Abstract
A monkeypox outbreak occurred in the United States in 2003. Patient's sera were sent to the Centers for Disease Control and Prevention as a part of outbreak response measures. Clinical and epidemiologic information was abstracted from the case investigation forms. Serum samples from patients were tested by using an immunoglobulin M (IgM)-capture and an IgG enzyme-linked immunosorbent assay ELISA against Orthopoxvirus antigen. The detection of antiviral IgG and IgM antibodies and the kinetics of the antiviral IgG and IgM antibody responses were evaluated. Patients were classified as confirmed, probable, or suspect cases or were excluded as cases based on laboratory test results and epidemiologic and clinical criteria. A total of 37 confirmed case patients with monkeypox were identified, and 116 patients were excluded as case patients based on molecular testing or insufficient epidemiology and clinical data to warrant classification as a suspect or probable case. Of 37 confirmed case patients, 36 had a known history (presence or absence) of smallpox vaccination. Of those, 29 of the 36 either had or developed an IgG response, while 34 of the 36 developed an IgM response, regardless of vaccination status. Serum collected ≥5 days for IgM detection or serum collected ≥8 days after rash onset for IgG detection was most efficient for the detection of monkeypox virus infection. IgM ELISA detects recent infection with orthopoxviruses and, in this case, recent infection with monkeypox virus. In addition, analysis of paired sera for IgG and IgM detected seroconversion, another indicator of recent infection. The ELISA results correlated with the virologic PCR and viral culture results, indicating its diagnostic capabilities for monkeypox and potentially other orthopoxvirus infections due to zoonotic transmission or bioterrorism events.
In 2003, a zoonotic outbreak of human monkeypox occurred in North America in association with infected prairie dogs (18, 7). The outbreak was the first time that this virus has caused disease in humans outside of Africa. Discovered in west and central Africa in 1970 as a smallpox-like zoonotic viral infection, monkeypox virus causes sporadic illness in human populations. The laboratory or clinical diagnosis of monkeypox in Africa has been difficult due to a lack of resources and coincidental community circulation of other agents, such as varicella-zoster virus (8).
During the 2003 outbreak in the United States, the methods of laboratory evaluation of individuals suspected of having monkeypox included PCR assays, electron microscopy (EM), immunohistochemistry, culture of material derived from rash specimens, and serologic testing for orthopoxvirus (OPXV)-specific antibodies. PCR testing is virus specific, discriminates between different OPXV species, and provides direct evidence of acute viral presence (14, 17). In contrast, serological testing has been used to evaluate exposure and immunity to OPXV but lacks a practical capacity to reliably differentiate between OPXV species (6, 2, 3, 4, 5, 1, 8). Although species-specific serologic assays for monkeypox have been described, they are technically complex and appear to lack reproducibility (9, 13, 12, 16, 15). The diagnostic uses of serological tests have therefore been limited to the use of immunoglobulin G (IgG) detection in the context of an epidemiologically defined outbreak (i.e., monkeypox), in regions of endemicity, and more recently, for vaccine efficacy testing. Assays used include enzyme-linked immunosorbent assay (ELISA); plaque reduction neutralization testing; and hemagglutination inhibition, complement fixation, and Western blot assays (10, 8, 6, 2, 3, 4, 5, 1, 13, 12, 16, 15). While these techniques are useful for population surveys and vaccine studies, they remain limited for determination of virus species and diagnosis of acute infection. In particular, the measure of acute-phase immunity by IgM assays has not been presented, nor has there been an analysis of acute-phase humoral responses during a human outbreak of OPXV infection.
In vaccinated populations, the use of IgG serology for diagnostic purposes can be problematic due to the longevity of IgG responses and subsequent cross-reactivity with other OPXVs (20). IgG-based serology for the diagnosis of recent infection relies on the testing of multiple samples from a patient to determine a rise or a fall in antibody levels as an indication of recent exposure. The development of an OPXV-specific IgM assay allows the detection of recent exposure (by “natural” infection or vaccination) to OPXV by measurement of the IgM class of antibody, which is indicative of acute-phase immune induction. In addition, serology provides an advantage over PCR in diagnostic capacity due to the stability and duration of antibody responses. PCR and traditional diagnostic testing typically require a rash tissue or biopsy sample for viral detection, while antibody detection allows a broader window for sample collection beyond the rash stage of illness, which may be critical in demonstrating disease presence retrospectively or from remote locales. The use of ELISA for the detection of OPXV-specific IgG and IgM provided diagnostic support for evaluation of the OPXV infections during the 2003 U.S. outbreak. This report also provides, for the first time, characterization of the acute-phase humoral response to an orthopoxvirus following natural infection by IgM and IgG ELISAs. A description of the kinetics of humoral immunity along with evidence of the diagnostic performance and utility of ELISA is described here.
MATERIALS AND METHODS
Serum samples.
Patient specimens, including lesion material and acute- and convalescent-phase sera, were sent to the Centers for Disease Control and Prevention (CDC) as a part of outbreak control and surveillance measures. Samples were obtained at various times during the patients' courses of illnesses; efforts were made to obtain two serum samples 4 to 6 weeks apart. Clinical and epidemiologic information was obtained by health care personnel or field investigators at the time of sample collection. Serum samples from suspect cases were tested by use of an IgM-capture ELISA and an IgG ELISA to detect OPXV-specific antibodies. The kinetics of the IgG and IgM antibody responses were evaluated. The diagnostic utility of the IgM assay was determined by comparison to those of molecular and culture-based methods. Molecular analyses were performed by PCR and have been described previously, along with the culture, EM, and immunohistochemistry techniques (1). A total of 37 serum samples were obtained from laboratory-confirmed cases. Of these, 36 patients had a defined history of smallpox vaccination status. This population of 36 confirmed case patients was used to evaluate the efficacies of the IgG and IgM ELISAs for their diagnostic potentials. Also used for this evaluation were sera from the 116 patients with a defined history of smallpox vaccination who had been excluded as case patients (http://www.cdc.gov/ncidod/monkeypox/casedefinition.htm). The sera from these 116 non-case patients were used to validate the efficacy of the assays by determining the false-positive rates for both IgG and IgM detection.
IgG ELISA.
For the IgG ELISA, microtiter plates (Immulon II) were coated with 100 μl of vaccinia virus (purified) at 1.2 × 105 PFU/well in carbonate buffer overnight at 4°C. The plates were then blocked for 30 min at room temperature with assay diluent (phosphate-buffered saline [PBS] plus 0.05% Tween 20 [PBST], 5% skim milk, 2% bovine serum albumin [BSA], and 2% goat serum), followed by washing three times with PBST. Patient serum samples were then added at a 1:100 dilution and incubated for 1 h at 37°C (a 1:100 dilution of serum was found to be optimal for single-dilution assays). The plates were washed, and goat anti-human IgG-horseradish peroxidase conjugate (Kirkegaard & Perry Laboratories [KPL], Gaithersburg, MD) was added at a 1:2,000 dilution for 1 h at 37°C. The plates were washed, tetramethylbenzidine one-component substrate was added, and development was allowed to proceed for 5 to 15 min. The plate reactions were stopped by addition of stop solution (KPL), and the optical densities (ODs) were read at 450 nm on an optical density reader (Molecular Devices Corporation, Sunnyvale, CA). The values reported represent the average for duplicate wells of each sample. Known positive and negative sera from smallpox vaccine recipients were used as assay controls. On each day that the assays were performed, cutoff values (COVs) for the ELISA were determined based on the mean plus 3 standard deviations for five negative control serum samples. Subsequent normalization was performed on a daily basis by subtracting the COV from the OD to provide the value for analysis, indicated as the OD minus the COV (OD − COV). By using this normalization, any resulting value above zero is positive. Receiver operating characteristic (ROC) plots were used to define the sensitivity and the specificity of the ELISA (11, 21). The ROC plot is a graph of sensitivity (probability of true positivity) versus 1 − specificity (probability of false positivity). Based on known positive values (laboratory-confirmed cases) and known negative values (laboratory-confirmed noncases), an ROC plot displays the percentages of sensitivity and specificity (y axis) at specified normalized COVs (OD − COVs), represented on the x axis. An OD COV of zero represents an assay result where the OD equals the COV. By this method, the sensitivity and the specificity of the ELISA may be optimized based on the efficiency of differentiating between positive and negative sample groups at various COVs.
IgM ELISA.
For the IgM ELISA, microtiter plates (Immulon II) were coated with 100 μl of a 1:800 dilution of goat anti-human IgM (KPL) diluted in PBS (pH 7.4) and were incubated for 1 h at 37°C. The plates were then washed five times with PBST (PBS plus 0.1% Tween 20) and blocked for 30 min at room temperature with assay diluent solution (PBST, 0.5% gelatin, 2% BSA, 5% skim milk, 2% normal goat serum). The plates were washed, and patient serum samples were added at a 1:50 dilution in assay diluent (a 1:50 dilution of serum was found to be optimal for single-dilution assays). The patient samples were incubated on the plates for 1 h at 37°C, followed by washing. Antigen (purified vaccinia virus; Wyeth, Madison, NJ) was then added at a concentration of 6.2 × 105 PFU/well (in diluent), and the plate was incubated for 1 h at 37°C. The plates were washed, and a 1:250 dilution of an anti-variola virus hyperimmune mouse polyclonal ascitic fluid (HMAF) was added for 1 h at 37°C (during assay development, HMAF was found to be superior to anti-vaccinia virus antibodies either produced at CDC or purchased commercially). The plates were washed, and a 1:6,000 dilution of goat anti-mouse IgG-horseradish peroxidase conjugate (KPL) was added at 37°C for 30 min. The plates were washed, and the tetramethylbenzidine one-component substrate was added for 5 to 20 min of development (KPL). The reactions were stopped by addition of stop solution (KPL), and the ODs were read at 450 nm on an optical density reader. The values reported represent the average for duplicate wells of each sample. Positive and negative control sera were used as assay controls. COV normalization (OD − COV) and ROC analysis were performed as described above for the IgG ELISA.
RESULTS
Kinetics of antibody response in confirmed cases.
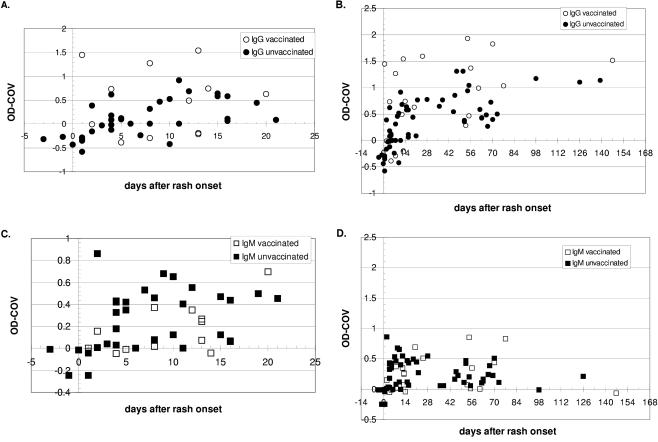
Cases confirmed by virologic identification of monkeypox virus in rash tissue specimens were used to define the temporal distributions of antibody responses in relation to the onset of clinical illness as a fever or a rash as well as vaccination history (Fig. 1). The antibody responses observed are described in reference to the date of rash onset reported for each confirmed case patient. The kinetics of the antibody responses after rash or fever onset are similar in profile, with a slightly longer interval between fever onset and initiation of the antibody response (fever occurs 2 to 3 days prior to rash onset). Analysis of the IgG and IgM profiles in the 36 confirmed cases was performed. Detection of both IgG and IgM reactivity within the first week after rash onset was observed in both vaccinated and nonvaccinated cases (Fig. 1). Positive IgG titers appeared as early as day 1 and day 2 after rash onset in vaccinated and unvaccinated cases, respectively (Fig. 1A). The apparent trend in OPXV IgG rise from days 0 to 56 after rash onset appeared to be of greater magnitude in the vaccinated population; otherwise, the overall IgG responses were similar in the two populations (Fig. 1B). Detectable IgG was observed out to day 147 and day 139 in the vaccinated and the unvaccinated case groups, respectively (Fig. 1B). Positive IgM titers were observed as early as day 2 after rash onset in both vaccinated and unvaccinated cases (Fig. 1C). In contrast to the IgG response, the trend of the OPXV IgM rise from day 0 to day 14 appears to be a more rapid onset in unvaccinated cases than in those who were vaccinated. Positive IgM titers were observed out to day 126 in unvaccinated cases, while vaccinated cases had detectable titers out to day 77 (Fig. 1D). Of particular interest is that IgM responses were observed in previously vaccinated cases with a profile similar to that observed in nonvaccinated cases (Fig. 1C and D), indicating the presence of an acute-phase induction against monkeypox virus even in cases previously vaccinated against smallpox virus (vaccinia virus) (see Discussion).
FIG. 1.
Kinetics of anti-OPXV IgG and IgM responses in confirmed cases with or without a history of smallpox vaccination after rash onset. Results are presented as OD COVs, where values above zero are considered positive. (A) Kinetics of IgG antibody responses in vaccinated and unvaccinated cases within the first 21 days after rash onset; (B) kinetics of IgG antibody responses for all time points in vaccinated and unvaccinated cases; (C) kinetics of IgM antibody responses in vaccinated and unvaccinated cases within the first 21 days after rash onset; (D) kinetics of IgM antibody responses for all time points in vaccinated and unvaccinated cases.
Qualitative IgG and IgM results for confirmed cases.
Of 36 confirmed cases, 29 (80.5%) were positive for IgG and 34 (94.5%) were positive for IgM at one or more time points when they were sampled during or after their illness (Table 1). Of the seven case patients for whom positive IgG titers were not recorded, all were sampled early (or at an unknown time) during the course of disease; sera from three of these seven case patients were obtained ≤3 days after rash onset, sera from two patients were obtained 8 days after rash onset, and the date of rash onset was unknown for two patients. Unexpectedly, one of these seven patients had a previous history of smallpox vaccination; the majority were “orthopoxvirus naive” and were sampled only once, early in the disease course. The two negative IgM results occurred in previously vaccinated individuals (both were IgG positive), with blood drawn at day 1 and day 147 after rash onset in one patient and day 4 after rash onset in the other (Table 1).
TABLE 1.
Serology results for 36 patients with confirmed cases of monkeypox infection from whom sera were collected
Three of seven cases were sampled on day 0, 2, or 3 after rash onset; two of seven were sampled on day 8 after rash onset; and two of seven cases had an unknown rash onset date.
The two cases were sampled on day 1 and day 147 and on day 4 after rash onset, respectively.
A total of 23 cases had paired serum samples collected during the outbreak. Of these 23, 15 had detectable antibody seroconversion. Of these 15, IgM seroconversion (from IgM negative to IgM positive) was detected in 8, while IgG seroconversion (from IgG negative to IgG positive) was detected in 13. Seroconversion for both IgM and IgG was detected in 6 of these 15 cases. Of five previously vaccinated cases with detectable seroconversion, one had detectable conversion to IgM alone, three had detectable seroconversion to IgG alone, and one had seroconversion to both IgM and IgG. Seroconversion from IgM positive in a day 38 sample after rash onset to IgM negative in a day 98 sample was observed in one case, indicating a loss of IgM titer. The exact or estimated day of seroconversion relative to the time of rash onset could not be determined since outbreak sample collection after rash or illness onset was not standardized and the time intervals were not similar in all cases. However, in two unvaccinated cases, seroconversion for both IgG and IgM was observed as early as day 4.
Qualitative IgG and IgM results in noncases.
Of 116 individuals who were tested during the outbreak but who were ultimately excluded as cases, 77 (67%) tested negative for IgG (Table 2). Of these, 7 of 77 (11%) individuals testing negative for IgG had previously been vaccinated and represent individuals with waning immunity from childhood vaccination. Of the 39 (34%) who tested positive for IgG, 31 had a history of smallpox vaccination, suggesting the presence of residual long-term antibodies; 6 of the remaining 8 were born before the cessation of smallpox vaccinations in the United States, and for these individuals, previous vaccination cannot be discounted. The remaining two were young enough never to have received the routine childhood vaccination and may represent either persons with background serological cross-reactivity or true cases for which sample collection during the outbreak was insufficient to confirm monkeypox (see Discussion).
TABLE 2.
Serology results for 116 noncases from whom sera were collected
Of the 39 cases, 31 had a history of smallpox vaccination (see Results).
Twenty-three of 25 of these positive results were considered equivocal, as determined by analysis of the IgM results for the confirmed cases (see Discussion), and 2 of 25 may have been true cases for whom sufficient sample collection was lacking for confirmation based on clinical and epidemiologic data.
Absolute IgM results (OD − COVs) indicated that 25 of 116 (22%) noncases tested positive for IgM. However, 23 of 25 of these positive results were considered “equivocal,” as determined by analysis of the IgM results for the confirmed cases (see below), and 2 of 25 may have been true cases who lacked sufficient sample collection for confirmation based on clinical and epidemiologic data (Table 2).
Sensitivity (and specificity) of IgG test.
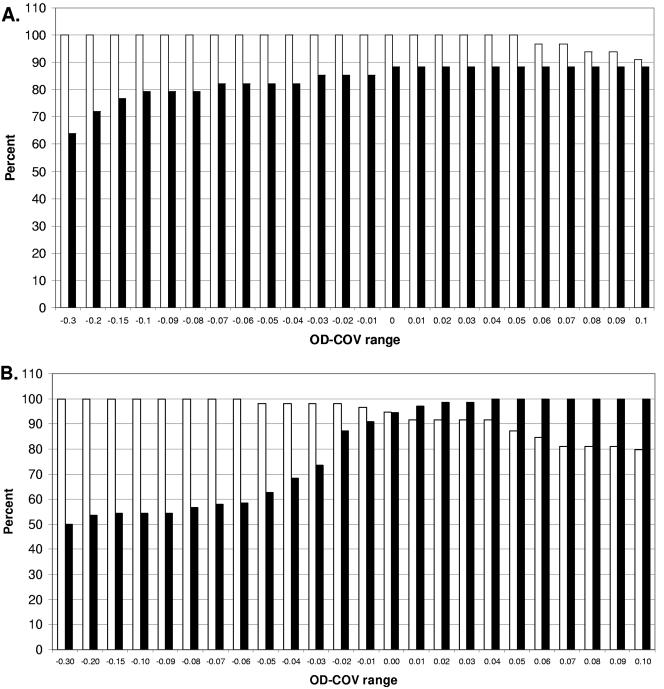
The efficiencies of the serology assays for the diagnosis of monkeypox was tested by determining the sensitivity and the specificity of the ELISA for the diagnosis of confirmed cases. ROC plots were used to define the assays' ability to discriminate between confirmed cases and noncases whose samples were submitted for testing during the outbreak (see Materials and Methods). To determine the sensitivity and the specificity of the IgG ELISA, samples sufficient for this purpose were selected based on elimination of residual vaccination-related IgG and the kinetics of the IgG response in nonvaccinated cases (Fig. 2A). By this strategy, confirmed cases and noncases who were never vaccinated and from whom serum samples were collected at least 14 days after rash onset were selected. The resulting data set included 30 serum samples from 18 confirmed cases and 23 serum samples from 19 noncases. Analysis of the ROC plot indicates that for the IgG ELISA, a sensitivity of 100% and a specificity of 88.5% were achieved by use of the designated OD − COV of 0.0 (Fig. 2A).
FIG. 2.
ROC plots of serology ELISA data for confirmed cases (positives) and noncases (negatives) with serum drawn ≥14 days after rash onset for IgG and >4 days to ≤78 days after rash onset for IgM. The x axis and the y axis represent the sensitivity (□) and the specificity (▪) of the ELISA, respectively. (A) ROC plot for IgG ELISA; (B) ROC plot for IgM ELISA.
Sensitivity (and specificity) of IgM ELISA.
Based on the kinetics of the IgM ELISA results for confirmed cases, only those with serum samples collected ≥4 days and <77 days after rash onset were included. This time frame was determined to be optimal for sufficient sampling based on the observed results and biological plausibility. Of the two confirmed cases who tested negative for IgM, one had serum drawn at days 1 and 147 after rash onset, while the other had a single serum sample collection at day 4 after rash onset (Table 1). Because serum collection occurred outside the optimal range for test accuracy, these cases were excluded from sensitivity and specificity testing. Likewise, noncases for inclusion in this analysis were subjected to the same standards. The resulting data set included 55 serum samples collected from 25 of the original 36 confirmed cases and 60 serum samples collected from 49 of the original 116 noncases. Analysis of the ROC plot indicates that for the IgM ELISA, a sensitivity of 94.8% and a specificity of 94.5% were achieved by use of the designated OD − COV of 0.0 (Fig. 2B).
To further define the efficacy of the IgM assay, analysis of 25 noncase samples that tested positive for IgM was performed. Equivocal assay results were observed for 23 of 25 noncase samples that tested positive for IgM. In an effort to define an equivocal result by using confirmed cases and noncases as comparison groups, the ROC plots of the normalized responses (OD − COVs) were used (Fig. 2B). The OD − COV in only 2 of 25 noncases positive for IgM was greater than 0.04. The ranges of the sensitivities and specificities were 94.8% and 94.5%, respectively, at an OD − COV of 0.0 to 92% and 100%, respectively, at an OD − COV of 0.04. Therefore, if a range of OD − COVs of 0.0 to 0.04 is considered equivocal, then all confirmed cases whose samples were collected between day 5 and day 77 are positive (nonequivocal) for IgM, with all but 2 noncase serum samples of 116 noncase serum samples testing either negative or equivocal for IgM. By using this range for equivocal results, the IgM ELISA provides 100% specificity while maintaining at least 92% sensitivity (Fig. 2B).
DISCUSSION
Characterization of humoral responses to orthopoxvirus infection has been limited primarily to the detection of IgG antibody through complement fixation, hemagglutination inhibition, agar precipitation, and enzyme-linked immunosorbent assays. More recent advances in ELISA allow more reliable and sensitive methods of detection of antibody against OPXV. Typically, confirmation of orthopoxvirus infections requires clinical evaluation of symptoms, followed by laboratory support for definitive virus identification through isolation and culture, detection of the virus by electron microscopy of lesion material, and molecular detection of viral nucleic acid by PCR. Serologic testing for IgG is used primarily to provide evidence of virus exposure following illness or vaccination and has a limited diagnostic ability due to uncertainties regarding the duration of the immune responses to previous orthopoxvirus exposures or vaccination. Likewise, serological testing has been useful to help define the range of permissive hosts for (specific) viruses among animal species through specific antibody detection as a marker for infection.
During the 2003 outbreak of monkeypox in the United States, multiple laboratory assays were used for diagnosis. In addition to PCR, serology was used for laboratory support of case confirmation. IgG ELISA was helpful for detection of exposure for a longer period after rash onset but was useful as a “stand-alone” only for individuals who had not previously been vaccinated against smallpox (vaccinia virus is the only other OPXV recognized to contact humans in the United States). In contrast, the IgM-capture ELISA was used to define the acute-phase humoral response and to provide evidence of recent exposure to and infection with monkeypox virus in an appropriate epidemiologic context. In addition, the IgM-capture ELISA was successfully used (with slight modifications) to test cerebrospinal fluid for the identification of monkeypox virus-induced viral encephalitis in a confirmed case during this outbreak (19). The validity of the assay is demonstrated by the correlation and the sensitivity of the results to those of confirmatory PCR and culture testing during this outbreak.
The IgM ELISA provided diagnostic identification for 94.5% of the PCR-confirmed cases (Table 1) and, along with IgG ELISA, allowed characterization of the acute-phase response following infection (Fig. 1). Use of PCR-confirmed cases as a positive subset of samples from the outbreak allows determination of the efficiencies of the tests. Tests such as viral culture, EM, or PCR testing are limited to the phase of illness that includes lesions containing virus or viral DNA. In contrast, serology provides a broader “window” of time to detect evidence of infection.
However, as with the sampling collection timing issues for culture, EM, and PCR, serology may have limitations as well. For detection of IgM, it is generally thought that early-phase serum collection is required, but the time immediately following infection may in fact precede immune induction. In this analysis we have found that sample collection less than 4 days after rash onset is sometimes problematic for IgM detection and that sample collection less than 14 days after rash onset is often problematic for IgG detection in unvaccinated individuals (Fig. 1). The utilization of paired serum specimens provided exceptional utility in interpretation of the assay, since seroconversion or changes in antibody levels could be measured. In fact, seroconversion was observed in 15 of the confirmed cases (with paired sera) by using analysis of negative IgM or IgG results to positivity or conversion from an IgM isotype to IgG. However, during an outbreak investigation, the use of paired serum specimens may not always be feasible, and evaluation of the serology for a single sample results requires a higher degree of scrutiny. During this outbreak, sampling between 5 and 77 days after rash onset allowed a 94.8% sensitivity and a 94.5% specificity for the IgM ELISA (Fig. 2B), and collections from day 14 after rash onset allowed a 100% sensitivity and a 88.5% specificity for the IgG ELISA (Fig. 2A).
Of particular interest is the detection of the IgM responses in individuals who were vaccinated against smallpox as children. The evidence of OPXV-specific IgM responses in previously vaccinated cases provides support for the potential use of this test to detect recent exposure to heterologous OPXV (non-vaccinia virus) as a surveillance tool for orthopoxvirus disease emergence or bioterrorism events involving such viruses, even in a previously vaccinated (vaccinia virus) population. Whether this observation is common in secondary exposures or vaccinations (vaccinia virus) or is specifically due to a heterologous virus challenge (naturally occurring) remains to be fully determined. However, detection of IgM responses in secondary or booster smallpox (vaccinia virus) vaccine recipients appears to be rare (CDC, unpublished data).
An advantage to serologic tests is that, unlike PCR, serologic tests are not limited to short targets within the genome that may be manipulated to avoid detection. Detection of antibody responses may be maintained even in the event of the use of a recombinant virus for intentional release due to the polyclonal nature of the immune response and the vast amount of homology among orthopoxviruses. Characterization of the acute-phase humoral responses and the use of the IgM assay for diagnostic capabilities, as described here, will provide important information and tools for responses to future outbreaks of orthopoxviruses through natural or bioterrorism events.
Acknowledgments
We thank CDC personnel Whitni Davidson and Jason Abel of the poxvirus program for database management, Mike Dillon and the CDC stat laboratory technicians for sample processing, and the entire CDC poxvirus laboratory group as well as the Bioterrorism Preparedness Response Program at CDC. We are also grateful for the collaborative efforts of the local, county, and state health departments of Wisconsin, Illinois, Indiana, Missouri, and Kansas.
REFERENCES
- 1.Arita, L., R. Gispen, S. S. Kalter, L. T. Wah, S. S. Marennikova, R. Netter, and I. Tagaya. 1972. Outbreaks of monkeypox and serological surveys in non-human primates. Bull. W. H. O. 46:625-631. [PMC free article] [PubMed] [Google Scholar]
- 2.Bremen J. G. 2000. Monkeypox: an emerging infection for humans?, p. 45-76. In W. M. Scheld, W. A. Craig, and J. M. Hughes (ed.), Emerging infections 4. ASM Press, Washington, D.C.
- 3.Breman, J. G., J. Bernadou, and J. H. Nakano. 1977. Poxvirus in West Africa nonhuman primates: serological survey results. Bull. W. H. O. 55:605-612. [PMC free article] [PubMed] [Google Scholar]
- 4.Breman, J. G., E. Coffi, J. H. Nakano, h. Godfrey, and J. G. Gautun. 1977. Human poxvirus disease after smallpox eradication. Am. J. Trop. Med. Hyg. 26:273-281. [DOI] [PubMed] [Google Scholar]
- 5.Breman, J. G., R. Kalisa, M. V. Steniowski, E. Zanotto, A. I. Gromyko, and I. Arita. 1980. Human monkeypox, 1970-79. Bull. W. H. O. 58:165-182. [PMC free article] [PubMed] [Google Scholar]
- 6.Esposito, J., and J. H. Nakano. 1992. Human poxviruses, p. 643-668. In E. H. Lennette (ed.), Laboratory diagnosis of viral infections, 2nd ed. Marcel Dekker, Inc., New York, N.Y.
- 7.Guarner J., B. J. Johnson, C. D. Paddock, W. Shieh, C. S. Goldsmith, M. G. Reynolds, I. K. Damon, R. L. Regnery, S. R. Zaki, and the Veterinary Monkeyox Virus Working Group. 2004. Monkeypox transmission and pathogenesis in prarie dogs. Emerg. Infect. Dis. 10:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutin, Y. J., R. J. Williams, P. Malfait, R. Pebody, V. N. Loparev, S. L. Ropp, M. Rodriguez, J. C. Knight, F. K. Tshioko, A. S. Khan, M. V. Szczeniowski, and J. J. Esposito. 2001. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg. Infect. Dis. 7:434-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jezek, Z., and F. Fenner. 1988. Human monkeypox. Monogr. Virol. 17:1-140. [Google Scholar]
- 10.Jezek, Z., J. H. Nakano, I. Arita, M. Mutombo, M. Szcenlowski, and C. Dunn. 1987. Serological survey for human monkeypox infectiosn in a selected population in Zaire. J. Trop. Med. Hyg. 90:31-38. [PubMed] [Google Scholar]
- 11.Karem, K. L., A. C. Poon, C. Bierl, R. Nisenbaum, and E. Unger. 2002. Optimization of a human papillomavirus-specific enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 9:577-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khodakevich, L., M. Szczenlowski, D. M. Mambu, Z. Jezek, S. S. Marennikova, J. H. Nakano, and F. Meier. 1987. Monkeyppox virus in relation to the ecological features surrounding human settlements in Bumba Zone, Zaire. Trop. Geogr. Med. 39:56-63. [PubMed] [Google Scholar]
- 13.Khodakevich, L., M. Szczenlowski, D. M. Mambu, Z. Jezek, S. S. Marennikova, J. H. Nakano, and D. Messenger. 1987. Role of squirrels in sustaining monkeypox virus transmission. Top. Geogr. Med. 39:115-122. [PubMed] [Google Scholar]
- 14.Li, Y., V. A. Olson, T. Laue, and I. K. Damon. Unpublished data.
- 15.Maltseva, N. N., and S. S. Marennikova. 1976. A method for serological differentation of closely related poxviruses. Acta Virol. 20:250-252. [PubMed] [Google Scholar]
- 16.Mal'tseva, N. N., S. S. Marennikova, J. Nakano, G. R. Matsevich, and N. A. Khabakhpashaeva. 1984. Data from a serological survey of the population of the Republic of Congo for the presence of antibodies to orthopoxviruses. II. The species identification of the antibodies by using a solid-phase variant of immunoenzyme method. Zh. Mikrobiol. Epidemiol. Immunobiol. 4:64-67. (In Russian.) [PubMed] [Google Scholar]
- 17.Olson, V. A., T. Laue, M. T. Laker, I. V. Babkin, C. Drosten, S. N. Shchelkunov, M. Niedrig, I. K. Damon, and H. Meyer. 2004. Real-time PCR system for detection of orthopoxviruses and simultaneous identification of smallpox virus. J. Clin. Microbiol. 42:1940-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reed, K. D., J. W. Melski, M. B. Graham, R. L. Regnery, M. J. Sotir, M. V. Wegner, J. J. Kazmierczak, E. J. Stratman, Y. Li, J. A. Fairley, G. R. Swain, V. A. Olson, E. K. Sargent, S. C. Kehl, M. A. Frace, R. Kline, S. L. Foldy, J. P. Davis, and I. K. Damon. 2004. The detection of monkeypox in humans in the Western Hemisphere. N. Engl. J. Med. 350:342-350. [DOI] [PubMed] [Google Scholar]
- 19.Sejvar, J. J., Y. Chowdary, M. Schomogyi, J. Stevens, J. Patel, K. Karem, M. Fischer, M. J. Kuehnert, S. R. Zaki, C. D. Paddock, J. Guarner, W. J. Shieh, J. L. Patton, N. Bernard, Y. Li, V. A. Olson, R. L. Kline, V. N. Loparev, D. S. Schmid, B. Beard, R. R. Regnery, and I. K. Damon. 2004. Human monkeypox infection: a family cluster in the midwestern United States. J. Infect. Dis. 190:1833-1840. [DOI] [PubMed] [Google Scholar]
- 20.Stienlauf, S., M. Shoresh, A. Solomon, T. Lublin-Tennenbaum, Y. Atsmon, Y. Meirovish, and E. Katz. 1999. Kinetics of formation of neutralizing antibodies against vaccinia virus following re-vaccination. Vaccine 17:201-204. [DOI] [PubMed] [Google Scholar]
- 21.Zweig, M. H., and G. Campbell. 1993. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin. Chem. 39:561-577. [PubMed] [Google Scholar]