochemical test; LRG, leucine-rich alpha 2 glycoprotein; CRP, C-reactive protein. Each Δ represents the amount of change. The line indicates the correlation coefficient between the changes of fecal/serum marker levels and the maximum MES obtained by Spearman’s rank correlation. The correlation values and P-values are shown in the upper left corner.
We also analyzed the correlation between the changes in the UCEIS and biomarker levels. Spearman’s rank correlations between the changes of UCEIS and the changes of fecal/serum marker levels were similar to the analysis results for changes of MES (supplemental Fig. 3).
Correlation between the changes in Geboes score and the changes in biomarker levels
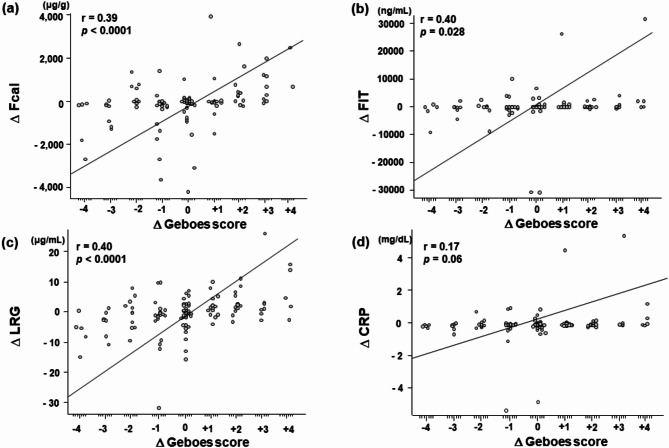
Because no biopsy specimens were obtained in 2 colonoscopy tests, 121 paired cases were analyzed pathologically. Among 121 cases, 45 paired cases showed improved Geboes scores (ΔGeboes score: -4, n = 5; -3, n = 7; -2, n = 11; -1, n = 22), 36 showed unchanged scores: (ΔGeboes score 0), and 40 showed worsened Geboes scores (ΔGeboes score: 1, n = 13; 2, n = 13; 3, n = 9; 4, n = 5).
The changes in the fecal/serum markers of the improved, unchanged, and worsened groups, respectively, were as follows: Fcal, -99 (-249–1.0) µg/g, -27 (-148–26) µg/g, 114 (3.0–784) µg/g; FIT, 0 (-828–0) ng/mL, 0 (0–15) ng/mL, 28 (0–785) ng/mL; LRG, -1.0 (-5.0–0.23) µg/mL, -0.11 (-1.93–2.16) µg/mL, 1.45 (0.08–4.53) µg/mL; and CRP, -0.01 (-0.08–0.04) mg/dL, 0 (-0.09–0.06) mg/dL, 0.02 (-0.02–0.09) mg/dL.
Spearman’s rank correlations between the changes in the Geboes score and the changes in fecal/serum marker levels were as follows: Fcal, r = 0.39, p < 0.0001; FIT, r = 0.40, p = 0.028; LRG, r = 0.40, p < 0.0001; CRP, r = 0.17, p = 0.06 (Fig. 3). The difference in correlation coefficients with LRG was compared with Fcal, FIT, and CRP, resulting in the following p-values: Fcal, p = 0.93; FIT, p = 1.00; CRP, p = 0.01, respectively. These results suggested that the changes in LRG were similarly correlated with the fecal markers and significantly more strongly correlated with the changes in histological activity than those in CRP levels.
Fig. 3.
Correlation between the changes in fecal/serum marker levels and the change in the maximum Geboes score. Correlation between the change in the maximum Geboes score and the changes in Fcal (a), FIT (b), serum LRG (c), and serum CRP (d) levels. Fcal, fecal calprotectin; FIT, fecal immunochemical test; LRG, leucine-rich alpha 2 glycoprotein; CRP, C-reactive protein. Each Δ represents the amount of change. The line indicates the correlation coefficient between the changes of fecal/serum marker levels and maximum Geboes score obtained by Spearman’s rank correlation. The correlation values and P-values are shown in the upper left corner.
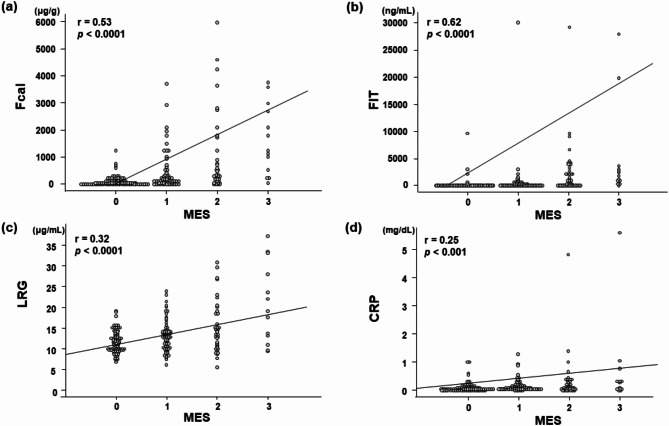
Correlation between the MES and the fecal/serum marker levels at 202 time points
The correlation between the MES and each biomarker level at 202 time points in 123 paired cases is shown in Fig. 4. One hundred fifty-two (75.2%) of 202 colonoscopies showed no or mild endoscopic activity (MES 0 or 1). Spearman’s rank correlations between the MES and the fecal/serum marker levels were as follows: Fcal, r = 0.53, p < 0.0001; FIT, r = 0.62, p < 0.0001; LRG, r = 0.32, p < 0.0001; and CRP, r = 0.25, p < 0.001. The detectability of endoscopic improvement (MES 0–1) in the ROC analysis is also shown (Supplemental Table 3). The cut-off values of LRG for endoscopic improvement (MES 0–1) was 14.8 µg/mL. These results suggested that fecal marker levels were more strongly correlated with the endoscopic activity than the serum markers at a single time point of measurement and had higher diagnostic rates, especially in determining endoscopic improvement.
Fig. 4.
Correlation between the fecal/serum marker levels and maximum MES. Correlation between the MES and the levels of Fcal (a), FIT (b), serum LRG (c), and serum CRP (d) at each analysis point. MES, Mayo Endoscopic Subscore; Fcal, fecal calprotectin; FIT, fecal immunochemical test; LRG, leucine-rich alpha 2 glycoprotein; CRP, C-reactive protein. The line indicates the correlation coefficient between the fecal/serum marker levels and maximum MES obtained by Spearman’s rank correlation. The correlation values and P-values are shown in the upper left corner.
Correlation between the Geboes score and the fecal/serum marker levels at 202 time points
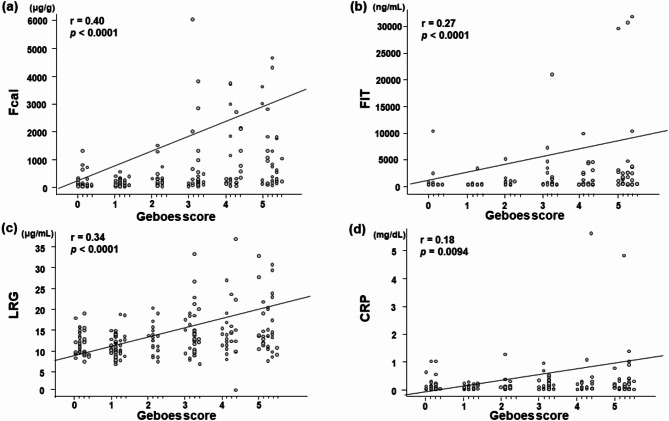
The correlations between the Geboes score and each biomarker level at 202 time points in 123 paired cases is shown in Fig. 5. Spearman’s rank correlations between the Geboes score and the fecal/serum marker levels were as follows: Fcal, r = 0.40, p < 0.0001; FIT, r = 0.27, p < 0.0001; LRG, r = 0.34, p < 0.0001; and CRP, r = 0.18, p = 0.0094. The detectability of histological remission (Geboes score < 2.1) in the ROC analysis of each biomarker is shown in Supplemental Table 4. The cut-off value of LRG for histological remission (Geboes score < 2.1) was 12.6 µg/mL. These results suggested that LRG was more strongly correlated with the histological activity than CRP.
Fig. 5.
Correlation between the fecal/serum marker levels and maximum Geboes score. Correlation between the maximum Geboes score and the levels of Fcal (a), FIT (b), serum LRG (c), and serum CRP (d). Fcal, fecal calprotectin; FIT, fecal immunochemical test; LRG, leucine-rich alpha 2 glycoprotein; CRP, C-reactive protein. The line indicates the correlation coefficient between the fecal/serum marker levels and the maximum Geboes score obtained by Spearman’s rank correlation. The correlation values and P-values are shown in the upper left corner.
Discussion
Our study showed that the changes in serum LRG between two consecutive colonoscopy tests were significantly correlated with the changes in the endoscopic and histologic activity, when presently available serum biomarkers are not sufficiently effective for monitoring the disease activity. To our knowledge, our results are unique in real-world clinical practice, in the point of view that comparing the changes in the serum LRG/CRP levels and the Fcal/FIT levels with the changes in the endoscopic and histologic scores in the same UC patient. LRG was not different from the fecal marker regarding the correlation with the changes in the endoscopic and histological activity. On the other hands, LRG was shown to have a stronger correlation with the changes in the endoscopic activity than CRP, and a significantly stronger correlation with the changes in the histologic activity. These findings confirmed that LRG was a highly useful serum marker.
Previous reports have already determined the correlation between serum LRG and the disease activity of UC using single-point analyses16,17. In addition, the comparison of the biomarkers including LRG with findings of multiple endoscopies has already been reported and discussed in a previous well-conceived prospective report18. In contrast to the previous studies, our report has the following advantages. First, most of our patients were in remission or mild disease in outpatient settings that enabled validation of usefulness of LRG in real clinical practice with not so large changes in disease activity. Second, all analyzed biomarkers including FIT, which was clinically useful but was not examined in the previous reports, were compared to the endoscopic and histologic findings. The results that the changes in the serum LRG had a strong correlation in with the trend in UC activity, similarly to fecal markers, are meaningful for daily clinical practice.
Based on the above results, we propose the following usage of each biomarker in clinical practice related to UC. In cases with high activity (MES2-3) during the induction period, CRP has been reported to be more strongly correlated with the MES in comparison to fecal markers26,27. In addition, Karashima et al. reported that in patients who showed endoscopic improvement within 1 year, the absolute value of LRG at 1 week after treatment initiation was significantly lower than in patients without endoscopic improvement, whereas no significant difference in the calprotectin values was observed until 8 weeks after treatment initiation. The heterogeneity in fecal samples in cases of diarrhea due to excessive disease activity may make measurements inaccurate. In these situations, however, LRG could be useful because CRP are likely to be negative during remission induction despite residual endoscopic activity.
In cases with low disease activity (MES 1), such as those with mild inflammation without clinical symptoms, serum CRP levels are often negative and are not useful9. On the other hand, Fcal and FIT may be significantly correlated with the change from low disease activity to improvement (MES 0–1)20,27,28. Based on the present study, the examination of serum LRG may also be useful for these cases. Especially when fecal markers do not show any significant decrease despite appropriate therapeutic intervention, the combined use of the changes in serum LRG with fecal markers may be useful because fecal markers yield false positive results due to the use of non-steroidal anti-inflammatory drugs, the presence of hemorrhoids, inflammatory polyps, colonic diverticulum, the infection with bacteria or viruses, the diurnal variation, or menstruation in women29–34. In determining endoscopic remission (MES 0) and assessing the maintenance of MES 0, fecal markers may be more useful than serum LRG4,5. However, once fecal markers become elevated with or without symptom relapse, the examination of changes in serum LRG may be useful for assessing the changes in disease activity.
The present study was associated with several limitations. First, since this was a single-center study, confirmation by multi-center studies is desirable. Second, with regard to patient selection, the inclusion was limited to patients who could undergo the total colonoscopy 2 or more times, bring stool samples or have disease activity safety to perform the bowel preparation. Therefore, the population was biased toward patients with relatively low inflammation (MES 0–1). Third, the intervals of colonoscopy and biomarker examinations were not fixed and different among individuals. Fourth, all cases with fecal hemoglobin concentrations of < 50 ng/mL were treated as 50 ng/mL in this study. More accurate data may be necessary for patients with hemoglobin concentrations of < 50 ng/mL.
In conclusion, the examination of the changes in serum LRG may be more useful and accurate than serum CRP for assessing the changes of UC disease activity, and shows similar clinical significance to the examination of fecal markers. Measurement of serum LRG can be performed by simple blood collection. Hence, LRG may have utility in the management of the chronic course of UC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Mr. Masanori Furukawa for their efforts in measuring serum LRG samples. We are also thankful to Ms. Mayumi Tokumitsu for her invaluable help in data input.
Abbreviations
- UC
Ulcerative colitis
- IBD
Inflammatory bowel disease
- CRP
C-reactive protein
- Fcal
fecal calprotectin
- FIT
Fecal immunochemical test
- LRG
Leucine-rich alpha 2 glycoprotein
- RA
Rheumatoid arthritis
- MES
Mayo endoscopic subscore
- ROC
Receiver Operating Characteristic
- AUC
Area under the curve
- PPV
Positive predictive value
- NPV
Negative predictive value
- IQR
Interquartile range
Author contributions
Conceptualization: S.H.; Methodology: Y.A., S.H.; Formal analysis and investigation: Y. A., S.H., Y.Y.; Writing - original and revised draft preparation: Y.A., S.H.; Writing - review and editing: Y.A., S.H., T.I., T.T., K.Takei., S.I., K.Takeuchi., M.T., J.T., H.K.; Resources: Y. A., E.Y.; Supervision: H.O., J.K., M.O. All authors have read and approved the final version to be published.
Funding
This work was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology, Japan (#20K12669 to S.H. and #22H02828 to M.O.).
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author and it is provided within the manuscript or supplementary information files.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mowat, C. et al. Guidelines for the management of inflammatory bowel disease in adults. Gut60, 571–607 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Frøslie, K. F., Jahnsen, J., Moum, B. A. & Vatn, M. H. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology133, 412–422 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Yoon, J. Y. et al. Correlations of creactive protein levels and erythrocyte sedimentation rates with endoscopic activity indices in patients with ulcerative colitis. Dig. Dis. Sci.59, 829–837 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Nakarai, A. et al. Evaluation of mucosal healing of ulcerative colitis by a quantitative fecal immunochemical test. Am. J. Gastroenterol.108, 83–89 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Takashima, S. et al. Evaluation of mucosal healing in ulcerative colitis by fecal calprotectin vs. fecal immunochemical test. Am. J. Gastroenterol.110, 873–880 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Tan, W. S., Tang, C. L. & Koo, W. H. Opportunistic screening for colorectal neoplasia in Singapore using faecal immunochemical occult blood test. Singap. Med. J.54, 220–223 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Wong, M. C. et al. Prospective cohort study of compliance with faecal immunochemical tests for colorectal cancer screening in Hong Kong. Prev. Med.57, 227–231 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Lehmann, F. S. et al. Clinical and histopathological correlations of fecal calprotectin release in colorectal carcinoma. World J. Gastroenterol.20, 4994–4999 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabay, C. & Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N Engl. J. Med.340, 448–454 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Naka, T. & Fujimoto, M. LRG is a novel inflammatory marker clinically useful for the evaluation of disease activity in rheumatoid arthritis and inflammatory bowel disease. Immunol. Med.41, 62–67 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Camilli, C., Hoeh, A. E., De Rossi, G. D., Moss, S. E. & Greenwood, J. LRG1: an emerging player in disease pathogenesis. J. Biomed. Sci.29, 6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Donnell, L. C., Druhan, L. J. & Avalos, B. R. Molecular characterization and expression analysis of leucine-rich alpha2-glycoprotein, a novel marker of granulocytic differentiation. J. Leukoc. Biol.72, 478–485 (2002). [PubMed] [Google Scholar]
- 13.Shirai, R., Hirano, F., Ohkura, N., Ikeda, K. & Inoue, S. Up-regulation of the expression of leucine-rich alpha2-glycoprotein in hepatocytes by the mediators of acute-phase response. Biochem. Biophys. Res. Commun.382, 776–779 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serada, S. et al. Serum leu cine-rich alpha-2 glycoprotein is a disease activity biomarker in ulcerative colitis. Inflamm. Bowel Dis.18, 2169–2179 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Serada, S. et al. iTRAQ-based proteomic identification of leucine-rich alpha-2 glycoprotein as a novel inflammatory biomarker in autoimmune diseases. Ann. Rheum. Dis.69, 770–774 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Yasutomi, E. et al. Leucine-rich alpha-2 glycoprotein as a marker of mucosal healing in inflammatory bowel disease. Sci. Rep.11, 11086 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimoyama, T., Yamamoto, T., Yoshiyama, S., Nishikawa, R. & Umegae, S. Leucine-Rich Alpha-2 glycoprotein is a Reliable serum biomarker for evaluating clinical and endoscopic Disease Activity in Inflammatory Bowel Disease. Inflamm. Bowel Dis.29, 1399–1408 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Shinzaki, S. et al. Leucine-rich alpha-2 glycoprotein is a potential biomarker to monitor disease activity in inflammatory bowel disease receiving adalimumab: PLANET study. J. Gastroenterol.56, 560–569 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karashima, R. et al. Early change in serum leucine-rich α-2-glycoprotein predicts clinical and endoscopic response in ulcerative colitis. Intest Res.22, 473–483 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiraoka, S. et al. Fecal immunochemical test and fecal calprotectin results show different profiles in Disease Monitoring for Ulcerative Colitis. Gut Liver. 15, 142–148 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tatsumi, Y. et al. Biomarkers for monitoring of changes in Disease Activity in Ulcerative Colitis. J. Clin. Med.12, 7165 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroeder, K. W., Tremaine, W. J. & Ilstrup, D. M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl. J. Med.317, 1625–1629 (1987). [DOI] [PubMed] [Google Scholar]
- 23.Ma, C. et al. Modeling endoscopic improvement after induction treatment with mesalamine in patients with mild-to-moderate Ulcerative Colitis. Clin. Gastroenterol. Hepatol.20, 447–454 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Travis, S. P. et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: the Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut61, 535–542 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magro, F. et al. Histologic Features of Colon Biopsies (Geboes score) Associated with Progression of Ulcerative Colitis for the First 36 months after Biopsy. Clin. Gastroenterol. Hepatol.12, 2567–2576 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Yoshimura, T. et al. Evaluation of Serum Leucine-Rich Alpha-2 Glycoprotein as a New Inflammatory Biomarker of Inflammatory Bowel Disease. Mediators Inflamm. 8825374 (2021). (2021). [DOI] [PMC free article] [PubMed]
- 27.Ishida, N. et al. C-reactive protein is superior to fecal biomarkers for evaluating colon-wide active inflammation in ulcerative colitis. Sci. Rep.11, 12431 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonoyama, H. et al. Capabilities of fecal calprotectin and blood biomarkers as surrogate endoscopic markers according to ulcerative colitis disease type. J. Clin. Biochem. Nutr.64, 265–270 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lasson, A. et al. The intraindividual variability of faecal calprotectin: a prospective study in patients with active ulcerative colitis. J. Crohns Colitis9, 26–32 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Husebye, E., Ton, H. & Johne, B. Biological variability of fecal calprotectin in patients referred for colonoscopy without colonic inflammation or neoplasm. Am. J. Gastroenterol.96, 2683–2687 (2001). [DOI] [PubMed] [Google Scholar]
- 31.Calafat, M. et al. High within-day variability of fecal calprotectin levels in patients with active ulcerative colitis: what is the best timing for stool sampling? Inflamm. Bowel Dis.21, 1072–1076 (2015). [DOI] [PubMed] [Google Scholar]
- 32.van Rheenen, P. F., Van de Vijver, E. & Fidler, V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ341, c3369 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fagerberg, U. L., Lööf, L., Merzoug, R. D., Hansson, L. O. & Finkel, Y. Fecal calprotectin levels in healthy children studied with an improved assay. J. Pediatr. Gastroenterol. Nutr.37, 468–472 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Van Turenhout, S. T. et al. Hemorrhoids detected at colonoscopy: an infrequent cause of false-positive fecal immunochemical test results. Gastrointest. Endosc. 76, 136–143 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author and it is provided within the manuscript or supplementary information files.