ABSTRACT
Growing evidence demonstrates that meditation practice supports cognitive functions, including attention and interoceptive processing, and is associated with structural changes across cortical networks, including prefrontal regions and the insula. However, the extent of subcortical morphometric changes linked to meditation practice is less appreciated. A noteworthy candidate is the pineal gland, a key producer of melatonin, which regulates circadian rhythms that augment sleep‐wake patterns and may also provide neuroprotective benefits to offset cognitive decline. Increased melatonin levels, as well as increased fMRI BOLD signal in the pineal gland, have been observed in meditators versus controls. However, it is not known if long‐term meditators exhibit structural changes in the pineal gland linked to the lifetime duration of practice. In the current study, we performed voxel‐based morphometry (VBM) analysis to investigate: (1) whether long‐term meditators (LTMs) (n = 14) exhibited greater pineal gland MRI‐derived signal intensity compared to a control group (n = 969), (2) a potential association between the estimated lifetime hours of meditation (ELHOM) and pineal gland signal intensity, and (3) whether LTMs show greater grey matter (GM) maintenance (BrainPAD) that is associated with pineal gland signal intensity. The results revealed greater pineal gland signal intensity and lower BrainPAD scores (younger brain age) in LTMs compared to controls. Exploratory analysis revealed a positive association between ELHOM and greater signal intensity in the pineal gland but not with GM maintenance as measured by BrainPAD score. However, greater pineal signal intensity and lower BrainPAD scores were correlated in LTMs. The potential mechanisms by which meditation influences pineal gland function, hormonal metabolism, and GM maintenance are discussed – in particular, melatonin's roles in sleep, immune response, inflammation modulation, and stem cell and neural regeneration.
Keywords: brain age, meditation, pineal gland
1. Introduction
The pineal is a highly vascularised, singular, unpaired gland, and its most well‐known function is the synthesis and release of the hormone melatonin, which participates in the regulation of the sleep‐wake cycle. Melatonin is also involved in regulating mood, and it possesses immune and neuroprotective functions [1], regulates neural stem cell production [2] and is one of the most powerful and available antioxidants in the human body, particularly in the brain [3]. There is also a concurrent popular belief, largely deriving from an occult or mystical perspective, that the pineal possesses a pivotal spiritual function, that it is a gateway to higher states of consciousness, and that it can be “activated” through various spiritual practices such as meditation or psychedelics. It is considered to be part of the physiological representation of the “third eye” by many people, most likely because it possesses anatomical similarities and shares phylogenetic history with the lateral and third eyes [4].
Putting mystical interpretation of pineal function aside, an interesting study [5] has nevertheless reported that the pineal gland, along with the entire corpora quadragemina, had considerably higher activation during meditation practice compared to quiet rest. Increased melatonin levels in meditators have also been observed in several studies [6, 7], suggesting the pineal gland has psychophysiological sensitivity [8]. These findings raise the possibility that the increased melatonin observed with meditation may, to some extent, be a result of potential remodelling of the pineal gland following repeated activation.
Melatonin levels generally decrease as we age (Karasek, 2001), and there is significant recent interest in the relationship of this age‐related decrease with accelerated cognitive decline and neurodegenerative processes. Findings have suggested that individuals with Alzheimer's Disease (AD) exhibit irregularities of melatonin and pineal gland function [9, 10]. Plasma concentration of melatonin is linearly correlated with volume of the pineal [11, 12, 13], suggesting that pineal tissue density could decrease with melatonin production decline. Given the sleep regulatory and brain maintenance roles of melatonin, this seems plausible. The ability of melatonin supplementation to halt or slow the effects of age‐related brain deterioration is also encouraging [14, 15].
In the present study, we examined the structural MRI signal intensity of the pineal using voxel‐based morphometry (VBM) in a cross‐section of meditators with varied levels of expertise. We hypothesised that if the pineal is activated consistently during meditative practice, a reasonable consequence could be structural remodelling of the pineal, and that these structural changes, if present, should exhibit a relationship with the total number of hours spent in meditation. As a secondary aim, we also investigated whether meditators exhibited greater grey matter maintenance in comparison to controls, as earlier research has suggested [16, 17].
2. Methods
2.1. Participants and Secondary Data
Data for the meditator group (14 individuals: 11 females, 3 males, ages 28–66) came from Hasenkamp Lab's prior work [18, 19]. Among the 14 subjects, six predominantly practised Shamatha, five engaged in other Tibetan styles (compassion and tong‐len), and three focused on Vipassana [20]. Notably, all meditation styles employed or included breath‐focused meditation. Healthy control data originated from various sources. A total of 135 young subjects (96 males, 39 females, ages 20–35) were from the LEMON data set ([21]–collected at Max Planck Institute for Human Cognitive and Brain Sciences). MRI data were downloaded from https://fcon_1000.projects.nitrc.org/indi/retro/MPI_LEMON/downloads/download_MRI.html. A total of 395 adult/elderly controls (168 males, 227 females, ages 56–95) were part of the ADNI 3 phase from IDA (https://ida.loni.usc.edu). A total of 398 individuals (249 males, 149 females) from BASE‐II [22] were accessed via https://www.mpib-berlin.mpg.de/research/research-centers/lip/projects/aging/base-ii. Lastly, 41 older adults (15 males, 26 females, ages 60–72) were provided by the University of Dallas Texas's Centre of Brain Health [23]. These datasets, totalling 969 healthy controls, were compared to 14 meditators. Data were downloaded and accessed between December 2020 and January 2023.
2.2. Neuroimaging Processing and BrainPAD Calculation
All the MRI images used in this study were 3 T high‐resolution T1‐weighted MRIs with a 1 mm³ voxel size. The T1 images were sourced from various datasets in NIfTI format and underwent the same processing and standardisation procedure. The 14 meditators and the 969 healthy controls were processed using the Computational Anatomy Toolbox 12–CAT12 (http://www.neuro.uni-jena.de/cat/) implemented in Statistical Parametric Mapping 12–SPM12 (https://www.fil.ion.ucl.ac.uk/spm/). The processing was run following the default CAT12 settings, except for the voxel size, which was set to 1 mm³ isotropic voxel size (matrix size/field of view [FOV]: 181 × 217 × 181). Upon completion of the processing, the images' total intracranial volume (TIV) was calculated, and the images were modulated and spatially oriented in the Montreal Neurological Institute (MNI) coordinate space. All the images had a quality score above 75% on the CAT12 quality assurance rating scale. Subsequently, the processed, normalised images of grey matter (GM) and white matter (WM) were smoothed with a 2 mm³ FWHM kernel. Brain Predicted Age Discrepancy (BrainPAD) is an objective measure of brain ageing, reflecting age‐related GM maintenance. Negative values imply a younger brain, while positive values signal accelerated GM degeneration. A score of zero indicates no age difference between chronological and brain GM age. BrainPAD computes age disparity using a defined GM trajectory in healthy subjects and was calculated here using the method of Boyle and colleagues [24]. Further details are provided in the supplementary materials.
2.3. Pineal Gland ROI Definition
To isolate the pineal gland in the analyses, a binary mask oriented in the MNI space was manually developed (voxel by voxel in FSLeyes–edit mode) (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). Using the average brain template available in CAT12 (derived from 555 healthy control subjects – “Template_T1_IXI555_MNI152_GS”; http://brain-development.org/) as an anatomical reference, and considering the pineal anatomical localisation described in the literature [25, 26], the pineal area was isolated voxel by voxel (1 mm³ isotropic voxel size) on the coronal plane, proceeding slice by slice until the boundaries of the corpora quadrigemina and using the pineal and Supra‐pineal Recesses as references. The binary mask (1 mm³ isotropic voxel size) did not encroach on the Splenium of the Corpus Callosum or the Thalamus. The binary ROI had the same matrix size (FOV) of 181 × 217 × 181 as the processed images from CAT12.
2.4. Structural Analyses in CAT12 and JASP
In the first instance (first branch), a voxel‐based morphometry (VBM) analysis investigated whether meditators (group A) had greater pineal signal intensity than controls (group B) via a two‐sample t‐test (controlling for age, gender, and TIV). The main hypothesis was tested in SPM contrast manager (1–1, group A > group B), isolating the pineal Gland using a binary mask by applying progressive statistical thresholds from p < 0.05 to stricter thresholds (p < 0.01; p < 0.001; p < 0.05 FWE corrected). As a control procedure, the opposite hypothesis was tested in SPM contrast manager (–1 1, group A < group B). Given the large discrepancy in sample size, the same analysis was repeated, comparing the meditator group with 15 additional matched controls from Hasenkamp data. Several other control analyses involving sample size and re‐sampling are reported in the supplementary materials–see supplementary methods and supplementary results section S1.
In the second instance (second branch), exploratory analysis investigated the relationship between meditation hours and pineal signal intensity. In a multiple regression model, ELHOM was entered as an independent variable while controlling for age, gender, and TIV. As in the first model, the pineal region was isolated with a binary mask, and the positive relationship with ELHOM was tested, applying thresholds for p < 0.05 to stricter thresholds. Similarly, another multiple regression model investigated a direct relationship between the pineal Gland and BrainPAD.
Lastly (third branch), to test whether meditators had reduced GM degeneration compared to controls, an ANCOVA model was created in JASP, treating BrainPAD as a dependent variable, group as an independent factor, and age, gender, and TIV as covariates. A post‐hoc Bonferroni correction was then applied. This analysis was carried out using 834 controls rather than 969 because Babayan et al. [21] refused to provide the exact ages of the 135 participants in the LEMON data set, making it impossible to calculate BrainPAD.
3. Results
Table 1a and Table 1b summarise the key demographic variables and brain parameters used in subsequent analyses.
Table 1a.
Groups descriptive statistics.
| Age | TIV | Pineal Gland | BrainPAD | |||||
|---|---|---|---|---|---|---|---|---|
| Controls | Meditators | Controls | Meditators | Controls | Meditators | Controls | Meditators | |
| Valid | 969 | 14 | 969 | 14 | 969 | 14 | 834 | 14 |
| Mean | 61.790 | 44.286 | 1449.399 | 1454.226 | 0.561 | 0.649 | 3.292 | ‐0.184 |
| Std. Deviation | 19.374 | 11.027 | 150.051 | 170.461 | 0.281 | 0.162 | 8.016 | 15.000 |
| Minimum | 22.500 | 28.000 | 1082.290 | 1243.460 | 0.271 | 0.354 | ‐30.205 | ‐23.927 |
| Maximum | 95.000 | 66.000 | 2134.020 | 1812.880 | 1.550 | 0.898 | 37.459 | 23.738 |
Note: Meditators group gender distribution: 11 females; 3 males.
Control group gender distribution: 491 females, 478 males.
Table 1b.
Meditators descriptive statistics.
| ELHOM | Age | TIV | BrainPAD | |||||
|---|---|---|---|---|---|---|---|---|
| F | M | F | M | F | M | F | M | |
| Valid | 11 | 3 | 11 | 3 | 11 | 3 | 11 | 3 |
| Mean | 1410.455 | 1297.667 | 42.909 | 49.333 | 1387.932 | 1697.307 | 3.257 | −12.799 |
| Std. Deviation | 1485.710 | 1044.139 | 9.843 | 16.042 | 111.005 | 120.234 | 15.089 | 4.514 |
| Minimum | 17.000 | 362.000 | 28.000 | 34.000 | 1243.460 | 1572.900 | −23.927 | −18.009 |
| Maximum | 3661.000 | 2424.000 | 62.000 | 66.000 | 1611.830 | 1812.880 | 23.738 | −10.054 |
Table 1a reports mean age, TIV, pineal gland signal intensity and BrainPAD for the meditation and control groups. Table 1b reports the mean ELHOM, Age, TIV and BrainPAD stratified by sex.
3.1. Differences Between Pineal Gland Signal Intensity in Meditators Versus Controls
As reported in Table 2a, by controlling for age, gender, and TIV, the meditator group exhibited greater pineal Gland MRI signal intensity compared to 969 healthy controls, at a statistical threshold of p < 0.05. Specifically, a cluster of 363 voxels (T = 5.40; FWE‐corrected p = 0.038) within the pineal region was significantly associated with meditation practice. As shown in Figure 1, the significant cluster matched the spatial localisation of the pineal Gland in MNI space (original SPM12 outputs are available in the supplementary materials in the results section). This result was corroborated by control analyses (see Table 2a) testing the alternative hypothesis (Meditators < Controls), which did not yield significant results. Similarly, another control analysis comparing the meditators to 15 matched controls from Hasenkamp et al.'s (2012) original data set revealed the same pattern of findings, albeit with weaker statistical power possibly due to the reduced sample size (see Table 2b). This result corroborates the main findings and suggest that the observed findings are not attributable to discrepancy in sample size. Moreover, additional analyses sampling control participants across different datasets consistently demonstrated greater pineal gland MRI signal intensity in the meditator group versus controls. For further details, refer to the supplementary materials–supplementary results section 1 ‘Control Analyses’.
Table 2.
MRI Structural analyses table: t‐test shows the results of the two‐samples t‐test comparing the structural variation of the pineal gland between a group of 14 meditators (group A) and 969 healthy controls (group B). The analyses tested both the hypothesis (Group A > B and Group B > A). The model was controlled for age, gender and total intracranial volume. The table reports the significant clusters of voxels for the differences observed between the two groups for the statistical threshold of p < 0.05. Pineal Gland structural difference survived multiple comparison corrections when was applied (FWE corr. p < 0.038).
| Two samples t‐test | side | MNI coordinates | Peak T valuea | Peak Z scoreb | p value uncorrc | FWEd | FDRe | Total number of voxels for p < 0.05f | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Meditators versus controls | x | y | Z | ||||||||
| Pineal Gland (A > B) | left | −2 | −34 | 0 | 5.40 | 5.36 | 0.000 | 0.038 | 0.000 | 363 | |
| Pineal Gland (A < B) | / | / | / | / | / | / | / | / | / | / | / |
Peak T value: T value of the most significant cluster of contiguous voxels.
Peak Z‐score: Z‐score of the most significant cluster of contiguous voxels.
p value uncorrected.
FWE = family wise error correction value.
FDR = false discovery rate correction value (q).
Total number of voxels outcoming in the ROI including all clusters of contiguous voxels.
Figure 1.

shows the significant cluster of voxels of the pineal region where meditator group exhibits greater signal intensity in comparison to the control group. The left portion shows the pineal cluster (in yellow‐orange) on axial 3D reconstruction of the brain. The right portion showcases the pineal average signal intensity between the two groups. The model is corrected for age, gender and total intracranial volume.
3.2. The Relationship Between Pineal Gland Signal Intensity and Hours of Meditation
An exploratory analysis was conducted to examine the association between ELHOM and pineal gland signal intensity. At a threshold of p < 0.01 (uncorrected), a small cluster of 16 voxels (T = 3.33, BF10 = 1926.853, R² = 0.795) localised within the pineal gland showed a positive association with greater ELHOM. Specifically, higher estimated hours of meditation practice were linked to increased pineal gland signal intensity (see Table 3 and Figure 2). Bayesian modelling provided very strong evidence for this relationship (BF10 = 1926.853, R² = 0.795). However, this cluster did not survive multiple comparison corrections (original SPM12 outputs are available in the supplementary materials–supplementary results section S2).
Table 3.
MRI structural analyses table: Multiple regression model–pineal gland and hours of meditation shows the results of the multiple regression model exploring potential positive relationship between Pineal Gland structural signal intensity and estimated lifetime hours of meditation (ELHOM) in a group of 14 meditators. The model was controlled for age, gender and total intracranial volume. The table reports the significant clusters of voxels for the statistical threshold of p < 0.05. Pineal gland (and supra‐pineal area) greater signal intensity is positive associated with greater number of hours of meditation. However, the result did not survive multiple comparison corrections when was applied.
| Multiple regression | side | MNI coordinates | Peak T valuea | Peak Z scoreb | p value uncorrc | FWEd | FDRe | Total number of voxels for p < 0.05 with max BF10 f | ||
|---|---|---|---|---|---|---|---|---|---|---|
| ELHOM | x | y | Z | |||||||
| Pineal Gland | right | 4 | −31 | 2 | 3.33 | 2.62 | 0.004 | 1.000 | 1.032 | 16 (BF101926.853 ‐ R² 0.795) |
Peak T value: T value of the most significant cluster of contiguous voxels.
Peak Z‐score: Z‐score of the most significant cluster of contiguous voxels.
P value uncorrected.
FWE = family wise error correction value.
FDR = false discovery rate correction value (q).
Total number of voxels outcoming in the ROI including all clusters of contiguous voxels (in brackets are reported Bayes Factors).
Figure 2.
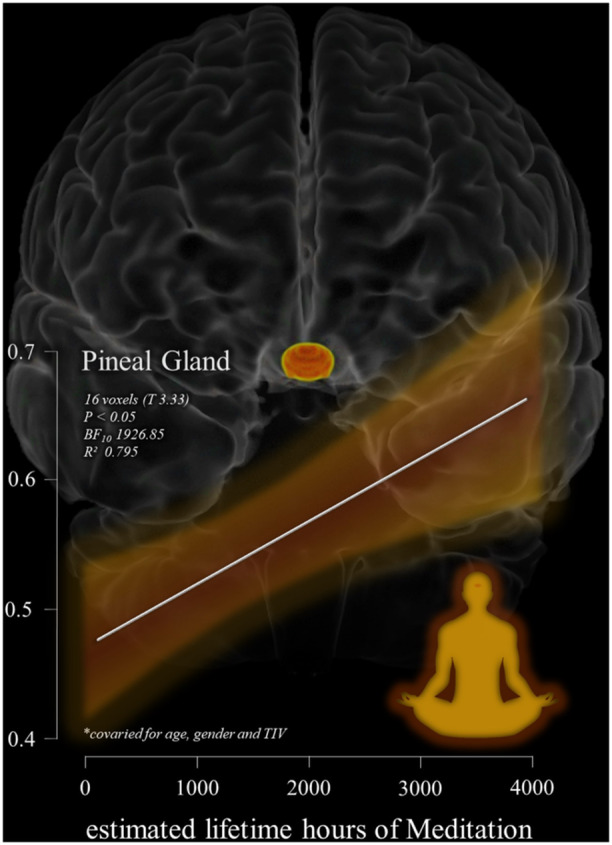
Shows the relation between the significant cluster of voxels in the pineal gland (highlighted in yellow‐orange on a 3D reconstruction of the brain–coronal view) associated with estimated lifetime hours of meditation (ELHOM) for the 14 individuals examined in the MRI structural multiple regression model. The model and the scatterplot are controlled for the effects of age, gender and total intracranial volume for the statistical threshold of p < 0.05. The Y axis of the scatterplot report pineal gland signal intensity values while X axis the estimated lifetime hours of meditation reported. The white regression line depicts the linear relationship and the orange shaded region shows the confidence interval.
Additionally, as detailed in the supplementary materials, greater pineal gland signal intensity was directly associated with lower BrainPAD scores (BF10 = 360.042, R² = 0.692).
3.3. Differences in Grey Matter Maintenance Between Meditators and Controls
As reported in Table 4, the ANCOVA model, controlling for age, gender, and total intracranial volume (TIV), demonstrated that meditators (n = 14) exhibited reduced grey matter (GM) degeneration compared to controls (n = 834). On average, controls had a predicted brain age that was 3 years older than their chronological age, while meditators showed GM aging consistent with their chronological age (F = 14.13, p < 0.001, d = 0.97).
Table 4.
Reporting the results of the ANCOVA model investigating differences in grey matter maintenance (BrainPAD) between meditators versus controls. Results shown that meditators exihbited reduced GM deneration in comparison to controls, on average controls had a brain 3 years older than their chronological age, while meditators shown GM aging consistent with their chronological age.
| ANCOVA ‐ BrainPAD–Grey matter maintenance ‐ Meditators versus Controls | |||||
|---|---|---|---|---|---|
| Cases | Sum of Squares | df | Mean Square | F | p |
| Group | 819.220 | 1 | 819.220 | 14.130 | < 0.001 |
| TIV | 3767.227 | 1 | 3767.227 | 64.980 | < 0.001 |
| Gender | 857.613 | 1 | 857.613 | 14.793 | < 0.001 |
| Age | 4516.990 | 1 | 4516.990 | 77.912 | < 0.001 |
| Residuals | 48873.404 | 843 | 57.976 | ||
| Note: Type III sum of squares. | |||||
| Descriptives ‐ BrainPAD |

|
|||
| Group | Mean | SD | N | |
| Controls | 3.292 | 8.016 | 834 | |
| Meditators | −0.184 | 15.000 | 14 | |
| Post Hoc comparisons | |||||||
|---|---|---|---|---|---|---|---|
| Mean difference | SE | t | Cohen's d | p bonf | |||
| Controls | Versus | Meditators | 7.925 | 2.108 | 3.759 | 0.970 | < 0.001 |
Note: Cohen's d does not correct for multiple comparisons. Results are averaged over the levels of: gender.
Additionally, as detailed in the supplementary materials, greater pineal signal intensity was directly associated with lower BrainPAD scores (BF10 = 360.042, R² = 0.692). There was no significant association between BrainPAD scores and ELHOM.
4. Discussion
We observed enhanced MRI signal intensity in the pineal gland in meditators compared to controls, with this effect being linearly related to the estimated lifetime hours spent in meditation. Additionally, we found better maintenance of grey matter (as measured by BrainPAD, a proxy for brain age relative to chronological age) in the meditation group compared to controls. These results align with previous findings [27, 28, 29, 30, 31]. Furthermore, greater pineal gland signal intensity was associated with reduced brain age in meditators. While the evidence for pineal MRI signal change remains preliminary, it suggests that meditation may lead to structural augmentation of the pineal gland. Prior studies have shown an increased fMRI BOLD response in the pineal gland during meditation (Liou et al., 2005) and higher plasma melatonin concentrations in advanced meditators compared to non‐meditators (Solberg et al., 2004), but to our knowledge, the current study is the first to demonstrate morphometric differences in this melatoninergic structure in long‐term meditation practitioners.
Our secondary findings—showing enhanced grey matter maintenance in meditators—are consistent with meta‐analyses showing preserved or increased grey matter across several cortical structures [32, 33, 34, 35].
Although the mechanisms underlying the relationship between meditation practice and hormonal changes are not well understood, there are several potential ways in which meditation might influence melatonin production and affect morphological changes in the pineal gland. First, meditation is often practiced in an undisturbed, dimly lit environment, and typically, though not exclusively, involves closing the eyes. Reduced light exposure, particularly from artificial sources, may enhance melatonin production over time [36]. However, a previous study demonstrated greater plasma melatonin concentrations following meditation compared to an eyes‐open control condition, even when light exposure was below the minimum intensity known to influence melatonin levels [37]. This suggests that reduced light exposure alone is not sufficient to explain meditation‐related effects on melatonin production.
Second, sleep duration or quality could be a mediating factor explaining the relationship between meditation and pineal signal intensity. Melatonin plays a key role in regulating sleep and wake cycles via circadian rhythms, and both melatonin supplementation and meditation have been shown to improve sleep quality [38]. It is plausible that improved sleep patterns resulting from regular meditation practice lead to more regular melatonin secretion, particularly during the night, which in turn could contribute to greater pineal tissue density (maintenance). This is supported by studies showing a linear correlation between melatonin plasma concentration and pineal volume [11, 12, 13, 39—where volume changes are inferred to indicate tissue density and reduced calcification.
Third, the stress‐reducing effects of meditation may also play a role in melatonin production. High levels of anxiety are known to suppress melatonin synthesis [40], and meditation may counteract this effect by promoting anxiolytic actions that stimulate greater melatonin production from the pineal gland.
Fourth, respiration is a key driver of cerebrospinal fluid (CSF) dynamics [41], and yogic breathing practices have been shown to modulate CSF in a pulsatile fashion [42]. Beneficial changes in the regularity and depth of respiration resulting from meditation could positively impact waste clearance in and around the pineal gland, which is directly exposed to the ventricular space. Similarly, increased CSF flow might facilitate the distribution of melatonin, which is released into the CSF. Increased ventricular dynamics could also generate a weak piezoelectric induction in the pineal via mechanical stimulation of calcite micro‐crystals, which are known to be present there [43, 44].
A final possibility is that habenular activation observed during meditation (quadrigemina study) might induce pineal gland activation via a previously documented efferent pathway from the medial habenula to the pineal [45, 46]. Indeed, around 15% of pineal cells have been shown to respond to habenular activation in a murine model [47].
Irrespective of the specific mechanism, structural augmentation of the pineal gland and higher melatonin secretion over time in meditators are likely to have benefits for cognitive aging. Notably, our finding that greater pineal signal intensity is associated with a younger brain age suggests that greater brain maintenance over time may be supported by the neuroprotective properties of melatonin secretion. As we age, the pineal gland shrinks [48] and produces less melatonin [49]. Pineal volume and melatonin production are further reduced in individuals with dementia [50, 51, 52, 53].
Melatonin plays a critical role in modulating immune and inflammatory responses, which are implicated in the early stages of Alzheimer's disease (AD). It exhibits both pro‐ and anti‐inflammatory properties, reducing the pro‐inflammatory actions of amyloid‐β peptides by enhancing α‐secretase activity and inhibiting β‐ and γ‐secretase [54, 55, 56, 57, 58]. Melatonin lowers Aβ peptide levels and inhibits amyloid plaque deposition in AD models, although its efficacy is most significant when administered early and is not likely to reverse AD once it has begun [59]. Additionally, melatonin mitigates oxidative stress as a potent antioxidant [1, 3, 60, 61] and may reduce tau pathology in AD [62, 63]. Melatonin also regulates neural stem cell proliferation [64] and differentiation [65], promoting neural plasticity. Thus, sustaining melatonin levels via practices like meditation may delay or reduce neurodegeneration, preserving brain health and function.
4.1. Limitations
The main limitations of this study are the small sample size of meditators and the cross‐sectional nature of the models, which limit the conclusions from preliminary observations that require further replication. A further limitation raised during review is that our initial analysis compared two groups with a class imbalance in sample size. To address this issue we repeated the analysis with an independent control group (n = 15) drawn from Hasenkamp et al. (2012), which provided a more balanced design, and we found the significant effect versus meditators remained. We also sampled from several different control datasets reliably demonstrating that pineal signal intensity significantly differed from the meditation group (see supplementary materials). Nevertheless, future studies should endeavour to include larger samples of meditators and employ longitudinal designs along with neuropsychological testing. Additionally, sociodemographic factors such as diet, physical fitness, and other lifestyle factors should be considered to better parse the role of meditation from other contributing factors to overall brain health and structural changes in the pineal gland.
While our findings replicate earlier evidence demonstrating reduced grey matter degeneration in meditators, the effect observed here is smaller than those reported by Luders et al. [17] and Adluru et al. [16]. This discrepancy may be attributed to differences in the methodologies used to estimate BrainAGE and BrainPAD in these studies. However, all studies report the same direction of effect: meditative practice is associated with a younger and better‐maintained brain.
Although we demonstrate structural differences in the pineal gland between meditators and controls and associate pineal gland signal intensity with meditation practice, further research is needed to clarify which specific elements of the meditative experience contribute to these morphometric effects. Variables such as diurnal light exposure, sleep quality, and emotional health may all play a role in the structural modification of the pineal gland through melatonin secretion, either directly from meditation or via other beneficial effects of the practice.
The relationship between reduced grey matter degeneration and meditation may reflect the influence of other factors contributing to overall brain health, such as education, socioeconomic status, and a possible tendency toward a healthier lifestyle (including diet, physical fitness, and meditation). These factors should be explored as potential confounding variables in future studies. Additionally, because meditation and mindfulness practices are increasingly popular, some control participants may have had previous meditation experience. Given the open‐access nature of the datasets used in this study, we have no information on the level of meditation experience in control participants. However, it is plausible that approximately 5% of control participants may have engaged in meditation practices [66], which should be considered in future research.
5. Conclusions
Our findings of greater pineal signal intensity, enhanced grey matter maintenance, and lower brain age in meditators, coupled with the extensive literature supporting the cortical and functional benefits of meditation, suggest that meditation could be beneficial for cognitive and neurophysiological aging. Given melatonin's roles in sleep regulation, antioxidant action, anti‐inflammatory effects, immune modulation, and neural plasticity, it is plausible that early pineal degeneration could contribute to age‐related brain degeneration. Structural preservation of the pineal gland via meditation may therefore offer cognitive benefits. More research is needed, but the structural benefits to the pineal gland appear to accumulate with practice. It remains unclear whether acute meditation interventions could provide similar benefits once cognitive and cortical degeneration have begun. Nonetheless, early and continuous meditation practice across the lifespan may offer the greatest potential for optimal cognitive health.
Author Contributions
Emanuele R. G. Plini: conceptualisation, formal analyses, visualisation, manuscript writing, interpretation. Michael C. Melnychuk: conceptualisation, analyses support, manuscript writing, interpretation and supervision. Paul M. Dockree: conceptualisation, manuscript edits, supervision.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Supporting information.
Supporting information.
Supporting information.
Acknowledgments
Project partly founded by the Irish Research Council—Irish Research Council Laureate Consolidator Award (2018‐23) IRCLA/2017/306 to Paul Dockree.
This work was further supported by ProApto which founded this study (more info: https://www.proapto-camouflage.com/).
This work reports data from the Berlin Aging Study II project, which was supported by the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung [BMBF]) under grant numbers #01UW0808, #16SV5536K, #16SV5537, #16SV5538, #16SV5837, #01GL1716A, and #01GL1716B. Another source of funding is the Max Planck Institute for Human Development, Berlin, Germany. Additional contributions (e.g., equipment, logistics, and personnel) are made from each of the other participating sites.
This reports data by the Centre of Brain Health – Dallas Texas which was supported by a grant from the National Institute of Health (RC1‐AG035954, R01‐NS067015, R01‐AG033106) and by grants from the T. Boone Pickens Foundation, the Lyda Hill Foundation, and Dee Wyly Distinguished University Endowment.
Thanks are extended to Francesca Fabbricatore for proofreading the manuscript and for the thoughtful comments provided.
Emanuele R.G. Plini and Michael C Melnychuk contributed equally to the study.
Data Availability Statement
The data that support the findings of this study are available in ADNI3 at https://adni.loni.usc.edu/data-samples/access-data/. These data were derived from the following resources available in the public domain: ‐ ADNI, https://adni.loni.usc.edu/data-samples/access-data/ ‐ Wendy Hasenkamp, https://wendyhasenkamp.net/ ‐ BASEII, https://www.base2.mpg.de/7549/data-documentation ‐ LEMON, https://fcon_1000.projects.nitrc.org/indi/retro/MPI_LEMON.html ‐ Sandra Chapman, https://centerforbrainhealth.org/
The pineal gland MNI mask developed for this study is available on reasonable request emailing Dr. Emanuele RG Plini (plinie@tcd.ie – emanuele.rg.plini@gmail.com). The significant cluster of voxels of the pineal gland are available in nifti format in the supplementary materials.
The data on Meditators were provided by Wendy Hasenkamp as described in the methods and they are accessible upon reasonable request: https://wendyhasenkamp.net
The ADNI and the LEMON datasets used are opensource and can be accessed referring to the information provided in the methods.
ADNI: https://adni.loni.usc.edu/data-samples/access-data/
LEMON: https://fcon_1000.projects.nitrc.org/indi/retro/MPI_LEMON.html
Data of the Berlin Aging Study II project can be accessed at the following link prior dedicated documentation: https://www.base2.mpg.de/7549/data-documentation
Data of the Dallas Centre of Brain Health: are available from Jeffrey S. Spence, Center for BrainHealth, University of Texas at Dallas, on reasonable request: https://centerforbrainhealth.org/.
References
- 1. Lee J. G., Woo Y. S., Park S. W., Seog D. H., Seo M. K., and Bahk W. M., “The Neuroprotective Effects of Melatonin: Possible Role in the Pathophysiology of Neuropsychiatric Disease,” Brain Sciences 9, no. 10 (2019): 285, 10.3390/brainsci9100285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yu X., Li Z., Zheng H., Ho J., Chan M. T. V., and Wu W. K. K., “Protective Roles of Melatonin in Central Nervous System Diseases by Regulation of Neural Stem Cells,” Cell Proliferation 50, no. 2 (2017): e12323, 10.1111/cpr.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hardeland R., “Melatonin and Microglia,” International Journal of Molecular Sciences 22, no. 15 (2021): 8296, 10.3390/ijms22158296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eakin R. M., The Third Eye (University of California Press, 1973). [Google Scholar]
- 5. Liou C. H., Hsieh C.‐W., Lee S.‐C., et al., “Correlation Between Pineal Activation and Religious Meditation Observed By Functional Magnetic Resonance Imaging,” Nature Proceedings (2007): 1. [Google Scholar]
- 6. Thambyrajah J. C., Dilanthi H. W., Handunnetti S. M., Disssanayake DWN, et al., “Serum Melatonin and Serotonin Levels in Long‐Term Skilled Meditators,” Explore 19, no. 5 (2023): 695–701. [DOI] [PubMed] [Google Scholar]
- 7. Harinath K., Malhotra A. S., Pal K., et al., “Effects of Hatha Yoga and Omkar Meditation on Cardiorespiratory Performance, Psychologic Profile, and Melatonin Secretion,” Journal of Alternative and Complementary Medicine 10, no. 2 (2004): 261–268. [DOI] [PubMed] [Google Scholar]
- 8. Massion A. O., Teas J., Hebert J. R., Wertheimer M. D., and Kabat‐Zinn J., “Meditation, Melatonin and Breast/Prostate Cancer: Hypothesis and Preliminary Data,” Medical Hypotheses 44, no. 1 (1995): 39–46, 10.1016/0306-9877(95)90299-6. [DOI] [PubMed] [Google Scholar]
- 9. Roy J., Wong K. Y., Aquili L., et al., “Role of Melatonin in Alzheimer's Disease: From Preclinical Studies to Novel Melatonin‐Based Therapies,” Frontiers in Neuroendocrinology 65 (2022): 100986. [DOI] [PubMed] [Google Scholar]
- 10. Song J., “Pineal Gland Dysfunction in Alzheimer's Disease: Relationship With the Immune‐Pineal Axis, Sleep Disturbance, and Neurogenesis,” Molecular Neurodegeneration 14, no. 1 (2019): 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mahlberg R., Tilmann A., Salewski L., and Kunz D., “Normative Data on the Daily Profile of Urinary 6‐Sulfatoxymelatonin in Healthy Subjects Between the Ages of 20 and 84,” Psychoneuroendocrinology 31 (2006): 634–641. [DOI] [PubMed] [Google Scholar]
- 12. Mahlberg R., Walther S., Kalus P., et al., “Pineal Calcification in Alzheimer's Disease: An in Vivo Study Using Computed Tomography,” Neurobiology of Aging 29 (2008): 203–209. [DOI] [PubMed] [Google Scholar]
- 13. Nölte I., Lütkhoff A. T., Stuck B. A., et al., “Pineal Volume and Circadian Melatonin Profile in Healthy Volunteers: an Interdisciplinary Approach,” Journal of Magnetic Resonance Imaging 30 (2009): 499–505. [DOI] [PubMed] [Google Scholar]
- 14. Li J., Somers V. K., Xu H., Lopez‐Jimenez F., and Covassin N., “Trends in Use of Melatonin Supplements Among US Adults, 1999‐2018,” Journal of the American Medical Association 327, no. 5 (2022): 483–485, 10.1001/jama.2021.23652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vincent B., “Protective Roles of Melatonin Against the Amyloid‐Dependent Development of Alzheimer's Disease: A Critical Review,” Pharmacological Research 134 (2018): 223–237. [DOI] [PubMed] [Google Scholar]
- 16. Adluru N., Korponay C. H., Norton D. L., Goldman R. I., and Davidson R. J., “Brainage and Regional Volumetric Analysis of a Buddhist Monk: A Longitudinal MRI Case Study,” Neurocase 26, no. 2 (2020): 79–90, 10.1080/13554794.2020.1731553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luders E., Cherbuin N., and Gaser C., “Estimating Brain Age Using High‐Resolution Pattern Recognition: Younger Brains in Long‐Term Meditation Practitioners,” NeuroImage 134 (2016): 508–513, 10.1016/j.neuroimage.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 18. Hasenkamp W., Wilson‐Mendenhall C. D., Duncan E., and Barsalou L. W., “Mind Wandering and Attention During Focused Meditation: A Fine‐Grained Temporal Analysis of Fluctuating Cognitive States,” NeuroImage 59, no. 1 (2012): 750–760, 10.1016/j.neuroimage.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 19. Hasenkamp W. and Barsalou L. W., “Effects of Meditation Experience on Functional Connectivity of Distributed Brain Networks,” Frontiers in Human Neuroscience 6 (2012): 38, 10.3389/fnhum.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Monaghan P. and Viereck E. G.; Meditation: The Complete Guide: Techniques from East and West to Calm the Mind, Heal the Body, and Enrich the Spirit. New World Library, Oct 5, 2011. ‐ Body, Mind & Spirit.
- 21. Babayan A., Erbey M., Kumral D., et al., “A Mind‐Brain‐Body Dataset of MRI, EEG, Cognition, Emotion, and Peripheral Physiology in Young and Old Adults,” Scientific Data 6 (2019): 180308, 10.1038/sdata.2018.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bertram L., Böckenhoff A., Demuth I., et al., “Cohort Profile: The Berlin Aging Study II (Base‐II),” International Journal of Epidemiology 43, no. 3 (2014): 703–712, 10.1093/ije/dyt018. [DOI] [PubMed] [Google Scholar]
- 23. Chapman S. B., Aslan S., Spence J. S., et al., “Distinct Brain and Behavioral Benefits From Cognitive vs. Physical Training: A Randomized Trial in Aging Adults,” Frontiers in Human Neuroscience 10 (2016): 338, 10.3389/fnhum.2016.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boyle R., Jollans L., Rueda‐Delgado L. M., et al., “Brain‐Predicted Age Difference Score Is Related to Specific Cognitive Functions: A Multi‐Site Replication Analysis,” Brain Imaging and Behavior 15 (2021): 327–345, 10.1007/s11682-020-00260-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haines D. E., Neuroanatomy, an Atlas of Structures, Sections and Systems (Lippincott Williams & Wilkins, 2004). 6th edn. [Google Scholar]
- 26. Mai J. K. and Paxinos G., The Human Nervous System (Cambridge, MA, USA: Academic Press, 2012). 3rd ed. [Google Scholar]
- 27. Hernández S. E., Dorta R., Suero J., Barros‐Loscertales A., González‐Mora J. L., and Rubia K., “Larger Whole Brain Grey Matter Associated With Long‐Term Sahaja Yoga Meditation: A Detailed Area by Area Comparison,” PLoS One 15, no. 12 (2020): e0237552, 10.1371/journal.pone.0237552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luders E., Cherbuin N., and Kurth F., “Forever Young(er): Potential Age‐Defying Effects of Long‐Term Meditation on Gray Matter Atrophy,” Frontiers in Psychology 5 (2015): 1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luders E., Toga A. W., Lepore N., and Gaser C., “The Underlying Anatomical Correlates of Long‐Term Meditation: Larger Hippocampal and Frontal Volumes of Gray Matter,” NeuroImage 45, no. 3 (2009): 672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pagnoni G. and Cekic M., “Age Effects on Gray Matter Volume and Attentional Performance in Zen Meditation,” Neurobiology of Aging 28, no. 10 (2007): 1623–1627. [DOI] [PubMed] [Google Scholar]
- 31. Vestergaard‐Poulsen P., van Beek M., Skewes J., et al., “Long‐Term Meditation Is Associated With Increased Gray Matter Density in the Brain Stem,” Neuroreport 20, no. 2 (2009): 170–174, 10.1097/WNR.0b013e328320012a. [DOI] [PubMed] [Google Scholar]
- 32. Fox K. C., Nijeboer S., Dixon M. L., et al., “Is Meditation Associated With Altered Brain Structure? A Systematic Review and Meta‐Analysis of Morphometric Neuroimaging in Meditation Practitioners,” Neuroscience and Biobehavioral Reviews 43 (2014): 48–73. [DOI] [PubMed] [Google Scholar]
- 33. Gard T., Hölzel B. K., and Lazar S. W., “The Potential Effects of Meditation on Age‐Related Cognitive Decline: A Systematic Review,” Annals of the New York Academy of Sciences 1307, no. 1 (2014): 89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marciniak R., Sheardova K., Čermáková P., Hudeček D., Šumec R., and Hort J., “Effect of Meditation on Cognitive Functions in Context of Aging and Neurodegenerative Diseases,” Frontiers in Behavioral Neuroscience 8 (2014): 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sumantry D. and Stewart K. E., “Meditation, Mindfulness, and Attention: A Meta‐Analysis,” Mindfulness 12 (2021): 1332–1349. [Google Scholar]
- 36. Lewy A. J., Wehr T. A., Goodwin F. K., Newsome D. A., and Markey S. P., “Light Suppresses Melatonin Secretion in Humans,” Science 210, no. 4475 (1980): 1267–1269, 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- 37. Tooley G. A., Armstrong S. M., Norman T. R., and Sali A., “Acute Increases in Night‐Time Plasma Melatonin Levels Following a Period of Meditation,” Biological Psychology 53, no. 1 (2000): 69–78. [DOI] [PubMed] [Google Scholar]
- 38. Nagendra R. P., Maruthai N., and Kutty B. M., “Meditation and Its Regulatory Role on Sleep,” Frontiers in Neurology 3 (2012): 54, 10.3389/fneur.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liebrich L. S., Schredl M., Findeisen P., Groden C., Bumb J. M., and Nölte I. S., “Morphology and Function: MR Pineal Volume and Melatonin Level in Human Saliva Are Correlated,” Journal of Magnetic Resonance Imaging 40, no. 4 (2014): 966–971, 10.1002/jmri.24449. [DOI] [PubMed] [Google Scholar]
- 40. Repova K., Baka T., Krajcirovicova K., et al., “Melatonin as a Potential Approach to Anxiety Treatment,” International Journal of Molecular Sciences 23, no. 24 (2022): 16187, 10.3390/ijms232416187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dreha‐Kulaczewski S., Joseph A. A., Merboldt K. D., Ludwig H. C., Gärtner J., and Frahm J., “Inspiration Is the Major Regulator of Human CSF Flow,” Journal of Neuroscience 35, no. 6 (2015): 2485–2491, 10.1523/JNEUROSCI.3246-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yildiz S., Grinstead J., Hildebrand A., et al., “Immediate Impact of Yogic Breathing on Pulsatile Cerebrospinal Fluid Dynamics,” Scientific Reports 12 (2022): 10894, 10.1038/s41598-022-15034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baconnier S., Lang S. B., Polomska M., Hilczer B., Berkovic G., and Meshulam G., “Calcite Microcrystals in the Pineal Gland of the Human Brain: First Physical and Chemical Studies,” Bioelectromagnetics 23, no. 7 (2002): 488–495, 10.1002/bem.10053. [DOI] [PubMed] [Google Scholar]
- 44. Lang S. B., Marino A. A., Berkovic G., Fowler M., and Abreo K. D., “Piezoelectricity in the Human Pineal Gland,” Bioelectrochemistry and Bioenergetics 41, no. Issue 2 (1996): 191–195, 10.1016/S0302-4598(96)05147-1. [DOI] [Google Scholar]
- 45. Herkenham M. and Nauta W. J. H., “Efferent Connections of the Habenular Nuclei in the Rat,” Journal of Comparative Neurology 187, no. 1 (1979): 19–47, 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- 46. Ronnekleiv O. K. and Moller M., “Brain‐Pineal Nervous Connections in the Rat: An Ultrastructure Study Following Habenular Lesion,” Experimental Brain Research 37 (1979): 551–562. [DOI] [PubMed] [Google Scholar]
- 47. Ronnekleiv O. K., Kelly M. J., and Wuttke W., “Single Unit Recordings in the Rat Pineal Gland: Evidence for Habenulo‐Pineal Neural Connections,” Experimental Brain Research 39 (1980): 187–192. [DOI] [PubMed] [Google Scholar]
- 48. Arunkumar K., “Age‐ and Sex‐ Related Changes in Pineal Gland: A Morphological and Histological Study,” American Journal of Internal Medicine 3 (2015): 10, 10.11648/j.ajim.s.2015030601.13. [DOI] [Google Scholar]
- 49. Karasek M. and Reiter R. J., “Melatonin and Aging,” Neuroendocrinology Letters 23 Suppl 1 (2002): 14–16. [PubMed] [Google Scholar]
- 50. Liu R. Y., Zhou J. N., van Heerikhuize J., Hofman M. A., and Swaab D. F., “Decreased Melatonin Levels in Postmortem Cerebrospinal Fluid in Relation to Aging, Alzheimer's Disease, and Apolipoprotein E‐epsilon4/4 Genotype,” Journal of Clinical Endocrinology and Metabolism 84, no. 1 (1999): 323–327, 10.1210/jcem.84.1.5394. [DOI] [PubMed] [Google Scholar]
- 51. Matsuoka T., Imai A., Fujimoto H., et al., “Reduced Pineal Volume in Alzheimer Disease: A Retrospective Cross‐Sectional MR Imaging Study,” Radiology 286, no. 1 (2018): 239–248, 10.1148/radiol.2017170188. [DOI] [PubMed] [Google Scholar]
- 52. Matsuoka T., Oya N., Yokota H., Akazawa K., Yamada K., and Narumoto J.. Alzheimer's Disease Neuroimaging Initiative ., “Pineal Volume Reduction in Patients With Mild Cognitive Impairment Who Converted to Alzheimer's Disease,” Psychiatry and Clinical Neurosciences 74, no. 11 (2020): 587–593, 10.1111/pcn.13103. [DOI] [PubMed] [Google Scholar]
- 53. Skene D., “Melatonin Rhythmicity: Effect of Age and Alzheimer's Disease,” Experimental Gerontology 38, no. 1–2 (2003): 199–206. [DOI] [PubMed] [Google Scholar]
- 54. Feng Z., Chang Y., Cheng Y., et al., “Melatonin Alleviates Behavioral Deficits Associated With Apoptosis and Cholinergic System Dysfunction in the APP 695 Transgenic Mouse Model of Alzheimer's Disease,” Journal of Pineal Research 37 (2004): 129–136. [DOI] [PubMed] [Google Scholar]
- 55. García‐Mesa Y., Giménez‐Llort L., López L. C., et al., “Melatonin Plus Physical Exercise are Highly Neuroprotective in the 3×Tg‐AD Mouse,” Neurobiology of Aging 33 (2012): 1124.e13–e29. [DOI] [PubMed] [Google Scholar]
- 56. Lahiri D. K., Chen D., Ge Y. W., Bondy S. C., and Sharman E. H., “Dietary Supplementation With Melatonin Reduces Levels of Amyloid Beta‐Peptides in the Murine Cerebral Cortex,” Journal of Pineal Research 36, no. 36 (2004): 224–231. [DOI] [PubMed] [Google Scholar]
- 57. Lahiri D. K., “Melatonin Affects the Metabolism of the β‐Amyloid Precursor Protein in Different Cell Types,” Journal of Pineal Research 26 (1999): 137–146. [DOI] [PubMed] [Google Scholar]
- 58. Matsubara E., Bryant‐Thomas T., Pacheco Quinto J., et al., “Melatonin Increases Survival and Inhibits Oxidative and Amyloid Pathology in a Transgenic Model of Alzheimer's Disease,” Journal of Neurochemistry 85 (2003): 1101–1108. [DOI] [PubMed] [Google Scholar]
- 59. Hardeland R., “Melatonin and Inflammation—Story of a Double‐Edged Blade,” Journal of Pineal Research 65, no. 4 (2018): e12525. [DOI] [PubMed] [Google Scholar]
- 60. Tordjman S., Chokron S., Delorme R., et al., “Melatonin: Pharmacology, Functions and Therapeutic Benefits,” Current neuropharmacology 15 (2017): 434–443, 10.2174/1570159x14666161228122115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Menczel Schrire Z., Phillips C. L., Duffy S. L., et al., “3‐Month Melatonin Supplementation to Reduce Brain Oxidative Stress and Improve Sleep in Mild Cognitive Impairment: A Randomised Controlled Feasibility Trial,” Journal of Pineal Research 76, no. 8 (2024): e70019, 10.1111/jpi.70019. [DOI] [PubMed] [Google Scholar]
- 62. Das R., Balmik A. A., and Chinnathambi S., “Melatonin Reduces GSK3β‐Mediated Tau Phosphorylation, Enhances Nrf2 Nuclear Translocation and Anti‐Inflammation,” ASN Neuro 12 (2020): 1759091420981204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wu Y.‐H. and Swaab D. F., “The Human Pineal Gland and Melatonin in Aging and Alzheimer's Disease,” Journal of Pineal Research 38, no. 3 (2005): 145–152, 10.1111/j.1600-079x.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- 64. Moriya T., Horie N., Mitome M., and Shinohara K., “Melatonin Influences the Proliferative and Differentiative Activity of Neural Stem Cells,” Journal of Pineal Research 42 (2007): 411–418, 10.1111/j.1600-079X.2007.00435.x. [DOI] [PubMed] [Google Scholar]
- 65. Chen X., Li X., Du Z., et al., “Melatonin Promotes the Acquisition of Neural Identity Through Extracellular‐Signal‐Regulated Kinases 1/2 Activation,” Journal of Pineal Research 57 (2014): 168–176, 10.1111/jpi.12153. [DOI] [PubMed] [Google Scholar]
- 66. Cramer H., Hall H., Leach M., et al., “Prevalence, Patterns, and Predictors of Meditation Use Among US Adults: A Nationally Representative Survey,” Scientific Reports 6 (2016): 36760, 10.1038/srep36760. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.
Data Availability Statement
The data that support the findings of this study are available in ADNI3 at https://adni.loni.usc.edu/data-samples/access-data/. These data were derived from the following resources available in the public domain: ‐ ADNI, https://adni.loni.usc.edu/data-samples/access-data/ ‐ Wendy Hasenkamp, https://wendyhasenkamp.net/ ‐ BASEII, https://www.base2.mpg.de/7549/data-documentation ‐ LEMON, https://fcon_1000.projects.nitrc.org/indi/retro/MPI_LEMON.html ‐ Sandra Chapman, https://centerforbrainhealth.org/
The pineal gland MNI mask developed for this study is available on reasonable request emailing Dr. Emanuele RG Plini (plinie@tcd.ie – emanuele.rg.plini@gmail.com). The significant cluster of voxels of the pineal gland are available in nifti format in the supplementary materials.
The data on Meditators were provided by Wendy Hasenkamp as described in the methods and they are accessible upon reasonable request: https://wendyhasenkamp.net
The ADNI and the LEMON datasets used are opensource and can be accessed referring to the information provided in the methods.
ADNI: https://adni.loni.usc.edu/data-samples/access-data/
LEMON: https://fcon_1000.projects.nitrc.org/indi/retro/MPI_LEMON.html
Data of the Berlin Aging Study II project can be accessed at the following link prior dedicated documentation: https://www.base2.mpg.de/7549/data-documentation
Data of the Dallas Centre of Brain Health: are available from Jeffrey S. Spence, Center for BrainHealth, University of Texas at Dallas, on reasonable request: https://centerforbrainhealth.org/.


